Oxidative damage in mild cognitive impairment and early Alzheimer's disease
Abstract
Increasing evidence supports a role for oxidative damage in the pathogenesis of Alzheimer's disease (AD). Multiple studies show significantly increased levels of lipid peroxidation and protein, DNA, and RNA oxidation in vulnerable regions of the brain of patients with late-stage AD (LAD). More recent studies of patients with amnestic mild cognitive impairment (MCI), the earliest clinical manifestation of AD, show similar patterns of oxidative damage. These observations suggest that oxidative damage to critical biomolecules occurs early in the pathogenesis of AD and precedes pronounced neuropathologic alterations. Because oxidative damage begins early in the progress of the disease, it represents a potential therapeutic target for slowing the onset and progression of AD. © 2007 Wiley-Liss, Inc.
Alzheimer's disease (AD) is the fourth leading cause of death in the United States, in the year 2000 affecting 4.5 million Americans (Hebert et al.,2003). Current statistics suggest that ∼3% of Americans between ages 65 and 74 years, 19% between ages 75 and 84 years, and 47% over age 85 years are victims of the disease (Evans et al.,1989). With the aging of the U.S. population and lack of preventive strategies, there may be 14 million Americans with AD by the year 2040 (Hebert et al.,2003). Clinically, AD is characterized by a progressive decline in multiple cognitive functions and is thought to begin with mild cognitive impairment (MCI), which is widely considered to be a transition between normal aging and dementia. The clinical criteria for the diagnosis of MCI are those of Petersen et al. (1999) and include 1) memory complaints, 2) objective memory impairment for age and education, 3) intact general cognitive function, 4) intact activities of daily living (ADLs), and 5) the subject not being demented. Objective memory test impairment is based on a score of ≥1.5 standard deviations from the mean of controls on the CERAD Word List Learning Task (Morris et al.,1989) and corroborated in some cases with the Free and Cued Selective Reminding Test. Current data suggest that conversion from MCI to dementia occurs at a rate of 10% to 15% per year (Petersen et al.,1999), with ∼80% conversion by the sixth year of follow-up, although ∼5% of MCI patients remain stable or revert to normal (Bennett et al.,2002; DeCarli,2003).
The major barrier to treating and eventually preventing AD is a lack of understanding of the etiology and pathogenesis of neuron degeneration and loss. Numerous etiologic/pathogenic mechanisms have been suggested for AD, including genetic defects (St. George-Hyslop et al.,1987; St. George-Hyslop,1994), the amyloid cascade hypothesis (Selkoe,1991), trace element toxicity (Markesbery and Ehmann,1994), mitochondrial defects (Wallace,1992), and the oxidative stress hypothesis (Coyle and Puttfarcken,1993). Among these hypotheses, the oxidative stress hypothesis is particularly appealing insofar as it encompasses several other hypotheses, including the involvement of redox active trace elements (iron and copper), mitochondrial dysfunction, and free radical and amyloid β peptide hypotheses. A large body of literature over the past 10–15 years suggests that free-radical-mediated damage is associated with vulnerable regions of the late-stage AD (LAD) brain and that these alterations play a role in the pathogenesis of the disease. Studies show that increased lipid peroxidation and protein, DNA, and RNA oxidation are present in multiple vulnerable regions of the LAD brain (Picklo et al.,2002; Markesbery et al.,2006; Nunomura et al.,2006; Sultana et al.,2006). Although these studies show that oxidative damage is present in LAD, it is unclear whether oxidative damage is a consequence of the disease or whether it occurs early in the pathogenesis, thus making it a potential therapeutic target.
With the description of MCI as a transition between normal aging and dementia, recent emphasis has focused on early disease detection and elucidation of pathogenic mechanisms with the hope of early treatment to stop or slow progression of the disease. The study of patients with MCI provides insight into the pathogenic features of AD that occur early in the disease.
REACTIVE OXYGEN SPECIES AND OXIDATIVE STRESS
The brain is particularly susceptible to oxidative damage because of its high oxygen consumption rate (∼1/5 of consumed oxygen), its status as a postmitotic tissue with high energy demands, the relatively limited antioxidant capacity compared with other tissues, the high abundance of polyunsaturated fatty acids, and the relatively high level of redox active metals, including iron. In general, the predominant source of reactive oxygen species (ROS) is mitochondrial oxidative phosphorylation in which molecular oxygen is converted to water along with the production of ATP. Unfortunately, ∼2% of oxygen utilized during oxidative phosphorylation is converted to superoxide radical (O2–; Halliwell and Gutteridge,1989) that is detoxified by mitochondrial manganese superoxide dismutase (Mn-SOD) to produce H2O2. In normally functioning cells, H2O2 is converted to water by glutathione peroxidase (GSH-Px) or catalase. However, in the presence of redox active metals (Fe and copper), H2O2 can be converted to reactive OH· via Fenton or Haber-Weiss reactions (Fig. 1; for review see Moreira et al.,2005). Previous studies show increased levels of Fe in hippocampus, cerebral cortex, and nucleus basalis of Meynert but not the cerebellum (for review see Markesbery and Ehmann,1994), a pattern that mirrors the distribution of pathologic features in AD, suggesting ample opportunity for the generation of ROS in these vulnerable brain regions. An additional source of ROS in AD is the presence of increased levels of amyloid β peptide (Aβ), a 40- or 42-amino-acid product of proteolytic cleavage of the amyloid precursor protein by β and γ secretases that is the major component of senile plaques in AD. A large body of literature shows that Aβ mediates oxidative damage to lipids, proteins, and DNA (for review see Moreira et al.,2005).
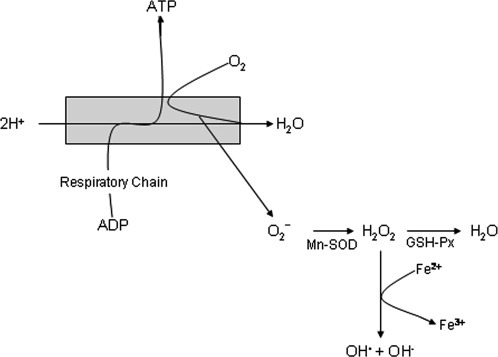
Reactive oxygen species generation during oxidative phosphorylation.
LIPID PEROXIDATION
Over the past 4 years, several studies have shown that there is increased oxidative damage to lipids in vulnerable regions of the MCI brain and in the brains of patients with early AD (EAD). The presence of relatively high concentrations of redox active trace elements and relatively low antioxidant capacities causes the brain to be particularly susceptible to free-radical-mediated lipid peroxidation. Attack of polyunsaturated fatty acids by free radicals leads to structural damage to membranes and generation of several aldehydic byproducts, including malondialdehyde, C3–C10 straight chain aldehydes, and α,β-unsaturated aldehydes including 4-hydroxy-2-nonenal (HNE) and acrolein. These α,β-unsaturated aldehydes show high reactivity with nucleophiles, including sulfhydryl groups of cysteine, histidine, and lysine (Esterbauer et al.,1991). Several studies show that HNE and acrolein are neurotoxic, can inhibit enzymes critical for neuron survival, and can induce changes in tau that are recognized by antibodies against altered tau present in neurofibrillary tangles in AD (Gomez-Ramos et al.,2003; Liu et al.,2005).
Lipid peroxidation also leads to production of isoprostanes and neuroprostanes. Although these molecules do not show toxicity, they serve as excellent markers of arachidonic and docosahexaenoic acid peroxidation in brain and cerebrospinal fluid (CSF; Montine et al.,2005). F2-isoprostane (F2-IsoP) levels in CSF also may be useful as a monitor of the efficacy of antioxidant treatment in AD (for review see Montine et al.,2005).
One of the first studies of lipid peroxidation in MCI evaluated levels of isoprostane 8,12 iso-iPF (2α)-VI in the plasma, urine, and CSF of MCI patients compared with age-matched control subjects and showed a statistically significant increase in this specific biomarker of increased lipid peroxidation in all three fluids of MCI patients compared with controls (Pratico and Sung,2004). Although these studies related a marker of lipid peroxidation present in CSF to MCI, brain levels were not measured. More recently, Keller et al. (2005) showed statistically significantly increased levels of thiobarbituric-acid-reactive substances and malondialdehyde in the temporal lobe of MCI patients compared with age-matched controls. Recently, levels of F2-IsoP, as a marker of free radical damage to arachidonic acid, and F4-neuroprostanes (F4-NP), as a marker of docosahexaenoic acid oxidation, were measured in frontal lobe, inferior parietal lobule, and occipital regions of longitudinally followed MCI patients and age-matched controls (Markesbery et al.,2005). Mean levels of F2-IsoP were significantly elevated in frontal lobe, inferior parietal lobule, and occipital lobe of MCI patients compared with controls and were comparable to levels observed in LAD subjects (Markesbery et al.,2005). F4-NP levels in the same patients were significantly elevated in inferior parietal lobule and occipital lobe in MCI compared with controls, and the levels approached those observed in LAD brain. In more recent studies, Williams et al. (2006) showed significantly elevated levels of HNE in the hippocampus/parahippocampal gyri (HPG), superior and middle temporal gyri (SMTG), and cerebellum of longitudinally followed MCI patients compared with cognitively normal control subjects. Levels of acrolein were also significantly elevated in SMTG of MCI patients compared with controls. Consistent with the studies of isoprostanes/neuroprostanes, levels of the neurotoxic lipid peroxidation markers in MCI were comparable to levels observed in LAD brain. Subsequent studies of protein-bound HNE showed similar results, with increased HNE-immunopositive proteins in MCI HPG and inferior parietal lobule compared with controls (Butterfield et al.,2006a).
Additional studies of Yao et al. (2005) showed that byproducts of 12/15-lipoxygenase peroxidation of arachidonic acid, including 12(s)-hydroxyeicosatetraenoic (HETE) acid and 15(s)-HETE, are significantly elevated in the CSF of MCI and LAD patients compared with controls. Overall, these studies suggest that lipid peroxidation is an early event in the pathogenesis of AD and that levels of oxidative damage are initially elevated in MCI and do not significantly increase with disease progression.
PROTEIN OXIDATION
Studies of protein oxidation in MCI have been more limited. Quantification of levels of protein carbonyls, a nonspecific indicator of protein oxidation, by Keller et al. (2005) showed increased protein oxidation in the SMTG of MCI patients compared with age-matched, cognitively normal control subjects. In subsequent studies, Butterfield et al. (2006b) used redox proteomics to identify specific oxidatively modified proteins in MCI including PIN-1, α-enolase, glutamine synthetase, and pyruvate kinase in the HPG of MCI patients compared with controls. Clearly, more research is needed to elucidate the possible contribution of protein oxidation to the pathogenesis of AD.
DNA OXIDATION
Free radical, particularly hydroxyl radical, attack of DNA can lead to strand breaks, DNA-DNA and DNA-protein cross-linking, and DNA base modification. Free-radical-mediated DNA alterations could contribute to alterations in protein production that further propagate neuron dysfunction and death. In general, mitochondrial DNA (mtDNA) is more susceptible to free-radical-mediated damage because of the proximity to ROS production, the lack of protective histones, the limited repair capacity, and the lack of significant regions of noncoding sequences. Using mass spectrometry, over 20 DNA base adducts have been identified, although most studies have focused on 8-hydroxy-2′-deoxyguanosine [measured as 8-hydroxyguanine (8-OHG)], which is formed through the C8 hydroxylation of guanosine and represents the predominant marker of DNA oxidation. Several studies have demonstrated significantly elevated 8-OHG in addition to other oxidatively modified bases in nuclear DNA (nDNA) and mtDNA in vulnerable regions of the LAD brain (for review see Markesbery and Lovell,2006).
In the only study to date to quantify oxidized DNA in brain specimens from short-post-mortem-interval autopsies of longitudinally followed amnestic MCI patients, we showed that 8-OHG was significantly elevated in nDNA from frontal and temporal lobes of MCI patients compared with age-matched controls (Wang et al.,2006). Levels of 5-hydroxycytosine were also significantly elevated in nDNA from frontal and temporal lobes and parietal lobules and in mtDNA of frontal lobe in MCI. Levels of 8-hydroxyadenine were also significantly elevated in nDNA of frontal and temporal lobes and parietal lobules in MCI patients compared with controls. Fapyadenine (4,6-diamino-5-formamidopyrimidine), which results from ring opening of 8-hydroxyadenine followed by one electron reduction, was significantly higher in nDNA and mtDNA of all three neocortical regions in MCI compared with control subjects. Comparison of neocortical DNA oxidation to levels of DNA oxidation in cerebellum using two-way ANOVA showed that 8-OHG (P < 0.04), fapyadenine (P < 0.001), and 5-hydroxycytosine (P < 0.004) in mtDNA were significantly increased in neocortical regions compared with cerebellum in MCI.
Perhaps the most interesting observation of the study was that levels of 8-OHG, 8-hydroxyadenine, and fapyguanine in MCI were comparable to those observed in LAD brain, suggesting that oxidative modification of nDNA and mtDNA occurs early in the pathogenesis of AD.
RNA OXIDATION
Several previous studies show increased oxidative RNA damage in a variety of neurological disorders, including LAD (for review see Nunomura et al.,2006). In addition, Nunomura et al. (2001) showed significantly increased cytoplasmic staining of 8-OHG in EAD brain that decreased as amyloid β peptide and neurofibrillary tangle burden increased. Increased RNA oxidation has also been observed in a presymptomatic subject who expressed a familial AD mutation (Nunomura et al.,2004) and in Down syndrome subjects with early AD pathology (Hofer et al.,2005). More recently, Ding et al. (2005) showed significantly elevated 8-OHG in MCI inferior parietal lobule but not cerebellum of well-characterized amnestic MCI patients that correlated with decreased ribosomal and transfer RNA and decreased protein synthesis capacity.
CONCLUSIONS AND FUTURE DIRECTIONS
Although previous studies suggest that oxidative damage to a variety of molecules is an early event in the pathogenesis of AD, they did not include analysis of brain specimens from patients with MCI, which likely represents the earliest detectable phase of the disease. As described above, recent studies analyzing specimens from well-documented amnestic MCI patients show oxidative damage to lipids, proteins, DNA, and RNA in vulnerable regions of the brain, which strongly suggests that oxidative damage is involved in the pathogenesis of neuron degeneration in AD. Unfortunately, the markers of oxidative damage in multiple biomolecules have not been measured in the same tissue specimens; therefore, the temporal profile of oxidative damage remains unclear. Because oxidative damage appears to be an early event, it may represent a potential therapeutic target for slowing progression or perhaps preventing onset of AD. In initial clinical trials of antioxidants in MCI, Petersen et al. (2005) showed that treatment of MCI patients with 2,000 IU of vitamin E alone did not provide any benefit. However, the study did not examine the combined effects of vitamins E and C, which have been shown to decrease lipid peroxidation (Montine et al.,2004) and have a protective effect in AD (Engelhart et al.,2002; Morris et al.,2002; Zandi et al.,2004). Because multiple biomolecules ranging from lipids to DNA are oxidized in MCI, it is unlikely that a single antioxidant would be sufficient to interrupt oxidative damage to all classes of molecules. Clearly, improved antioxidants or combinations of antioxidants are necessary to decrease oxidative damage in MCI and LAD.
Acknowledgements
The authors thank Ms. Paula Thomason for technical and editorial assistance.




