Hypoglutamatergic activity in the STOP knockout mouse: A potential model for chronic untreated schizophrenia
Abstract
In mice, the deletion of the STOP protein leads to hyperdopaminergia and major behavioral disorders that are alleviated by neuroleptics, representing a potential model of schizophrenia. The reduction of the glutamatergic synaptic vesicle pool in the hippocampus could reflect a disturbance in glutamatergic neurotransmission in this model. Here we examined potential disturbances in energy metabolism and interactions between neurons and glia in 15-week-old STOP KO, wild-type, and heterozygous mice. Animals received [1-13C]glucose and [1,2-13C]acetate, the preferential substrates of neurons and astrocytes, respectively. Extracts from the whole forebrain and midbrain were analyzed by HPLC, 13C and 1H NMR spectroscopy. Amounts and labeling of most metabolites were unchanged. However, glutamine concentration and amount of [4,5-13C]glutamine derived from [1,2-13C]acetate significantly decreased by 17% and 18%, respectively, in STOP KO compared with wild-type mice. The amount of [4-13C]glutamate was decreased in STOP KO and heterozygous compared with wild-type mice. γ-Aminobutyric acid labeling was not influenced by the genotype. Because STOP-deficient mice have a lower synaptic vesicle density, less glutamate is released to the synaptic cleft, leading to decreased stimulation of the postsynaptic glutamate receptors, reflecting increased glutamine metabolism only in the vicinity of the postsynapse of STOP KO mice. © 2007 Wiley-Liss, Inc.
Over the past 20 years, a neurodevelopmental origin for schizophrenia has become the prevailing pathogenic hypothesis for the disorder (Harrison, 1999; Marenco and Weinberger, 2000). Schizophrenia is regarded as a disease expressed mainly at the synapse level on the basis of both the hypothesis of a functional disconnection (Friston, 2002) and the data from genetic studies using DNA microarrays (Mirnics et al., 2001). For a long time, the “dopaminergic hypothesis” of schizophrenia based on the hyperactivity of the dopaminergic system was the prevailing hypothesis for the disease. Indeed, it is well known that amphetamine and cocaine that release or inhibit the reuptake of dopamine, respectively, induce psychosis (Seeman, 1987). However, a dysfunctioning of other neurotransmission systems has also been postulated, and a more recent view includes disturbances also at the level of serotonin and glutamate neurotransmissions (Carlsson et al., 2001).
The “glutamatergic hypothesis” of schizophrenia comes from the observation that N-methyl-D-aspartate (NMDA) glutamate-receptor antagonists, such as phencyclidine (Halberstadt, 1995) and ketamine (Lahti et al., 1995), cause strong psychotomimetic effects, with hallucinations and psychomotor signs. In contrast to dopaminergic agonists, which mimic only the positive symptoms of schizophrenia, NMDA antagonists produce the whole spectrum, including negative and cognitive symptoms. The proposed mechanisms for a dysfunction of glutamatergic neurotransmission include interactions between the dopaminergic and the glutamatergic systems (Carlsson et al., 2001), excitotoxic neuronal damage in hippocampus and cortex (Deutsch et al., 2001), and alterations in glutamine and glutamate metabolism (Brenner et al., 2005; Kondziella et al., 2005; Eyolfsson et al., 2006). It has been suggested that the dysregulation of dopamine transmission in schizophrenia may be secondary to alterations in glutamatergic NMDA receptor-mediated transmission (Carlsson et al., 2004; Olney and Farber, 1995). Indeed, in healthy volunteers receiving ketamine, the amplitude of amphetamine-induced dopamine release was significantly enhanced compared with control conditions (Kegeles et al., 2000). Thus, the elevated dopamine release seen in schizophrenic patients after amphetamine administration may well be secondary to a failure in glutamatergic control of dopamine neurons. This hypothesis is supported by experimental studies in rats (Miller and Abercrombie, 1996).
Glutamate is the most important excitatory neurotransmitter in the mammalian brain. In the basal state, 60–75% of the total cortical glucose utilization corresponds to glutamatergic neuron activity, 10–15% reflects γ-aminobutyric acid (GABA)-ergic neurotransmission, and 10–15% reflects glial needs (Sibson et al., 2001; Lebon et al., 2002; Shulman et al., 2004). The synthesis of glutamate and GABA in neurons is closely connected to astrocytic metabolism, and the homeostasis of glutamate is crucial to brain function for many reasons. First, fast removal of glutamate from the synaptic cleft by astrocytes is necessary for short glutamate action on the postsynaptic target cell and thereby precise information signaling. Second, a high extracellular concentration of glutamate is neurotoxic, and excessive glutamate release may participate in the pathophysiology of many brain disorders, possibly including schizophrenia (Deutsch et al., 2001). Third, because neurons lack the main anaplerotic enzyme in the brain, pyruvate carboxylase (Shank et al., 1985), they depend on astrocytic supply of tricarboxylic acid (TCA) cycle intermediates, because the constant loss of amino acid neurotransmitters from neurons would otherwise lead to restriction of neurotransmitter precursors (Sonnewald et al., 1993). After being released from neurons, glutamate is cleared from the synapses by astrocytes, which transform glutamate to glutamine via glutamine synthetase, which is an astrocyte-specific enzyme (Norenberg and Martinez-Hernandez, 1979). Astrocytes release glutamine into the extracellular space, where it is taken up by neurons and converted back to glutamate and GABA or enters the TCA cycle via 2-oxoglutarate to provide carbon skeletons for the synthesis of other metabolites. This shuttling between astrocytes and neurons is called the “glutamate-glutamine cycle” (Berl and Clark, 1983).
An excellent tool with which to study metabolic pathways and glial–neuronal metabolic interactions is 13C nuclear magnetic resonance spectroscopy (NMRS; for review see Sonnewald and Kondziella, 2003). The simultaneous injection of [1,2-13C]acetate and [1-13C]glucose allows study of astrocytic and neuronal metabolism in the same animal (Taylor et al., 1996). In the present work, this technique was applied to the characterization of neuronal and astrocytic metabolism in STOP (for stable tubule only polypeptide) protein knockout (KO) mice, which have been proposed to be a “meaningful model for the study of the pathophysiology of schizophrenia” (Brun et al., 2005). The STOP protein is involved in the cold stability of microtubules, synaptic plasticity, and neurotransmission (Andrieux et al., 2002). Deletion of the STOP protein leads to a decrease in synaptic vesicle density in hippocampal CA1 terminals, impaired long-term potentiation, and depression at the level of Schaffer collaterals-CA1 pyramidal cell synapses. STOP KO mice are also characterized by disorganized activity with frequent shifts between hyperlocomotion and prostration, anxiety-related behavior, inability to perform object-recognition tasks and social withdrawal (Andrieux et al., 2002). These mice also exhibit increased dopaminergic neurotransmission and increased efflux of dopamine in the nucleus accumbens upon stimulation (Brun et al., 2005). Taken together, these studies indicate the association between limbic hyperdopaminergy and hippocampal hypoglutamatergy in these mice (Andrieux et al., 2002; Brun et al., 2005). To study the cerebral metabolism of glutamate further, we explored the metabolic fate of [1-13C]glucose and [1,2-13C]acetate via 13C and 1H NMRS and HPLC in the brain of wild-type (WT), heterozygous, and STOP KO mice. Acetate and glucose are both precursors for acetyl-CoA that enters the TCA cycle. Whereas acetyl-CoA from glucose is metabolized more in the neuronal TCA cycle (Qu et al., 2000), acetate is metabolized predominantly in astrocytes, because it is selectively taken up into these cells (Waniewski and Martin, 1998).
MATERIALS AND METHODS
STOP KO male mice (STOP−/−), heterozygous (STOP+/−), and control WT littermates (STOP+/+), 15 weeks old, were generated as previously described (Andrieux et al., 2002). Mice were housed eight per cage and maintained in quiet, uncrowded facilities (room temperature of 22°C ± 1°C) on a 12 hr light-dark schedule (7:00 AM lights on), humidity 60%, and given unlimited access to lab chow and water. Males only were used for these experiments to eliminate confounding effects of variable estrogen level on neuronal excitability (Murphy et al., 1998). All animal experimentation was performed in accordance with the rules of the European Committee Council Direction of November 24, 1986 (86/69/EEC), and the French Department of Agriculture (Licence No. 67–97). The animals (10 in each group) were injected intraperitoneally with [1-13C]glucose (543 mg/kg, 0.3 M solution) and [1,2-13C]acetate (504 mg/kg, 0.6 M solution), followed by decapitation 15 min later. The heads were immediately frozen in liquid nitrogen and stored at −80°C. Brains were removed from the slightly thawed skull, and, because of their small size and limitations of sensitivity of the method, the forebrain/midbrain (cerebrum) including cerebral cortex and subcortical regions was used. The brainstem and cerebellum were discarded. The tissue was homogenized in 7% (w/v) perchloric acid and centrifuged at 4,000g for 5 min. The procedure was repeated, and the supernatants were pooled and neutralized with 1 M KOH, followed by lyophilization.
High-Pressure Liquid Chromatography
Amino acids in cell extracts and medium were quantified by HPLC on a Hewlett Packard 1100 system (Agilent Technologies, Palo Alto, CA). The amino acids were precolumn derivatized with o-phthaldialdehyde (Geddes and Wood, 1984) and subsequently separated on a Zorbax SB-C18 (4.6 × 250 mm, 5 μm) column from Agilent using a phosphate buffer (50 mM, pH 5.9) and a solution of methanol (98.75%) and tetrahydrofurane (1.25%) as eluents. The separated amino acids were detected with fluorescence and quantified by comparison with a standard curve derived from standard solutions of amino acids run after every 12 samples.
13C NMR Spectroscopy
Proton-decoupled 125.77-MHz 13C NMR spectra were obtained with a Bruker DRX-500 spectrometer after the samples had been redissolved in 300 μl D2O containing ethylene glycol 0.1% as an internal standard. Scans were accumulated with a 30° pulse angle and a 25-kHz spectral width with 64,000 data points. The number of scans was 9,000. The acquisition time was 1.308 sec, the relaxation delay 0.5 sec.
1H NMR Spectroscopy
A DRX-500 spectrometer was used to obtain 1H NMR spectra with a sweep width of 8 kHz with 32,000 data points. The pulse angle was 90°, the acquisition time 2.045 sec, and the relaxation delay 10 sec. The number of scans was 250. Water suppression was set at the residual H2O resonance.
Labeling Patterns
Most of the singlet peaks in the NMR spectrum (Fig. 1) represent label from [1-13C]glucose. In contrast, the doublets seen in the spectrum are mostly derived from [1,2-13C]acetate and thus astrocytic metabolism (Waniewski and Martin, 1998). [1-13C]glucose is converted to pyruvate via glycolysis and can form [3-13C]alanine and [3-13C]lactate. Pyruvate may enter the TCA cycle via [2-13C]acetyl-CoA, which will lead to the formation of [4-13C]glutamate and glutamine or [2-13C]GABA. If the 13C label stays for a second turn in the TCA cycle the [2-13C] or [3-13C] positions of glutamate and glutamine or the [3-13C] or [4-13C] positions of GABA can be labeled. Alternatively, in astrocytes, pyruvate can be carboxylated by pyruvate carboxylase (PC) to oxaloacetate, which can lead to the synthesis of [2-13C]glutamate and glutamine or [4-13C]GABA. [1,2-13C]Acetate can also be converted to acetyl-CoA, however, the product, [1,2-13C]acetyl-CoA, will have two 13C atoms resulting in doublet formation. Thus, [4,5-13C]glutamate and glutamine or [1,2-13C]GABA are formed (Fig. 2). After the second turn of the TCA cycle, this label will be in the [1,2-13C] or [3-13C] positions of glutamate and glutamine and the [2-13C] or [3-13C] positions of GABA. Glutamine is labeled more from [1,2-13C]acetate (doublet) than from [1-13C]glucose (singlet); the opposite is the case for glutamate and GABA. Alanine, lactate, N-acetylaspartate (NAA) in the C-6 position, and succinate are labeled mainly from glucose. Creatine, taurine, and the aspartate group in N-acetylaspartate are not labeled during the 15 min period of the present experiment; the naturally abundant 13C gives rise to the observed singlets in Figure 1. Because both acetyl-CoA and oxaloacetate can be labeled or unlabeled, the number of possible isotopomers of the TCA-cycle-derived metabolites is large, and only compounds derived from the first and the second turns are presented in Figure 2. Metabolic ratios were calculated, but no differences were detected between groups (for details see Brenner et al., 2005; Melø et al., 2006).
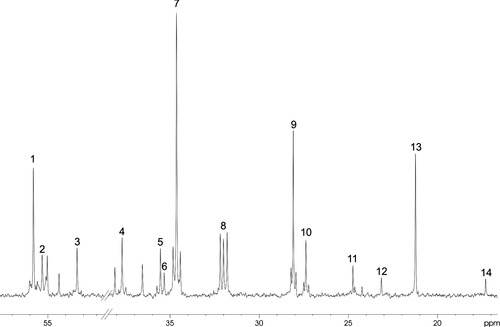
13C NMR spectrum of cerebrum extract from STOP KO mice injected with [1,2-13C]acetate and [1-13C]glucose. Peak assignments; 1: glutamate C-2; 2: glutamine C-2; 3: aspartate C-2; 4: aspartate C-3; 5: GABA C-2; 6: succinate C-2/C-3; 7: glutamate C-4; 8: glutamine C-4; 9: glutamate C-3; 10: glutamine C-3; 11: GABA C-3; 12: N-acetylaspartate C-3; 13: lactate C-3; 14: alanine C-3. The singlets are derived mostly from [1-13C]glucose and the doublets in the spectrum from [1,2-13C]acetate.
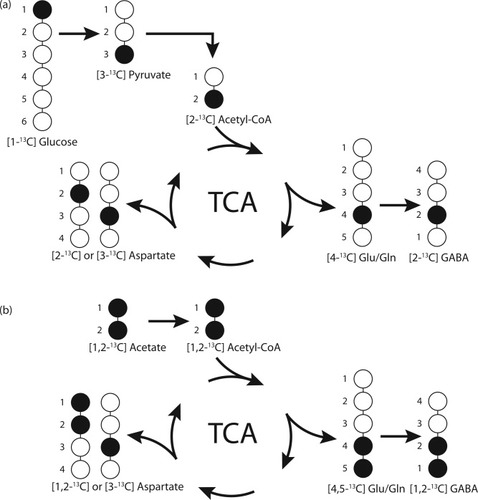
Schematic representation of 13C labeling of glutamate, glutamine, GABA, and aspartate originating from [1-13C]glucose (a) or [1,2-13C]acetate (b). Solid circles represent 13C and empty circles 12C. TCA, tricarboxylic acid cycle; Glu, glutamate; Gln, glutamine.
Data Analysis
The amounts of 13C in the different metabolites were quantified from integrals of the relevant peaks obtained from NMR spectra, with ethylene glycol as an internal standard. Factors for nuclear Overhauser and relaxation effects were applied to all spectra. Amounts of metabolites were quantified either from 1H NMR spectra using ethylene glycol as internal standard and correcting for number of protons or by HPLC. All results are given as mean ± SD. Statistical analyses were performed via two-tailed, unpaired Student's t-test; P < 0.05 was considered significant.
RESULTS
In the three genotypes, brain levels of all amino acids related to the TCA cycle and taurine, glutathione, succinate, lactate, and NAD+ were similar. The only difference was a significant (17%) decrease in the cerebral concentration of glutamine of STOP KO compared with WT mice (Table I).
| Wild type | STOP+/− | STOP KO | |
|---|---|---|---|
| Glutamate | 11.6 ± 0.9 | 11.7 ± 0.8 | 11.6 ± 0.6 |
| GABA | 2.1 ± 0.4 | 1.9 ± 0.3 | 2.0 ± 0.2 |
| Glutamine | 3.5 ± 0.4 | 3.3 ± 0.4 | 2.9 ± 0.3* |
| Aspartate | 3.3 ± 0.4 | 3.4 ± 0.4 | 3.4 ± 0.3 |
| Alanine | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.1 |
| Taurine | 10.1 ± 1.2 | 9.9 ± 1.3 | 10.8 ± 1.1 |
| Glutathione | 1.4 ± 0.2 | 1.4 ± 0.4 | 1.4 ± 0.4 |
| NAA | 7.6 ± 0.5 | 7.1 ± 0.7 | 6.9 ± 0.5 |
| Succinate | 0.4 ± 0.04 | 0.4 ± 0.05 | 0.4 ± 0.05 |
| Lactate | 7.2 ± 1.1 | 6.8 ± 1.3 | 6.8 ± 1.4 |
| NAD+ | 0.3 ± 0.06 | 0.3 ± 0.05 | 0.3 ± 0.05 |
- † Metabolites in brain extracts from mice measured using two methods: HPLC (alanine, taurine, glutathione) or 1H NMRS (others). Data represent means ± SD of 10 mice in each group.
- * P < 0.05, statistically significant difference from wild-type mice.
Injection of [1-13C]glucose and [1,2-13C]acetate led to efficient labeling of many metabolites (Fig. 1). In particular, labeling of glutamate and glutamine C-3 and C-4, GABA C-2 and C-3, and aspartate and lactate C-3 are shown in Figure 1. Labeling patterns from [1-13C]glucose and [1,2-13C]acetate from the first and second turns of the TCA cycle are shown in Figure 2. The amount of [4,5-13C]glutamine, derived from [1,2-13C]acetate, was significantly decreased by 18% in STOP KO compared with WT mice (Fig. 3). The amount of [4,5-13C]glutamate tended to be decreased in STOP+/− and STOP−/− mice compared with control animals, but the difference was not significant. Levels of [1,2-13C]GABA were similar in the three genotypes. Label from [1-13C]glucose in the form of [4-13C]glutamate and [4-13C]aspartate was decreased by 16% and 21%, respectively, in KO STOP compared with wild-type mice, whereas the labeling of [2-13C]GABA and [4-13C]GABA did not depend on the genotype. The pyruvate carboxylase/dehydrogenase (PC/PDH) ratios were calculated, and no significant differences between the groups were detected (data not shown). Generally, carboxylation was low in all groups.
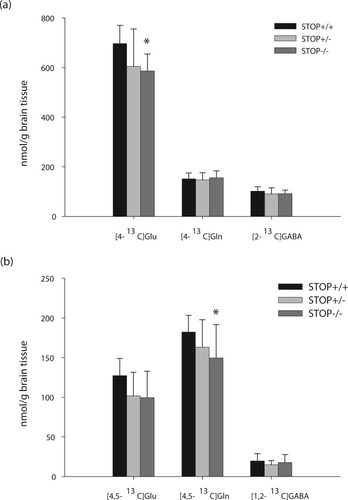
Amount of 13C labeled metabolites (nmol/g tissue) derived from [1-13C]glucose (a) and [1,2-13C]acetate (b). Data represent means ± SD of 10 mice in each group. *P < 0.05, statistically significant difference from wild-type mice.
DISCUSSION
The cytosolic step of neuronal glutamate and astrocytic glutamine metabolism were affected in the STOP KO mouse, whereas mitochondrial metabolism appeared unchanged. We propose that glutamine metabolism is decreased in major parts of the astrocytes of STOP KO mice, but unaltered in areas close to the presynapse and decreased in the vicinity of the postsynapse.
The STOP protein is a protein involved in the cold stability of microtubules, synaptic plasticity and neurotransmission (Andrieux et al., 2002). Different isoforms of the STOP protein are expressed in neurons and astrocytes (Galiano et al., 2004) and it has been shown that in knockout mouse embryos, microtubule cold stability was lost in both neurons and glial cells while being partly preserved in heterozygous mice (Andrieux et al., 2002). This mouse model was used in the present study and a decreased level of glutamine was found in the cerebrum of homozygous KO compared with WT mice. The glutamine level in heterozygous mice was not significantly different from both wild-type and knockout mice, indicating that reduced amounts of the STOP protein are sufficient for maintaining glutamine synthesis. As in STOP deficient mice, reduced amounts of glutamine were found in the left anterior cingulate cortex of patients with chronic schizophrenia in addition to reduction in glutamate (Theberge et al., 2003). This decrease of glutamate and glutamine could possibly be an effect of long term treatment with neuroleptics, but the authors argue that this is unlikely and the present study supports this view. Two 1H NMR spectroscopy studies of patients with never treated first episode schizophrenia, showed increased levels of glutamine in the medial prefrontal cortex and the left anterior cingulate cortex and thalamus, respectively (Bartha et al., 1999; Theberge et al., 2002). Taken together, these reports indicate that first episode schizophrenic patients have an increased glutamine level in specific areas of the brain, whereas chronic patients have decreased levels of glutamate and glutamine. Thus, it appears that the 15-week-old STOP KO mice mimic chronic schizophrenia but not first episode schizophrenia, and hence STOP KO mice might be a good model for chronic schizophrenia.
In the present study, a significant decrease was found in the amount of [4,5 13C]glutamine which is derived from [1,2-13C]acetate and therefore from astrocytic metabolism, indicating decreased glutamine synthesis in the STOP KO mice. A similar decrease was observed in the rat model of schizophrenia induced by the chronic injection of MK-801 (Kondziella et al., 2005). Pyruvate carboxylation, another astrocyte specific pathway, was not changed compared with WT or heterozygous mice. It is conceivable that changes in microtubule stability will affect cytosolic enzymes such as glutamine synthetase (GS), whereas pyruvate carboxylase (PC), located on/in mitochondria was not affected. Other cytosolic enzymes, those related to glycolysis appeared unaffected, since the amounts of lactate and alanine were unchanged. The osmolyte taurine, the antioxidant glutathione and energy metabolism related NAD+ were unchanged as well. As stated in the introduction, the astrocytes supply neurons with glutamine which can be converted to glutamate by the mitochondrial enzyme phosphate activated glutaminase (PAG). It is also important to note in this context that most of the glutamate in brain is located in glutamatergic neurons (Ottersen and Storm Mathisen 1986). Since the amount of [4,5-13C]glutamine was decreased in the present study in STOP KO compared with WT mice, a decreased level of [4,5-13C]glutamate would be expected, since the latter is directly derived from [4,5-13C]glutamine via PAG. However, no decrease was observed. The amount of [4,5-13C]glutamate in STOP KO animals was at the control level. This could be due to decreased catabolism of [4,5-13C]glutamate, increased PAG activity or normal release of [4,5-13C]glutamine, despite lower intracellular amounts. The fact that also [1,2-13C]GABA, another neuronal metabolite derived from [4,5-13C]glutamine, was unchanged, indicates that the latter situation is most likely occurring.
The labeling (data not shown) and concentrations of the metabolites associated with primarily neuronal mitochondria, such as N-acetylaspartate, succinate and aspartate (Baslow, 2003; Palaiologos et al., 1988; Sonnewald et al., 1996) were similar in STOP KO, heterozygous and WT mice. A decrease was found in glutamate labeled from [1-13C]glucose, i.e. [4-13C]glutamate which is derived from neuronal metabolism, in the STOP KO mice compared with WT. A decrease in [4-13C]glutamate concentration was also observed in the chronic MK-801 rat model of schizophrenia (Kondziella et al., 2005). Levels of [2-13C]GABA were unchanged in the STOP KO mice but decreased in MK-801-treated rats. The decrease in [4-13C]glutamate in the STOP KO mice could indicate that the cytosolic glutamate synthesis from 2-oxoglutarate was impaired as a result of microtubule instability. Glutamate in neurons (mostly [4-13C]glutamate) is accumulated into vesicles and released. As mentioned above, this glutamate can be converted to [4-13C]glutamine in the astrocytes for future transport to neurons. However, in spite of a reduction in the amount of [4-13C]glutamate, no change was detected in [4-13C]glutamine concentration. This could possibly reflect increased GS activity, which is inconsistent with the decrease in GS activity that we postulated above in the light of decreased labeling of [4,5-13C]glutamine. However, the cerebral metabolism of glutamate in astrocytes is compartmentalized and there have to be domains, which release glutamine, that is taken up by the neuronal cell body (Fig. 4). We postulate that GS activity is decreased in regions of the astrocyte that are close to the nonsynaptic parts of neurons (Fig. 4, 1), leading to decreased amounts of [4-13C]glutamine. However, in the synaptic region other mechanisms apply (Fig. 4, 3–5). Glutamate is released from vesicles docking the presynaptic membrane. Previous reports have shown that STOP KO mice have a lower synaptic vesicle density (Andrieux et al., 2002). This indicates that less glutamate is released to the synaptic cleft leading to decreased stimulation of the postsynaptic glutamate receptors. Upon depolarization, the activation of NMDA receptors by glutamate allows Ca2+ influx through the ion channel. Ca2+ entry into the postsynaptic neuron activates nitric oxide synthase (NOS) through a second messenger pathway involving calmodulin (Kosenko et al., 2003). Nitric oxide (NO) synthesized in the postsynaptic neuron does not only affect the post- and presynaptic neuron but also the nearby astrocyte resulting in tonic inhibition of GS (Kosenko et al., 2003). Reduced presynaptic glutamate release would then, through disinhibition of the astrocytic GS, cause increased glutamine production in the astrocyte (Fig. 4, 3–5). Indeed the unchanged amount of [4-13C]glutamine in the presence of decreased [4-13C]glutamate could support this hypothesis. Modification of GS activity due to changes in NMDA receptor stimulation has been reported previously by Brenner et al. (2005). In that study, rats received a single injection of the NMDA receptor antagonist MK-801, a model for schizophrenia; increased GS activity was postulated in astrocyte processes close to the postsynaptic neuron (Brenner et al., 2005).
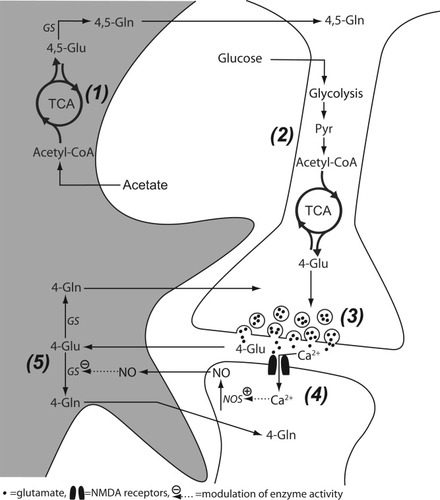
Schematic representation of the glutamate-glutamine cycle related interactions of an astrocyte with a pre- and postsynaptic neuron. 1: [1,2-13C]acetate is taken up by astrocytes, but not by neurons. Through the tricarboxylic cycle (TCA), [1,2-13C]acetatyl CoA is converted to [4,5-13C]glutamate. Glutamine synthetase (GS) converts [4,5-13C]glutamate to [4,5-13C]glutamine. We postulate that the STOP KO mice have a decreased GS activity in this nonsynaptic region of the astrocyte. 2: Glucose is taken up mostly by neurons. [1-13C]glucose is after several steps converted to [4-13C]glutamate. In the STOP KO mice neuronal cytosolic glutamate synthesis is decreased. 3: Microtubule instability in the STOP KO mice probably leads to reduced synaptic glutamate release. 4: NMDA receptors are activated by synaptic glutamate and allow influx of Ca2+ into the postsynaptic dendrite. Ca2+ activates the enzyme nitric oxide synthase (NOS). 5: NO may diffuse into the astrocyte where it normally inhibits GS. In the STOP KO mice, reduced activation of NMDA receptors by glutamate leads to reduced NO production resulting in less inhibition of GS. This will be the case only in the part of the astrocyte close to the synapse. 4,5-Gln, [4,5-13C]glutamine; 4,5-Glu, [4,5-13C]glutamate; 4-Gln, [4-13C]glutamine; 4-Glu, [4-13C]glutamate; GS, glutamine synthetase; NO, nitric oxide; NOS, nitric oxide synthase; Pyr, pyruvate; TCA, tricarboxylic acid cycle.
In conclusion, the results of the present study support previous indications that STOP KO mice have reduced synaptic glutamate release and are in a hypoglutamatergic state. Hypoglutamatergia is thought to be an important causal mechanism of schizophrenia. STOP KO mice also show signs of hyperdopaminergia (Brun et al., 2005). This, together with the reduction in glutamine levels, makes the STOP KO mouse a valid model of chronic schizophrenia and supports the hypothesis that dysregulation of dopamine in schizophrenia coexists with and may be secondary to alterations in glutamatergic NMDA receptor-mediated transmission (Carlsson et al., 2004; Olney and Farber, 1995).
Acknowledgements
The excellent technical assistance of Bich-Thuy Pham-Lê and Bente Urfjell is greatly acknowledged.




