Among the branched-chain amino acids, only valine metabolism is up-regulated in astrocytes during glutamate exposure
Abstract
Glutamate homeostasis during glutamatergic neurotransmission is predominantly maintained via functioning of the glutamate-glutamine cycle. However, the glutamate-glutamine cycle explains only the fate of the carbon atoms but not that of the accompanying transfer of nitrogen from neurons to astrocytes. In this respect, a putative branched-chain amino acid (BCAA) shuttle has been suggested for transfer of amino nitrogen. Metabolism of BCAAs was investigated in cultured cerebellar astrocytes in a superfusion paradigm employing 15N-labeled leucine, isoleucine, or valine. Some cultures were exposed to pulses of glutamate (50 μM; 10 sec every 2 min; 75 min in total) to mimic conditions during glutamatergic synaptic activity. 15N labeling of glutamate, aspartate, glutamine, alanine, and the three BCAAs was determined by using mass spectrometry. Incorporation of 15N into intracellular glutamate from [15N]leucine, [15N]isoleucine, or [15N]valine amounted to about 40–50% and differed only slightly among the individual BCAAs. Interestingly, label (%) in glutamate from [15N]valine was not decreased upon exposure to exogenous glutamate, which was in contrast to a marked decrease in labeling (%) from [15N]leucine or [15N]isoleucine. This suggests an up-regulation of transamination involving only valine during repetitive exposure to glutamate. It is suggested that valine in particular might have an important function as an amino acid translocated between neuronal and astrocytic compartments, a function that might be up-regulated during synaptic activity. © 2007 Wiley-Liss, Inc.
The discovery of intercellular compartmentation of glutamine and glutamate pools, related to astrocytes and neurons, respectively, led to the suggestion of a glutamate-glutamine cycle operating between glutamatergic neurons and astrocytes (van den Berg and Garfinkel, 1971; Benjamin and Quastel, 1972; Berl and Clarke, 1983; Ottersen et al., 1992). As shown in Figure 1, released neurotransmitter glutamate is taken up into surrounding astrocytes, transformed into glutamine by glutamine synthetase (GS), and released into the extracellular space from which it is taken up into neurons and transformed back to glutamate by phosphate-activated glutaminase (PAG). Note in Figure 1 that, for each molecule of glutamate recycled, one molecule of ammonium is assimilated in the astrocytes and one molecule of ammonium is produced in the neurons. This ammonium must be translocated back to the astrocytes for detoxification, because an elevated ammonium concentration has detrimental effects on a number of cellular functions, which may include inhibition of pyruvate dehydrogenase and/or α-ketoglutarate dehydrogenase (Lai and Cooper, 1986; Zwingmann et al., 2003). Basically, translocation of ammonium may take place by different means; the ammonium may simply diffuse (as NH3) or be transported (as ammonium ion; NH4+) across the cell membranes. Ammonium can diffuse across lipid membranes, and it has been shown that ammonium is transported by K+/Cl− cotransporters, as reviewed by Marcaggi and Coles (2001). However, rather than by a combination of simple and facilitated diffusion, an apparently more attractive way of translocating ammonium between neuronal and astrocytic compartments is via an amino acid shuttle. A shuttle system based on the branched-chain amino acids (BCAAs) as the amino acids translocated from neurons to astrocytes has been proposed (Yudkoff et al., 1996a, b; Yudkoff, 1997; Bixel et al., 1997, 2001; Hutson et al., 1998; Lieth et al., 2001). This shuttle, as outlined in Figure 1, is based on several observations, among others the distribution of isoforms of the branched-chain amino acid aminotransferase (BCAT), with the cytosolic variant being present in neurons and the mitochondrial in astrocytes (Bixel et al., 1997, 2001). Furthermore, BCAAs, not ammonium, were found to be amino group donors for glutamate biosynthesis in astrocytes (Gamberino et al., 1997; Hutson et al., 1998). In line with this, oxidation of the branched-chain keto acids (BCKAs) is low, and 70% of the leucine taken up into cultured astrocytes during 30 min of incubation is released as the cognate keto acid (Yudkoff et al., 1996a). In a putative glutamate/glutamine-BCAA shuttle, the ammonium released in the neuronal PAG reaction is fixed by glutamate dehydrogenase (GDH) and transaminated by BCATc into a BCAA. This BCAA is released and taken up into astrocytes in which the process is reversed, the released ammonium being used by the GS reaction. In this way, ammonium is cycled between the neuronal and the astrocytic compartments. Inherent in this hypothesis is the fact that there should be an activity-dependent coupling between glutamate-glutamine cycle activity and activity in the ammonium-carrying amino acid shuttle. This has been addressed experimentally in the present work employing cultured astrocytes in a superfusion paradigm. The cultures were superfused in the presence of 15N-labeled BCAAs in combination with the cognate keto acid. The keto acid was included for two main reasons: first, to mimic the presence of a BCAA shuttle in which neurons release the cognate keto acid into the extracellular space and, second, to make sure that the keto acid is present in an amount sufficient for the BCAT reaction to operate. Some cultures were exposed to short pulses of glutamate to mimic glutamatergic synaptic activity. Cell extracts were subsequently analyzed for content and 15N-labeling of intracellular glutamate, aspartate, alanine, and glutamine as well as the precursor.
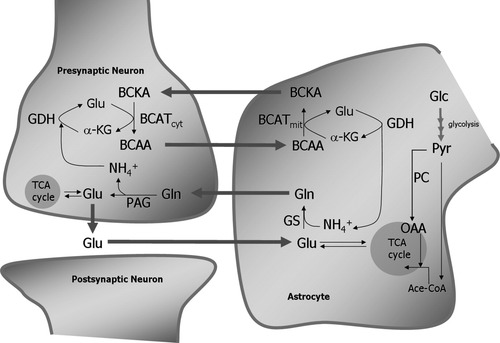
Schematic representation of a glutamate/glutamine-BCAA shuttle working at the glutamatergic synapse. Released neurotransmitter glutamate (Glu) is predominantly taken up into astrocytes, where it is amidated to glutamine (Gln) by glutamine synthetase (GS) using free ammonium and returned to the neurons. In the neurons, the phosphate-activated glutaminase (PAG) reaction regenerates glutamate and produces ammonium. Glutamate might to some extent be metabolized in the tricarboxylic acid (TCA) cycle of both neurons and astrocytes. Ammonium fixed in the glutamate dehydrogenase (GDH) reaction in neurons is transaminated into the branched chain keto acid (BCKA) to form the cognate branched chain amino acid (BCAA), which is exported to astrocytes. In astrocytes, the process is reversed, and the α-keto acid is transported to the neurons. Two cell-specific isoforms of branched-chain aminotransferase (BCAT) are involved in the transaminations. Ace-CoA, acetyl-CoA; BCAT, branched-chain aminotransferase (mit, mitochondrial; cyt, cytosolic isoforms); Glc, glucose; α-KG, α-ketoglutarate; OAA, oxaloacetate; PC, pyruvate carboxylase; Pyr, pyruvate.
MATERIALS AND METHODS
Materials
Seven-day-old NMRI mice were obtained from Taconic M&B (Ry, Denmark). Plastic tissue culture dishes were purchased from NUNC A/S (Roskilde, Denmark), fetal calf serum from SeraLab Ltd. (Sussex, United Kingdom). Culture medium and poly-D-lysine (MW >300,000) were from Sigma Chemical Co. (St. Louis, MO). Penicillin was from Leo (Ballerup, Denmark). Isotopically labeled compounds were either from Cambridge Isotopes Laboratories, Inc. (Cambridge, MA) or Isotec (a subsidiary of Sigma Chemical Co.); all were >98% enriched. The Phenomenex (Torrance, CA) EZ:faast LC-MS kit was used for amino acid analysis. All other chemicals used were of the purest grade available from regular commercial sources.
Preparation of Mouse Cerebellar Astrocyte Cultures
Cerebellar astrocytes were cultured from dissociated cerebella from 7-day-old mice as detailed by Hertz et al. (1989) and Waagepetersen et al. (2000). Cultures were prepared by mechanically dissociating the dissected cerebella by squeezing the tissue through 80-μm nylon sieves into the culture medium and subsequently seeding the resulting cell suspension in 80- or 25-cm2 culture flasks (15 or 5 ml/flask corresponding to 4 or 0.8 animals, respectively). The cells were cultured in a slightly modified Dulbecco's medium containing 6 mM glucose and 2.5 mM glutamine (Hertz et al., 1982). The culture medium was changed twice per week and maintained for 3 weeks before experiments were performed. During the last week of the culturing period, dibutyryl-cAMP (final concentration 0.25 mM) was added to the culture medium, which induces morphological and biochemical differentiation (Juurlink and Hertz, 1985; Hertz et al., 1989).
Superfusion Experiments
All experiments were performed by employing a superfusion paradigm. The culture medium was replaced with a Tris-buffered, Mg2+-free saline solution (50 mM Tris at pH 7.4, 5 mM KCl, 135 mM NaCl, 1.0 mM CaCl2, 37°C). Subsequently, the cultures were placed in a superfusion system (Drejer et al., 1987). The cell layer was covered with a nylon mesh (80 μm), and the culture flasks were superfused (4–5 ml/min) with Tris-buffered saline containing 1 mM [15N]leucine and 1 mM 4-methyl-2-oxopentanoic acid, 1 mM [15N]isoleucine and 1 mM 3-methyl-2-oxopentanoic acid, or 1 mM [15N]valine and 1 mM 3-methyl-2-oxobutanoic acid. To mimic synaptic activity, the superfusion medium was repetitively alternated to a similar medium containing glutamate (50 μM). The cell cultures were superfused for 75 min and exposed to 34 pulses of glutamate every 2 min lasting for 10 sec each. The cell cultures were subsequently washed twice with ice cold phosphate-buffered saline and metabolites were extracted using 70% v/v ethanol. The cell extracts were lyophilized and redissolved in water for analysis of content and labeling of intracellular metabolites.
Biochemical Analysis
Amino acids were quantified by reversed-phase HPLC employing precolumn, online o-phthaldialdehyde derivatization, and fluorescence detection (excitation 350 nm, detection 450 nm) in principle as described by Geddes and Wood (1984). Mass spectrometric analysis was performed by employing an LC-MS system consisting of a Shimadzu LCMS-2010 mass spectrometer coupled to a Shimadzu 10A VP HPLC system. The Phenomenex EZ:faast amino acid analysis kit for LC-MS was employed for analysis of labeling in relevant amino acids. Protein content was determined according to Lowry et al. (1951) using bovine serum albumin as the standard.
Data Analysis
All labeling data were corrected for natural abundance of isotopes and isotopic enrichment was calculated according to Biemann (1962). Data analysis was performed in Microsoft Excel 2003 and GraphPad Prism v4.01. All data are presented as averages ± SEM. Differences between groups were analyzed by employing one-way ANOVA, followed by Bonferroni's multiple-comparisons tests. P < 0.05 was considered statistically significant.
RESULTS
[15N]leucine and [15N]isoleucine labeled glutamate, glutamine, aspartate, and alanine to similar extents, except for double labeling of glutamine, which was tree times higher compared with [15N]leucine than [15N]isoleucine (Tables I, II). As expected, the highest extent of labeling, approximately 50%, was observed in glutamate labeled after the operation of BCAT. Labeling of aspartate and alanine, which occurs in subsequent transaminations, was 40% and 30%, respectively. Monolabeling of glutamine was similar to that of alanine. Interestingly, in comparing single and double labeling of glutamine from [15N]leucine and [15N]isoleucine, the double labeling constituted almost 15% of the single labeling from [15N]leucine but only 3% of that from [15N]isoleucine.
| Amino acid | Content (nmol/mg protein) | Mono label (%) | Double label (%) | |||
|---|---|---|---|---|---|---|
| Control | Rep. glu. | Control | Rep. glu. | Control | Rep. glu. | |
| Glutamine | 0.9 ± 0.1 | 1.3 ± 0.1 | 24.9 ± 2.8 | 18.0 ± 2.8* | 3.8 ± 0.1 | 2.6 ± 0.4 |
| Glutamate | 10.5 ± 0.9 | 24.6 ± 4.8* | 52.4 ± 1.2 | 25.1 ± 4.2* | — | — |
| Aspartate | 1.8 ± 0.1 | 3.1 ± 0.7 | 40.8 ± 0.7 | 19.3 ± 2.7* | — | — |
| Alanine | 3.3 ± 0.1 | 3.5 ± 0.1 | 27.7 ± 2.4 | 11.7 ± 1.2* | — | — |
| Leucine | 14.6 ± 1.0 | 18.4 ± 0.8 | 94.8 ± 0.3 | 92.3 ± 0.5 | — | — |
- † Cerebellar astrocytes prepared as described in Materials and Methods were superfused in the presence of [15N]leucine (1.0 mM) and ketoleucine (4-methyl-2-oxopentanoic acid; 1.0 mM). The effect of repetitive pulses of glutamate (50 μM; 10 sec) was investigated (see Materials and Methods). Results are averages of content and percentage excess labeling of intracellular amino acids ± SEM of four individual cultures.
- * Statistically significant differences (one-way ANOVA followed by Bonferroni's multiple comparison test; P < 0.05).
| Amino acid | Content (nmol/mg protein) | Mono label (%) | Double label (%) | |||
|---|---|---|---|---|---|---|
| Control | Rep. glu. | Control | Rep. glu. | Control | Rep. glu. | |
| Glutamine | 1.4 ± 0.1 | 1.6 ± 0.1 | 28.6 ± 3.2 | 16.5 ± 0.6* | 0.9 ± 0.3 | 0.7 ± 0.0 |
| Glutamate | 18.6 ± 1.1 | 46.9 ± 4.9* | 47.9 ± 0.4 | 17.0 ± 0.7* | — | — |
| Aspartate | 2.3 ± 0.1 | 1.0 ± 0.2 | 40.8 ± 0.3 | 15.1 ± 0.6* | — | — |
| Alanine | 4.4 ± 0.5 | 5.1 ± 0.7 | 32.3 ± 1.3 | 16.1 ± 0.6* | — | — |
| Isoleucine | 15.6 ± 0.9 | 12.5 ± 1.5 | 94.9 ± 0.4 | 92.8 ± 0.4 | — | — |
- † Cerebellar astrocytes prepared as described in Materials and Methods were superfused in the presence of [15N]isoleucine (1.0 mM) and ketoisoleucine (3-methyl-2-oxopentanoic acid; 1.0 mM). The effect of repetitive pulses of glutamate (50 μM; 10 sec) was investigated (see Materials and Methods). Results are averages of content and percentage excess labeling of intracellular amino acids ± SEM of four individual cultures.
- * Statistically significant differences (one-way ANOVA followed by Bonferroni's multiple comparison test; P < 0.05).
All amino acids except for double labeling of glutamine (and monolabeling in the presence of [15N]leucine) showed a decrease of approximately 50% in label from [15N]leucine and [15N]isoleucine following exposure to glutamate. Label in the precursors was more than 90% showing no effect of exposure to glutamate and a minor importance of reamination of the cognate keto acids. The content of glutamate was increased two- to threefold in cultures treated with glutamate in the presence of [15N]leucine or [15N]isoleucine. In contrast, when [15N] valine was the precursor, label in glutamate was not reduced upon exposure to glutamate (Table III), although the glutamate content increased fourfold. This was accompanied by significant increases in the labeling of aspartate and alanine after exposure to glutamate by 30% and 100%, respectively. This increase in labeling was not reflected in the intracellular amounts of aspartate and alanine. Upon exposure to glutamate, monolabeling of glutamate was twice that of glutamine, which was in contrast to the use of leucine and isoleucine as precursors, in which case they were identical. The labeling of valine was, as observed for [15N]isoleucine and [15N]leucine, unaffected by exposure to glutamate.
| Amino acid | Content (nmol/mg protein) | Mono label (%) | Double label (%) | |||
|---|---|---|---|---|---|---|
| Control | Rep. glu. | Control | Rep. glu. | Control | Rep. glu. | |
| Glutamine | 1.0 ± 0.1 | 1.4 ± 0.1 | 25.9 ± 1.2 | 24.5 ± 1.8 | 0.9 ± 0.3 | 2.9 ± 0.7 |
| Glutamate | 14.9 ± 0.3 | 66.6 ± 12.4* | 41.5 ± 0.9 | 40.3 ± 0.6 | — | — |
| Aspartate | 1.6 ± 0.1 | 0.9 ± 0.1 | 28.5 ± 1.2 | 37.3 ± 0.5* | — | — |
| Alanine | 4.9 ± 0.7 | 4.0 ± 0.2 | 20.6 ± 1.2 | 43.6 ± 1.4* | — | — |
| Valine | 18.8 ± 1.0 | 15.2 ± 0.8 | 90.2 ± 1.3 | 92.2 ± 0.2 | — | — |
- † Cerebellar astrocytes prepared as described in Materials and Methods were superfused in the presence of [15N]valine (1.0 mM) and ketovaline (3-methyl-2-oxobutanoic acid; 1.0 mM). The effect of repetitive pulses of glutamate (50 μM; 10 sec) was investigated (see Materials and Methods). Results are averages of content and percentage excess labeling of intracellular amino acids ± SEM of four individual cultures.
- * Statistically significant differences are indicated by asterisks (one-way ANOVA followed by Bonferroni's multiple comparison test; P < 0.05).
DISCUSSION
Maintaining intercellular ammonium homeostasis during synaptic activity as well as global brain ammonium homeostasis is of vital importance for proper functioning of the brain. The latter is maintained primarily by a combination of influx of BCAAs and efflux of glutamine (see, e.g., Oldendorf, 1971; Smith et al., 1987; Yudkoff, 1997; Hawkins et al., 2002), whereas intercellular ammonium homeostasis linked to the glutamate-glutamine cycle may be maintained via a glutamate/glutamine-BCAA shuttle (see Fig. 1 and the introductory paragraph for references). The astrocytic part of this shuttle was investigated in the present study. The paradigm of introducing pulses of glutamate to mimic the presence of an active glutamatergic synapse may induce several cellular events in the astrocyte: 1) some or most of the glutamate taken up is transformed into glutamine and released to the medium, both uptake and glutamine synthesis being energy requiring; 2) some of the exogenous glutamate may enter the mitochondria to be oxidatively metabolized involving pyruvate recycling for complete oxidation; 3) transaminations will be affected by uptake/accumulation of exogenous glutamate and BCKAs; 4) flux through the mitochondrial GDH reaction producing α-ketoglutarate and ammonium will increase in the face of accumulation of glutamate; and, finally, 5) leucine (present in some experiments) and ADP act both individually and synergistically as allosteric activators of GDH (Plaitakis and Zaganas, 2001; Zaganas et al., 2001), which may be important in an experimental paradigm inducing cellular energy consumption via glutamate uptake and glutamine synthesis.
Transamination of the three BCAAs was extensive, as evidenced by the degree of labeling of glutamate, glutamine, aspartate, and alanine from the 15N-labeled BCAAs. As might be expected, the label in glutamate (and consequently in aspartate and alanine) from [15N] leucine and [15N]isoleucine decreased upon exposure to glutamate. Label in the precursors did not change, even though the label in glutamate decreased, suggesting a minor importance for reamination of the BCKAs, which is consistent with previous findings (Yudkoff et al., 1994). However, no decrease in label of glutamate was observed when [15N]valine was the precursor, and, in addition, labeling of both aspartate and alanine actually increased. Furthermore, upon exposure to glutamate in the presence of [15N]valine, the content of glutamate increased four- to fivefold (with no change in percentage labeling), compared with two- to threefold in the presence of [15N]Leucine or [15N]isoleucine. Overall, this suggests that valine was metabolized to a greater extent than leucine or isoleucine during exposure to glutamate mimicking synaptic activity, insofar as both the label in the glutamate pool and the amount of glutamate were increased. This increased synthesis of labeled glutamate from [15N]valine is in line with a role for valine as an amino group nitrogen donor for astrocytic glutamate and subsequent glutamine synthesis during synaptic activity, as has been suggested as a major role of the BCAAs (Yudkoff, 1997; Lieth et al., 2001). The differential effect of glutamate on the metabolism of isoleucine and leucine compared with valine might be explained by differences in kinetic parameters of the BCAT reaction (Yudkoff, 1997). Uptake of the BCAAs may be increased during release of glutamine, insofar as the system L amino acid exchanger located in the astrocytic cell membrane has been suggested to work in this way, exchanging glutamine for external BCAAs as well as alanine (Deitmer et al., 2003). Such a mechanism may be operating differentially for the three BCAAs and may favor uptake of valine. In addition, differential properties in release of the cognate keto acids may play a role as well, as the ketovaline may be released more avidly than ketoleucine and ketoisoleucine. However, this parameter was not analyzed in the present work.
The GDH reaction producing α-ketoglutarate and ammonium from glutamate is an important step in the astrocytic part of the glutamate/glutamine-BCAA shuttle (Fig. 1), in that the glutamate that is formed in the BCAT reaction must be oxidatively deaminated by GDH to produce the ammonium for glutamine synthesis by the cytosolic GS reaction. Thus, in relation to a putative BCAA shuttle, the activity of GDH for formation of 15NH4+ from [15N]glutamate (derived from [15N]BCAAs) is important to evaluate. It was not determined in the present study the extent to which the monolabeling of glutamine is in the amino or the amide group. However, given the high level of label in glutamate, monolabeling in glutamine is probably mainly [2-15N]glutamine synthesized from [15N]glutamate using unlabeled NH4+. However, the extent of double labeling of glutamine may reveal some insight, in that this is possible only if [2-15N]glutamate is amidated using 15NH4+. Double labeling of glutamine was generally modest but was most pronounced from [15N]leucine, which may be explained by the fact that GDH is activated by leucine (Plaitakis and Zaganas, 2001; Zaganas et al., 2001). The extent of double labeling suggests importance of fixing ammonium, generated by GDH activity, into glutamine. No significant changes were observed after exposure to glutamate, which may indicate that there is no activity-dependent transfer of 15N from [15N]BCAAs to the amide group of glutamine. A likely increased ADP concentration resulting from cellular energy consumption via glutamate uptake and glutamine synthesis had no significant effect on labeling obtained from the operation of GDH. Furthermore, as glutamine may be released and thus not measured in the present study, it can not be concluded whether there is an activity-dependent coupling between glutamate-glutamine cycling and transfer of ammonium from the BCAAs into the amide group of glutamine, although this must be considered very likely. In this respect, the finding that valine is handled very differently from leucine and isoleucine makes it tempting to speculate that valine plays an important role in astrocytic metabolism different from that of leucine and isoleucine. In relation to a putative BCAA shuttle accompanying the glutamate-glutamine cycle, the fact that metabolism of valine was increased upon exposure to repetitive pulses of glutamate might indicate that valine is important for intercellular ammonium homeostasis during synaptic activity. Interestingly, it has been suggested that a specific enzyme responsible for transamination of valine may be present (Yudkoff et al., 1994), which is supported by a clinical finding of hypervalinemia (Tada et al., 1967). To elucidate the role of valine in ammonium transfer between glutamatergic neurons and astrocytes, studies employing cocultures of neurons and astrocytes or brain miniprisms representing intact neuronal-astrocytic networks may be warranted.
Acknowledgements
The authors are grateful to Dr. Marc Yudkoff (University of Pennsylvania, Philadelphia) for providing fruitful comments regarding these results. The expert technical assistance of Ms. Lene Vigh is cordially acknowledged.




