Expression of zinc finger transcription factor Bcl11A/Evi9/CTIP1 in rat brain
Abstract
Bcl11A/Evi9/CTIP1, a Kruppel-like zinc finger gene, plays an important role in B-cell development. In addition to expression in B lymphocytes, Bcl11A/Evi9/CTIP1 is also highly expressed in the brain, although its function there is still unclear. In the present study, regional and subcellular distributions of Bcl11A/Evi9/CTIP1 in rat brain were investigated by immunostaining and biochemical fractionation. Using antibodies recognizing the first 18 amino acid residues of Bcl11A/Evi9/CTIP1, the distribution of 2 isoforms of Bcl11A/Evi9/CTIP1 gene products, Bcl11A-L/Evi9a and Bcl11A-S/Evi9c, was examined. In rat brain, both Bcl11A-L/Evi9a and Bcl11A-S/Evi9c were expressed, although the amount of Bcl11A-S/Evi9c protein was higher. Bcl11A-S/Evi9c was widely expressed in different regions of the rat brain. In contrast, Bcl11A-L/Evi9a was more restricted, being expressed in the cerebral cortex, hippocampus, and olfactory bulb. At the subcellular level, biochemical fractionation and confocal analysis of adult rat brain revealed that, in addition to being in the nuclei of neurons, fractions of Bcl11A-L/Evi9a and Bcl11A-S/Evi9c could be found in extranuclear locations. Double staining with the synaptic marker synaptophysin indicated a synaptic distribution of Bcl11A/Evi9/CTIP1. Postsynaptic density was also biochemically purified and subjected to immunoblotting using Bcl11A/Evi9/CTIP1 antibodies. The results showed that Bcl11A-L/Evi9a was enriched in the PSD I and PSD II fractions. In contrast, only a trace amount of Bcl11A-S was detected in PSD fractions. Our study also indicated that a fraction of Bcl11A/Evi9/CTIP1 was present in the cytoplasm, even at synapses. To regulate gene expression in the nuclei, nuclear translocation of Bcl11A/Evi9/CTIP1 may be one of the mechanisms controlling neuronal Bcl11A/Evi9/CTIP1 function. © 2007 Wiley-Liss, Inc.
The Bcl11A/Evi9/CTIP1 gene is expressed specifically in hematopoietic tissue and brain (Nakamura et al.,2000; Avram et al.,2002; Leid et al.,2004). It acts as a transcription repressor through 2 mechanisms: by directly binding to its DNA target sequence, 5′-GGCCGG-3′ (Avram et al.,2002). and by interacting with and repressing other sequence-specific transcription factors, such as COUP-TFs (Avram et al.,2000). In mouse hematopoietic cells, knockout of Bcl11A/Evi9/CTIP1 results in the loss of B lymphocytes and alterations in several types of T cells (Liu et al.,2003), suggesting that Bcl11A/Evi9/CTIP1 plays an essential role in normal lymphoid development. The detailed mechanism underlying the function of Bcl11A/Evi9/CTIP1 in lymphocyte development is not clear. There are indications that an interaction with Bcl6, another B-cell proto-oncogene product (Nakamura et al.,2000), and the recruitment of the class III histone deacetylase SIRT1 (Senawong et al.,2005) are involved in Bcl11A function in B lymphocytes.
Through alternative splicing, human Bcl11A gene encodes 3 isoforms: Bcl11A-S, Bcl11A-L, and Bcl11A-XL (Fig. 1A, also in Satterwhite et al.,2001). In the mouse, the Bcl11A homologs, Evi9 (Nakamura et al.,2000) or CTIP1 (Avram et al.,2000), also encode 3 proteins: Evi9a, Evi9b, and Evi9c (Nakamura et al.,2000). Evi9a in mouse is equivalent to Bcl11A-L in human, Evi9c is the mouse homolog of Bcl11A-S, and Evi9b uses an internal translation initiation site and only encodes the region corresponding to amino acid residues 287–773 of Evi9a. Expression of different transcripts of Bcl11A/Evi9/CTIP1 is complicated and has not been fully explored. So far, the Evi9b transcript has not been identified in humans, whereas the Bcl11A-XL transcript has not been found in the mouse or the rat (Satterwhite et al.,2001), although Bcl11A-XL was shown to be the most abundant isoform in human lymphoid tissues (Liu et al.,2006; Pulford et al.,2006; Weniger et al.,2006). These observations suggest a tissue- or species-specific expression of different Bcl11A transcripts.
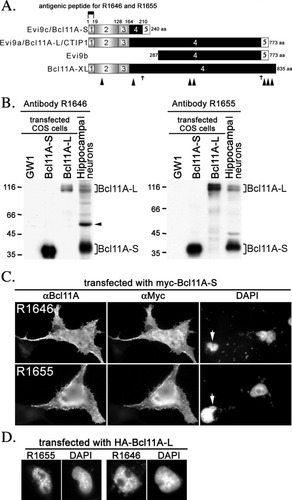
Characterization of Bcl11A antibodies. A: Schematic structure of Bcl11A/Evi9/CTIP1. The Bcl11A/Evi9/CTIP1 gene has a total of 5 exons. Exon usage of the different forms of Bcl11A are shown. Exon 1 encodes amino acid residues 1–18; exon 2 covers amino acid residues 19–127; exon 3 extends from amino acid residue 128 to 164. Exon 4 of the Bcl11A gene has 2 internal splicing sites, indicated by small arrows. Bcl11A-S/Evi9c uses the first internal splicing site in exon 4 and Bcl11A-L/Evi9a the second internal splicing site. Exon 4 is the last exon used in Bcl11A-XL as exon 5 is not used. Large arrowheads mark the positions of zinc fingers. The C-terminal end of the Bcl11A-XL protein has 3 unique zinc fingers. The antigenic peptide epitope generating peptide antibodies R1646 and R1655 corresponds to the first 18 amino acid residues encoded by exon 1 of the Bcl11A/Evi9/CTIP1 gene. B: Immunoblotting using affinity-purified Bcl11A antibodies R1646 and R1655. Total cell lysates of cultured hippocampal neurons and COS cells transfected with Bcl11A-S, Bcl11A-L, and vector GW1 alone, as indicated, were separated by SDS-PAGE and then immunoblotted using R1655 and R1646 antibodies, as indicated. The arrowhead points to the cross-reactive protein species around 50 kDa. Data shown are representative of 3 independent experiments. C: Subcellular distribution of Bcl11A-S revealed by immunostaining. COS cells were transfected with myc-tagged Bcl11A-S and double-stained with anti-myc and Bcl11A antibodies, as indicated. Bcl11A is visualized with FITC staining, myc-tag is visualized with Cy3 staining, and chromosomal DNA is labeled by DAPI. Arrows indicate untransfected cells. Data shown are representative of 3 independent experiments. D: Nuclear distribution of Bcl11A-L revealed by immunostaining. COS cells were transfected with HA-tagged Bcl11A-L and immunostained using Bcl11A antibodies and counterstained with DAPI, as indicated. Data shown are representative of 3 independent experiments.
Although Bcl11A/Evi9/CTIP1 is highly expressed in brain and interacts with COUP-TF1 (Avram et al.,2000), an important orphan nuclear receptor for development of the nervous system (Zhou et al.,1999), the function of Bcl11A/Evi9/CTIP1 in the brain is unknown. In the present study, we used immunohistochemical and biochemical approaches to analyze the distribution of Bcl11A/Evi9/CTIP1 proteins in rat brain. The results showed that Bcl11A/Evi9/CTIP1 proteins were widely distributed in different regions of the brain. Interestingly, in addition to being expressed in the nucleus, a fraction of Bcl11A/Evi9/CTIP1 proteins were present in the cytoplasm and even at synapses. This suggests that nuclear translocation may be involved in the regulation of Bcl11A/Evi9/CTIP1 in neurons. Alternatively, it is also possible that in addition to transcriptional repression, Bcl11A/Evi9/CTIP1 proteins perform another, as-yet-unknown function in the cytoplasm of neurons.
Hereafter, we use the human gene symbols to refer to Evi9/CTIP1/Bcl11A transcripts in order to avoid nomenclature conflicts.
MATERIALS AND METHODS
Antibodies.
The synthetic peptide corresponding to the N-terminal first 18 amino acid residues of Bcl11A-S was conjugated to KLH and used as the immunogen for antibodies R1646 and R1655. The peptide antibodies were affinity- purified by Sulfolink column (Pierce) and conjugated with antigenic peptide.
Animals and Housing.
All animal experiments were carried out with the approval of and in strict accordance with the guidelines of the Academia Sinica Institutional Animal Care and Utilization Committee (AS IACUC) and the Council of Agriculture Guidebook for the Care and Use of Laboratory Animals. Animals were sacrificed by CO2 inhalation or anesthetized with ketamine (Sigma, 0.1 mg/g of body weight) and xylazine (Sigma, 0.01 mg/g of body weight). All efforts were made to minimize animal suffering and to reduce the number of animals required.
Plasmid Construction.
The human Bcl11A-S coding region was PCR amplified and inserted between the KpnI and SalI sites of vector GW1-CMV (British Biotechnology) to express Bcl11A-S in mammalian cells. Because the Bcl11A-L coding sequence contains an EcoRI site in order to subclone Bcl11A-L into the EcoRI site of vector GW1-CMV, sticky-end PCR (Zeng,1998; Shih et al.,2002) was used to amplify the Bcl11A-L coding sequence from a human EST clone with GenBank accession number BC021098 (I.M.A.G.E. 5087967). The EcoRI sites for subcloning purposes were introduced in the primers for sticky-end PCR reaction.
Transfection, Immunostaining, and Immunoprecipitation of COS Cells.
Transfection, immunostaining, and immunoprecipitation of COS-7 cells were performed as described previously (Hsueh and Sheng,1999b) with some modifications. Briefly, transfected cells were harvested 18 and 24 hr after transfection for analysis of Bcl11A-S and Bcl11A-L, respectively. For staining, 2% paraformaldehyde was used for fixation for 15 min. Fixed cells were then permeabilized with Tris-buffered saline (TBS) containing 0.1%Triton-X-100 for 1 min. For coimmunoprecipitation of Bcl11A-S and Bcl11A-L, the DNA ratio of Bcl11A-L and myc-Bcl11A-S for transfection was 3:1. Twenty-four hours after transfection, cells were harvested and solubilized in TBS containing 0.5% Triton X-100, 20 mM DTT, and proteinase inhibitors. Monoclonal myc tag antibody (9B11, Cell Signaling) was then added into cell lysates for immunoprecipitation at 4°C for 4 hr. The immunoprecipitates were then washed with the following solutions: TBS containing 0.5% Triton X-100, once; TBS containing 0.1% Triton X-100, once; TBS containing 0.1% Triton X-100 and 0.5M LiCl, once; TBS containing 0.1% Triton X-100, once; and TBS containing 10 mM Tris (pH 7.4), twice. The precipitates were then analyzed by SDS-PAGE, followed by immunoblotting using antibody R1655.
Hippocampal Culture and Immunofluorescence Staining.
After trypsinization and mechanical dissociation, hippocampal cells from embryonic day 18–19 rat embryos were cultured in growth medium (Neurobasal medium, supplemented with 2% B27 supplement, 0.5 mM glutamine, and 12.5 μM glutamate) at a density of 250,000 cells/cm2. Then 18–21 day later, neurons were subjected to immunofluorescence staining. Cultured neurons were washed 3 times with PBS and then fixed with 4% paraformaldehyde and 4% sucrose in PBS for 15 min. Cells were further permeabilized with ice-cold methanol in −20°C for 15 min. After a 60-min incubation in PBS with 10% bovine serum albumin, cells were incubated with primary antibody overnight at 4°C. After washing 3 times with PBS, cells were incubated with cy3-conjugated antimouse and FITC-conjugated antirabbit antibodies at room temperature for 2 hr. After a final wash, cells were analyzed under a fluorescence microscope (DMRE, Leica, Wetzlar, Germany), and digitized images were processed for publication with Adobe Photoshop (Adobe, San Jose, CA).
Immunostaining of Rat Brain.
Floating sections of rat brain of different ages were prepared and processed as described previously (Hsueh et al.,1998; Hsueh and Sheng,1999a). For DAB staining, brain sections were permeabilized with 50% ethanol in TBS for 30 min. Endogenous peroxidase activity was depleted by incubating with 1% H2O2 in TBS for 30 min. After blocking with 3% horse serum at room temperature for 1 hr, sections were incubated with primary antibody at a final concentration of 1 μg/mL in TBS containing 1% horse serum at room temperature overnight. After washing, biotin-conjugated secondary antibody (Vector Labs) was added for an additional 2 hr of incubation. The ABC reaction (Vectastain Elite ABC kit, Vector Labs) was performed according to the manufacturer's instructions. Color reaction products were developed in TBS containing 0.05% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma, St. Louis, MO) and 0.03% H2O2 for about 3 min. For fluorescence staining, H2O2 pretreatment was skipped and FITC- or Cy3-conjugated secondary antibody was used. The sections were then analyzed by a Zeiss confocal microscope LSM 510 with narrow-band filters and processed using Adobe Photoshop with minimal contrast/brightness adjustment applied to the entire images.
Biochemical Fractionations.
High-purity nuclear fractions of adult brain were prepared as described previously (Gorski et al.,1986). The purity (>90%) of the nuclear fractions was monitored during the process by microscopy. A series of subcellular fractions of cortex and hippocampus from adult rat brain were prepared as described previously (Huttner et al.,1983; Lin et al.,2006). Briefly, minced rat brain tissue was homogenated using Dounce homogenizer with a loose pestle. Total homogenate of rat brain was first centrifuged at 800g to remove nuclei, unbroken cells, and other large debris (P1). The supernatant was centrifuged at 9,200g to obtain a crude synaptosomal fraction (P2), which was subsequently lysed with hypotonic buffer and centrifuged at 25,000g to pellet the lysed synaptosomal membrane fraction (LP1). The supernatant (LS1) was then centrifuged at 165,000g to obtain a crude synaptic vesicle fraction (LP2) and a soluble fraction (LS2). The supernatant (S2) above the P2 fraction was centrifuged at 165,000g to obtain a cytosolic soluble fraction (S3) and a light membrane fraction (P3). Equal amounts of proteins from each fraction were separated by SDS-PAGE and analyzed by immunoblotting using Bcl11A and PSD-95 antibodies. For PSD (postsynaptic density) preparation, the P2 fraction was applied to a step gradient with 0.85M, 1M, and 1.2M sucrose solutions, as described previously (Cho et al.,1992). The interface between the 1M and 1.2M sucrose solutions was collected and then solubilized by 0.5% Triton-X-100 and subjected to centrifugation at 100,000g to pellet the PSD I fraction. The PSD I fraction was further solubilized with either Triton-X-100 again or 3% sarcosyl and centrifuged at 100,000g again to pellet the PSD II and PSD III fractions, respectively.
RESULTS
Characterization of Bcl11A Antibodies
To analyze distribution of Bcl11A proteins in rat brain, we first generated the Bcl11A antibodies R1646 and R1655 by immunization of rabbits with synthetic peptide corresponding to the first 18 amino acid residues of Bcl11A. Because this sequence is shared across different forms of the Bcl11A protein, apart from Evi9b, the antibodies were expected to recognize these different forms of Bcl11A, (Fig. 1A). Antibody specificity was confirmed by immunoblotting rat hippocampal neurons and COS cells transfected with human Bcl11A-S, Bcl11A-L, or vector control. Both R1646 and R1655 antibodies recognized the short and long forms of human and rat Bcl11A proteins (Fig. 1B). The size of Bcl11A-S on SDS-PAGE was around 37 kDa (Fig. 1B). Multiple bands around 116 kDa were revealed by both R1655 and R1646 in Bcl11A-L-transfected COS cells as well as in hippocampal neurons (Fig. 1B). These protein species all represent Bcl11A-L protein, because they were not present in COS cells transfected with vector control or Bcl11A-S (Fig. 1B). These variations in SDS-PAGE mobility of human Bcl11A-L proteins might be a result of posttranscriptional modification. Both Bcl11A-S and Bcl11A-L were recognized by our Bcl11A antibodies, although antibody R1646 also nonspecifically recognized a protein species at about 50 kDa, which was not recognized by antibody R1655. This suggests that R1655 is more specific than R1646.
We then tested if these antibodies could recognize Bcl11A-S and Bcl11A-L in immunostaining. COS cells were transfected with myc-tagged Bcl11A-S and double-stained with myc and Bcl11A antibodies. The results show that both R1646 and R1655 antibodies labeled myc tag–positive cells (Fig. 1C). Both the cytoplasm and nuclei of COS cells had Bcl11A-S proteins (Fig. 1C). The immunoreactivity of Bcl11A antibodies was specific, as the antibodies did not stain untransfected COS cells (Fig. 1C, arrows). Similar to immunoblotting, these Bcl11A antibodies also recognized Bcl11A-L (Fig. 1D). Consistent with the results of previous studies (Avram et al.,2000; Nakamura et al.,2000), we found that Bcl11A-L proteins were highly concentrated in the granular structures of the nucleus. These results not only support the specific recognition of Bcl11A by our antibodies but also reveal the differential subcellular distribution of Bcl11A-S and Bcl11A-L in transfected COS cells.
Distribution of Bcl11A in Brain
The expression pattern of Bcl11A in rat brain was then examined using these Bcl11A antibodies (Fig. 2A). Similar to the expression of RNA in mouse (Nakamura et al.,2000), expression of both Bcl11A-S and Bcl11A-L was higher in postnatal day 1 (P1) rat brain than in adult rat brain. In addition, Bcl11A-S seemed more abundant than Bcl11A-L, although that could be a result of the lower transfer efficiency of Bcl11A-L. Bcl11A-S protein was widely expressed in both adult and P1 brains, although levels were relatively lower in thalamus, cerebellum, and brain stem (Fig. 2A). In adult brain, Bcl11A-L was highly concentrated in the olfactory bulb, cerebral cortex, and hippocampus. In P1 rat brain, a higher amount of Bcl11A-L was detected in the olfactory bulb, cerebral cortex, hippocampus, and striatum (Fig. 2A).
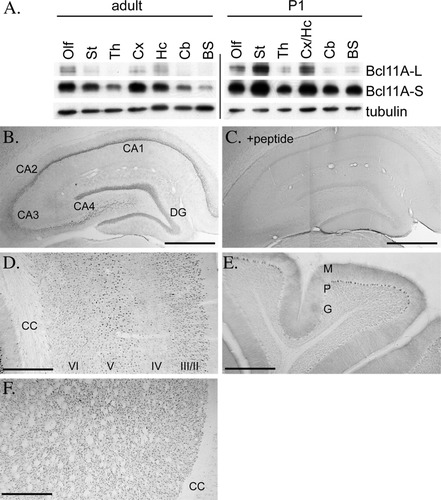
Expression of Bcl11A in rat brain. A: Immunoblotting analysis of Bcl11A expression in rat brain. Total extracts of different regions of rat brains were purified from adult and P1 rats, and expression of Bcl11A-L and Bcl11A-S was examined with R1655 antibody, as indicated. Tubulin served as an internal control (Olf, olfactory bulb; St, striatum; Th, thalamus; Cx, cerebral cortex; Hc, hippocampus; Cb, cerebellum; BS, brain stem). Data shown are representative of 2 independent experiments. B–C: DAB stain of adult hippocampus using antibody R1655. Antigenic peptide preabsorption was carried out in (C) to demonstrate the specificity of staining. D: DAB stain of adult cerebral cortex using antibody R1655. E: DAB stain of adult cerebellum using antibody R1655. F: DAB stain of adult striatum using antibody R1655. Roman numerals specify cortical cell layers (DG, dentate gyrus; CC, corpus callosum; M, molecular layer; P, Purkinje cell layer; G, granule cell layer). Data shown in B–F are representative of 3 independent experiments. Scale bar = 50 μm.
The distribution of Bcl11A in adult rat brain was then examined by immunohistochemistry. Because our antibodies recognized both proteins, the rat brain immunostaining represented the sum of Bcl11A-L and Bcl11A-S protein levels. Consistent with immunoblotting analysis, Bcl11A proteins were widely distributed in adult rat brain with higher intensity in the cerebral cortex (Fig. 2D), hippocampus (Fig. 2B), and striatum (Fig. 2F) shown by DAB staining. In hippocampal formation, Bcl11A proteins were expressed in all regions including CA1, CA2, CA3, CA4, and the dentate gyrus, with lower or more diffuse signals in the CA3 and CA4 regions (Fig. 2B). In the cerebral cortex, Bcl11A proteins were mainly concentrated in the pyramidal neurons in layers II, III, V, and VI (Fig. 2D). In the cerebellum, Bcl11A immunoreactivity was only observed in Purkinje cells (Fig. 3E). Two pieces of evidence support the specificity of the staining pattern of our Bcl11A antibodies. First, preadsorption with antigenic peptide completely blocked R1655 immunoreactivity (Fig. 2C). And second, the protein distribution pattern revealed in the present study was similar to the expression of Bcl11A mRNA revealed by in situ hybridization in mouse brain (Leid et al.,2004).
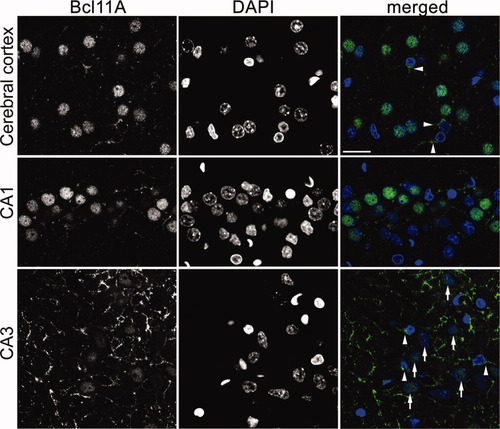
Confocal analysis of Bcl11A expression in adult rat brain. Indirect fluorescence staining using antibody R1655 was performed. Alexa-488-stained Bcl11A appear green; DAPI-counterstained nuclei appear blue. Expression of Bcl11A in the cerebral cortex and CA1 and CA3 regions of the hippocampus was examined. Arrowheads indicate neurons expressing cytoplasmic Bcl11A; arrows indicate cells expressing nuclear Bcl11A. Data shown are representative of 3 independent experiments. Scale bar = 20 μm.
Confocal Analysis of Bcl11A Distribution in Neurons
Although Bcl11A-L was highly concentrated in the nuclei of transfected COS cells (Fig. 1D), Bcl11A-S was expressed in both the cytoplasm and nuclei of transfected COS cells (Fig. 1C). We then determined the subcellular distribution of Bcl11A proteins in neurons. Therefore, confocal imaging was performed using rat brain tissues. In adult cerebral cortex and the hippocampus CA1 region, Bcl11A immunoreactivity was mainly in the nuclei of neurons, counterstained by DAPI (Fig. 3). However, a small but clear Bcl11A signal was also detected outside the nuclei in some cells in the cerebral cortex and the CA1 region (such as the cells indicated by arrowheads in Fig. 3). In the CA3 region of hippocampus, the cytoplasmic Bcl11A signal was even stronger. The relative level of Bcl11A in the nuclei in CA3 region was lower than that in the CA1 region and the cerebral cortex (Fig. 3). Bcl11A immunoreactivity in the cytoplasm was relatively punctate, rather than evenly distributed, suggesting a potential synaptic distribution of Bcl11A in the cytoplasm. To confirm this speculation, double staining of Bcl11A and a synaptic marker, synaptophysin (SVP38), was performed in cultured hippocampal neurons. Consistent with what we observed in the brain slices, we found a significant amount of Bcl11A outside the nuclei of cultured neurons. Moreover, Bcl11A immunoreactivity in the cytoplasm was very punctate in the mature hippocampal culture (Fig. 4A) and overlapped the synaptophysin (SVP38) (Fig. 4C,D), which labeled the presynaptic buttons (Fig. 4B). This result provided support for there being a fraction of Bcl11A at synapses. Some Bcl11A spots were not colocalized with SVP38, which may represent a population of synapses without SVP38 or nonsynaptic aggregates of Bcl11A in the cytoplasm.
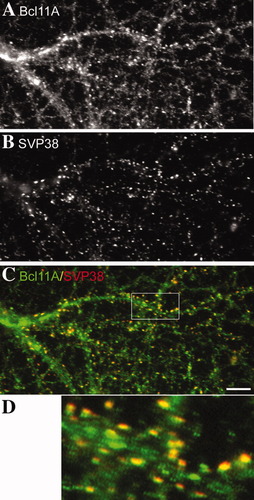
Synaptic distribution of Bcl11A in neurons revealed by immunofluorescence staining. Cultured hippocampal neurons were subjected to double immunostaining using R1655 and synaptophysin (SVP38) antibodies after 21 DIV. FITC-stained Bcl11A appears green; CY3-stained SVP38 appears red; areas of colocalization of these 2 proteins appear yellow. Data shown are representative of 2 independent experiments. Scale bar: = 10 μm.
Subcellular Distribution of Bcl11A Identified by Biochemical Fractionation
The immunofluorescence staining indicated that a small but significant amount of Bcl11A was in the cytoplasm or even at synapses. To confirm this and to determine which form of Bcl11A was in the cytoplasm and which in the nuclei of neurons, adult rat brains were separated into different subcellular fractions using 3 biochemical fractionation approaches. The first experiment isolated a high-purity nuclear fraction by centrifugation using sucrose cushioning. The total homogenate (H) and high-purity nuclear fraction (N) were then analyzed by immunoblotting using the R1655 antibody. Bcl11A-S and Bcl11A-L proteins were distinguished from each other by size on SDS-PAGE. The result showed that both Bcl11A-S and Bcl11A-L proteins were concentrated in the nuclear fraction of the adult rat brain, although the amount of Bcl11A-L in the nuclear fraction was higher than the amount of Bcl11A-S (Fig. 5A). In addition to monitoring the nuclear fraction under the microscope during preparation (data not shown), immunoblotting using an antibody against the postsynaptic protein PSD-95 was also performed to ensure the purity of the nuclear fraction. There was PSD-95 was in the total homogenate but not in the nuclear fraction (Fig. 5A, lowest panel), confirming the purity of our nuclear fraction. The second experiment used a series of centrifugations to isolate crude synaptic membrane (LP1), crude synaptic vesicle (LP2), light membrane (P3), and soluble cytosol (S3) fractions. Immunoblotting was then used to examine the distribution of Bcl11A-S and Bcl11A-L in these fractions. Bcl11A-S protein was widely distributed in different fractions, with the highest level in the light membrane fraction and moderate levels in the crude synaptic membrane and crude synaptic vesicle fractions (Fig. 5B). Distribution of Bcl11A-L was slightly different from that of Bcl11A-S. A large amount of Bcl11A-L was found in the crude synaptic membrane fraction, with moderate levels in the light membrane and crude synaptic vesicle fractions (Fig. 5B). The lowest expression of both Bcl11A-S and Bcl11A-L proteins occurred in the soluble cytosol fraction (Fig. 5B). As observed in the immunoblots of cultured hippocampal neurons, Bcl11A-L purified from brains was present as multiple protein species around 116 kDa (Fig. 5B). SDS-PAGE on less dense gels (7.5%) clearly revealed 3 or 4 protein bands immunopositive for Bcl11A-L around 116 kDa. The relative intensity of these protein bands differed among the subcellular compartments, suggesting differential posttranslational modification of Bcl11A-L in different fractions. Immunoblotting of PSD-95 (Fig. 5B) was performed again to ensure the quality of these biochemical fractions (Hsueh and Sheng,1999a). The presence of Bcl11A-S and Bcl11A-L proteins in the crude synaptic membrane and crude synaptic vesicle fractions is consistent with the observation that Bcl11A immunoreactivity was colocalized with synaptophysin in cultured hippocampal neurons, confirming a synaptic distribution of BCl11A. To further elucidate this, the presence of Bcl11A proteins in the postsynaptic density (PSD) fractions was examined. The result showed that Bcl11A-L was enriched in the PSD I and PSD II fractions (Fig. 5C). In contrast, the PSD I and PSD II fractions had only a trace amount of Bcl11A-S (Fig. 5C). This analysis suggested that Bcl11A-L is the major form of postsynaptic Bcl11A protein.
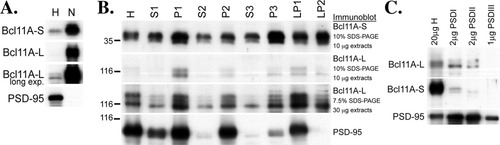
Subcellular distribution of Bcl11A in rat brain revealed by biochemical fractionation. A: High-purity nuclear fraction (N) purified by sucrose cushion and an equal amount of total homogenate (H) were analyzed by immunoblotting with R1655 and PSD-95 antibodies as indicated. Data shown are representative of 2 independent experiments. B: A series of subcellular fractions purified by a series of centrifugations (see detailed procedure in Materials and Methods section) were also analyzed by immunoblotting with R1655 and PSD-95 antibodies (H, total homogenate; P1, nuclei, unbroken cells, and other large debris; S1, supernatant above P1; P2, crude synaptosomal fraction; S2, supernatant above P2; S3, cytosolic soluble fraction; P3, light membrane fraction; LP1, lysed synaptosomal membrane fraction; LP2, crude synaptic vesicle fraction). To obtain a clear signal for Bcl11A-L, the experiment was repeated using 30-μg extracts on 7.5% SDS-PAGE. Data shown are representative of 4 independent experiments. C: Expression of Bcl11A in PSD fractions was determined by immunoblotting. PSD-95 was used to control for the quality of fractionation. Data shown are representative of 2 independent experiments.
Interaction between Bcl11A-S and Bcl11A-L
A fusion protein pull-down experiment showed that the region of the first 171 amino acids of Bcl11A is the self-interacting domain (Avram et al.,2002). To examine the interaction between Bcl11A-S and Bcl11A-L in cells, Bcl11A-L and myc-tagged Bcl11A-S were coexpressed in COS cells. Myc-tag antibody was then used to precipitate myc-tagged Bcl11A-S. The precipitates were further analyzed by R1655 antibody to visualize both Bcl11A-S and Bcl11A-L. The result showed that myc antibody not only precipitated myc-tagged Bcl11A-S but also brought down Bcl11A-L from the cell extract (Fig. 6). This interaction was specific as shown by myc antibody not precipitating Bcl11A-L in the absence of myc-tagged Bcl11A (Fig. 6). These data indicated that Bcl11A-S forms a complex with Bcl11A-L in cellular conditions.
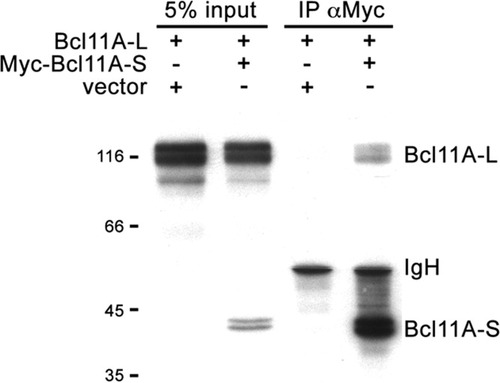
Interaction between Bcl11A-S and Bcl11A-L proteins in COS cells. Coimmunoprecipitation between Bcl11A-S and Bcl11A-L. COS cells were transfected with Bcl11A-L, myc-tagged Bcl11A-S, or vector control, as indicated. Cell extracts were then immunoprecipitated with myc-tag antibody. The precipitates were analyzed by immunoblot using R1655 antibody. Data shown are representative of 2 independent experiments.
DISCUSSION
In this report, analyses of immunostaining and biochemical fractionation demonstrated that Bcl11A-L and Bcl11A-S are differentially expressed in the adult rat brain. Bcl11A-S proteins are widely distributed in different regions of the brain; Bcl11A-L proteins are predominantly found in the cerebral cortex, hippocampus, and olfactory bulb. The Bcl11A-S and Bcl11A-L proteins also differ in their subcellular distribution. Although both proteins are present in the nuclei and cytoplasm of neurons, Bcl11A-L is concentrated at synapses, as indicated by the presence of Bcl11A-L in the PSD fractions. Only a very small amount of Bcl11A-S was found in the PSD fractions. Because Bcl11A-S forms heterooligomers with Bcl11A-L, Bcl11A-S may be drawn to specific subcellular regions by its interaction with Bcl11A-L. The overall amount of Bcl11A-S is higher that of Bcl11A-L; therefore, Bcl11A-S proteins free from interaction with Bcl11A-L are able to be distributed to other subcellular regions.
Bcl11A was originally identified as a proto-oncogene that is overexpressed in classical Hodgkin's lymphoma (Satterwhite et al.,2001; Martin-Subero et al.,2002) and BXH2 murine myeloid leukemia (Nakamura et al.,2000). It also physically interacts with another proto-oncogene, Bcl6 (Nakamura et al.,2000). Bcl11A is highly expressed at the embryonic stage, with expression gradually decreasing during development (Nakamura et al.,2000). In normal adult tissues, the highest expression of Bcl11A is in the brain (Avram et al.,2000; Nakamura et al.,2000). It is unclear why proto-oncogenes have higher expression than neurons, which are postmitotic and quiescent. It seems unlikely that Bcl11A regulates expression of genes involved in cell division, which many oncogenes are. Identification of downstream Bcl11A target genes will assist in the elucidation of the molecular function of Bcl11A in lymphocytes and neurons.
A single Bcl11A gene expresses different gene products by alternative splicing or by using an internal promoter. The results of the present study suggest that Bcl11A-S and Bcl11A-L are the two major forms of Bcl11A in neurons. Of these two forms, Bcl11A-S is more abundant than Bcl11A-L in the brain. In human lymphoid cells and tumors, the longest transcript product, Bcl11A-XL, is the most abundant form (Liu et al.,2006; Weniger et al.,2006). These data indicate that different forms of Bcl11A protein products have different species preferences or patterns of tissue expression, which may be involved in regulation of Bcl11A activity, as different forms of Bcl11A have different biochemical properties. For instance, Bcl11A-XL contains three extra zinc fingers at the C-terminal region. It is unclear if these extra zinc fingers are able to recognize a DNA sequence or if they act as a protein–protein interacting domain. If so, Bcl11A-XL is expected to have functions in addition to those of Bcl11A-L, which may account for the unique properties of Bcl11A-XL in lymphocytes. In addition, evidence suggests that Bcl11A-S acts as a dominant-negative mutant of Bcl11A-L and Bcl11A-XL. A fusion protein pull-down experiment from another study (Avram et al.,2000) and our coimmunoprecipitation experiment indicate that Bcl11A-S forms a heterooligomer with Bcl11A-L. However, Bcl11A-S contains only the first two zinc fingers. So far, there is no evidence that Bcl11A-S binds to DNA. And also, Bcl11A-L has been shown to interact with the type III histone deacetylase SIRT1 through the region of amino acids 194–378 to repress gene expression (Senawong et al.,2005). Because this fragment is missing in Bcl11A-S, Bcl11A-S is not expected to interact with SIRT1 and thus cannot efficiently repress gene expression. Therefore, Bcl11A-S may attenuate the transcriptional activity of Bcl11A-L by forming a heterooligomer with Bcl11A-L. The same mechanism could also apply to Bcl11A-XL. If this speculation is correct, regulation of Bcl11A-S expression should modulate the transcriptional activity of Bcl11A in cells. Additional studies are needed to address this point.
In summary, our study has revealed the regional and subcellular distribution of Bcl11A proteins in rat brain and provides fundamental information for studying the function of this gene in the brain.
Acknowledgements
We thank Sue-Ping Lee for technical assistance with confocal microscopy and Dr. Harry Wilson for English editing.




