Characterization of thromboxane A2 receptor signaling in developing rat oligodendrocytes: Nuclear receptor localization and stimulation of myelin basic protein expression
Abstract
The present work investigates the role of thromboxane A2 (TXA2) receptors in the development of oligodendrocytes (OLGs). The results demonstrate that the proteins of the TXA2 signaling pathway, i.e., cyclooxygenase (COX-1), TXA2 synthase (TS), and TXA2 receptor (TPR) are expressed in the developing rat brain during myelination. Furthermore, culture of OLG progenitor cells (OPCs) revealed that the expression levels of these proteins as well as TXA2 synthesis increase during OLG maturation. Separate studies established that activation of TPRs by the agonist U46619 increases intracellular calcium in both OPCs and OLGs as visualized by digital fluorescence imaging. Immunocytochemical staining demonstrated that TPRs are localized in the plasma membrane and perinuclear compartments in OPCs. However, during OLG differentiation, TPRs shift their localization pattern and also become associated with the nuclear compartment. This shift to nuclear localization was confirmed by biochemical analysis in cultured cells and by immunocytochemical analysis in developing rat brain. Finally, it was found that U46619 activation of TPRs in maturing OLGs resulted in enhanced myelin basic protein (MBP) expression. Alternatively, inhibition of endogenous TPR signaling led to reduced MBP expression. Furthermore, TPR-mediated MBP expression was found to be associated with increased transcription from the MBP promoter using a MBP-luciferase reporter. Collectively, these findings suggest a novel TPR signaling pathway in OLGs and a potential role for this signaling during OLG maturation and myelin production. © 2006 Wiley-Liss, Inc.
Oligodendrocytes (OLGs), the myelin-producing cells of the central nervous system (CNS), form the insulating sheaths required for saltatory nerve impulse propagation. These cells develop from precursors in the embryonic and postnatal subventricular zone and undergo extensive migration and proliferation before terminal differentiation (Goldman, 1995; Miller, 1996; Orentas and Miller, 1998; Belachew et al., 2003). Multiple extracellular factors are known to influence progenitor cell proliferation, differentiation, and survival through interaction with specific oligodendrocyte progenitor cell (OPC) or OLG receptors. These signaling molecules appear to serve as local stimuli to facilitate directed oligodendrocyte proliferation, migration, and myelination. Some of these growth factors and neurotransmitters include platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1), ciliary neurotrophic factor (CNTF), neurotrophins, glutamate, and adenosine, among others (McMorris et al., 1986; Noble et al., 1988; Raff et al., 1988; Bogler et al., 1990; Louis et al., 1993; Barres et al., 1994a, b; Yuan et al., 1998; Stevens et al., 2002; Du and Dreyfus, 2002). In vivo, these exogenous factors derived from neurons, microglia, and/or astrocytes act in a cooperative signaling gradient to influence OLG development and function (Barres and Raff, 1994; Du and Dreyfus, 2002; Bozzali and Wrabetz, 2004). However, little is known regarding endogenous modulators of OLG development acting in a paracrine or autocrine manner.
In different cell types, metabolites of arachidonic acid, e.g., eicosanoids, have been shown to serve in such an autocoid manner. The eicosanoids are produced through cellular stimuli that activate phospholipase A2 and phospholipase C, leading to the liberation of membrane-esterified arachidonic acid. Free arachidonic acid is first metabolized by the cyclooxygenases (COX-1 or COX-2) and then by terminal prostaglandin synthases to produce the prostaglandins (PGD2, PGE2, PGF2α, PGI2) and thromboxane A2 (TXA2). Consequently, these eicosanoid signaling lipids may be released by stimulation of different CNS cell types and participate in the local modulation of OLG and myelin homeostasis. With regard to eicosanoid function, it is known that activation of phospholipases also leads to the production of mediators of inflammation, oxidative stress, and cell damage in the brain and other tissues (Farooqui et al., 2004; Brault et al., 2004). Thus, arachidonic metabolites have been implicated in increasing neuromicrovascular permeability, infiltration of immune cells, and apoptotic/necrotic cell death as well as neurodegeneration. On the other hand, our recent work suggests that one of these eicosanoids, notably, thromboxane A2 (TXA2), might have beneficial effects on OLG-lineage cells. Specifically, we showed that TXA2 receptors (TPR) are enriched in the myelinated fibers of the optic tract, striatum, and internal capsule (Borg et al., 1994). Subsequently, it was shown that this labeling pattern derived from OLGs (Blackman et al., 1998, 1999). Most recently, we have provided evidence that TPR activation promotes the proliferation of OPCs and the survival of mature oligodendrocytes, suggesting a role for TPR signaling in each of these events (Lin et al., 2005). In the present studies, we further characterized TPR localization and signaling during OLG development. Additional studies investigated whether this signaling pathway can modulate the synthesis of the myelin component myelin basic protein (MBP) during OLG differentiation. Our results suggest a novel role for endogenous TXA2/TPR signaling during OLG development.
MATERIALS AND METHODS
Materials
The stable PGH2/TXA2 mimetic U46619 and TPR antagonist SQ29,548 were purchased from Cayman Chemical (Ann Arbor, MI). The stable TPR antagonist BM13,505 was obtained from Boehringer-Mannheim (Mannheim, Germany). The B104 and CG-4 cell lines were kindly provided by Dr. Ravi Tikoo (Cornell University, New York, NY). The A2B5 hybridoma cell line was obtained from ATCC (Manassas, VA). The rat myelin basic protein-luciferase (rat MBP-Luc) plasmid was a gift of Dr. Robin Miskimins (University of South Dakota, Vermillion, SD). PDGF and FGF-2 were obtained from Peprotech (Rocky Hill, NJ). Laminin-2/merosin was from Chemicon (Temecula, CA). Prolong Anti-Fade mounting medium, Fura-2AM, and DAPI were from Molecular Probes (Eugene, OR). Cell culture materials and other reagents were from Fisher Scientific (Hanover Park, IL) or Sigma-Aldrich Chemicals (St. Louis, MO). Pregnant female and male breeder Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN).
Antibodies
Rabbit anti-TPR Ab and rabbit antithromboxane synthase (TS) Ab were from Cayman Chemical. Mouse anti-O4 Ab and mouse anti-MBP Ab were obtained from Chemicon. Mouse anti-2′,3′-cyclic nucleotide phosphodiesterase (CNP) was purchased from Sigma-Aldrich. Goat anticyclooxygenase-1 (COX-1) Ab, mouse antihistone H1 Ab, goat antiactin Ab, goat anti-rabbit IgG (H+L) horseradish peroxidase (HRP) conjugate, goat anti-mouse IgG (H+L) HRP conjugate, and rabbit anti-goat IgG (H+L) HRP conjugate were from Santa Cruz Biotechnology (Santa Cruz, CA). Goat anti-rabbit immunoglobulin (IgG; H+L) Alexa Fluor 488 conjugate, goat anti-mouse IgG (H+L) Alexa Fluor 568 conjugate, and donkey anti-goat IgG (H+L) Alexa Fluor 488 conjugate Abs were from Molecular Probes.
Cell Culture
Mixed glial cultures were prepared from 1–2-day-old Sprague-Dawley rat pups by using a modification of the method of McCarthy and de Vellis (1980). Briefly, the neonatal rats were killed according to protocols approved by the IACUC at the University of Illinois at Chicago. Cerebral hemispheres were harvested and placed in ice-cold Leibovitz-15 medium. The meninges were removed, and the tissue was mechanically dissociated, trypsinized for 30 min, and filtered sequentially through 145-μm and 30-μm nylon mesh. The cell suspension was then plated onto poly-L-lysine (0.1 mg/ml)-coated tissue culture flasks and grown in complete medium [Dulbecco's modified Eagle's medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS), 4.5 g/liter glucose, 1 mM pyruvate, 6 mM glutamine, 10,000 U/ml penicillin, and 10,000 μg/ml streptomycin]. The medium was changed every 2–3 days. After the cultures had grown to confluence (10–14 days), the flasks were shaken on a rotary shaker at 200 rpm for 1 hr at 37°C to remove loosely adherent microglia. The medium was changed, and the flasks were shaken for an additional 18–20 hr at 220 rpm at 37°C to obtain OPCs in suspension. The suspension was collected, and the cells were plated onto bacterial-grade petri dishes for 1 hr to remove contaminating astrocytes and microglia, which preferentially adhere to the dish. The nonadherent cells were collected, resuspended in complete medium, and plated onto poly-L-lysine-coated dishes or coverslips. After 4 hr, the medium was replaced with DMEM (4.5 g/liter glucose, 1 mM pyruvate, 6 mM glutamine) containing a modification of the N1 supplements: 5 μg/ml human apotransferrin, 5 μg/ml bovine insulin, 16.1 μg/ml putrescine, 5 ng/ml sodium selenite, 10 ng/ml biotin, 1 mg/ml bovine serum albumin, 35% B104 cell line conditioned medium (B104 CM), and 10 ng/ml FGF-2 to promote OPC expansion and prevent differentiation. In some cases, OPCs were expanded for 2–3 days before experiments. In all experiments, cultures were found to be >95% pure as visualized by immunostaining for OLG-lineage markers and glial fibrillary acidic protein (GFAP) staining for type-1 astrocytes. OPCs were allowed to differentiate into mature OLGs by removal of the B104 CM and culture in differentiation medium (DM): DMEM containing 50 μg/ml human apotransferrin, 5 μg/ml bovine insulin, 16.1 μg/ml putrescine, 5 ng/ml sodium selenite, 30 nM triiodothyronine, 15 nM hydrocortisone, 10 ng/ml biotin, 1 mg/ml bovine serum albumin, and 0.5% FBS for 6–10 days. In this culture system, OPCs stained positively for the progenitor cell marker A2B5. After induction of differentiation, day-2 OLGs stained positively for the immature cell marker O4, and day 10 OLGs expressed the mature cell marker MBP. The CG-4 progenitor cell line was cultured and passaged in N1 medium containing 35% B104 CM (Louis et al., 1993). CG-4 cells were induced to differentiate in differentiation medium as described for the primary cells.
In experiments to examine the role of TPRs in OLG development, freshly plated OPCs or CG4 cells were grown for 24 hr in B104 CM medium before vehicle or drug stimulation. Treatments were performed in a minimal differentiation medium containing insulin, sodium selenite, biotin, triiodothyronine, and BSA at the above-mentioned concentrations. Under all conditions, the ethanol concentration was 0.0095%, and drugs were replenished by changing half the medium daily.
Immunocytochemistry
Cell culture.
Cells were fixed with 4% paraformaldehyde in Hank's balanced salt solution containing 10 mM HEPES (HBSS-HEPES) for 20 min at room temperature and washed three times with 100 mM glycine and three times with HBSS-HEPES. Cells were incubated overnight with primary antibodies in permeabilization buffer (5% goat serum, 0.1% Triton X-100, 0.2% BSA in HBSS-HEPES) at 4°C. Primary antibodies were diluted as follows: rabbit anti-TPR (1:400), rabbit anti-TS (1:2,500), goat anti-COX-1 (1:500), mouse anti-A2B5 hybridoma supernatant (1:50), mouse anti-O4 (1:250), and mouse anti-MBP (1:500). The coverslips were washed three times in HBSS-HEPES and incubated with either goat anti-rabbit Alexa Fluor 488- or goat anti-mouse Alexa-568-conjugated secondary antibody at 1:1,000 dilution (Molecular Probes) for 1 hr at room temperature in the dark. Cells were further stained with DAPI (2 μg/ml) for 5 min before coverslips were mounted on glass slides. For cell counting analysis, each experimental condition was performed on triplicate coverslips and at least 500 cells/coverslip were counted at ×63 magnification. Each experiment was repeated on at least three individual culture preparations.
Tissue sections.
Sprague-Dawley rats were anesthetized with ether and intracardially perfused with cold 4% (0.1 M) Sorenson's phosphate-buffered paraformaldehyde. Brains were removed, fixed overnight, and washed with PBS. The tissue was then cryoprotected in sucrose (15–30% w/v) before embedding in OCT, frozen on dry ice, and stored at –80°C until further use. Cryostat sections 10–12-μm were collected on chrome-alum-gelatin-coated glass slides. The sections were placed in a humidifying chamber for 1 hr. Blocking buffer (PBS containing 0.2% Tween-20 and 3% FBS) was added dropwise to the sections and incubated for 2 hr at room temperature. The sections were then washed three times in PBS and incubated with cold methanol for 3–6 min. After washing, the sections were incubated with the primary antibodies (1:200) in blocking buffer at 4°C overnight. Secondary goat anti-rabbit Alexa Fluor 488- and goat anti-mouse Alexa-568-conjugated antibodies were used at 1:500 dilution, and sections were further stained with DAPI. The sections were then mounted with antifade medium and coverslips. All images were acquired with a Zeiss LSM 510 Meta laser scanning confocal microscope.
Immunoblotting
Rat brain tissue.
Sprague-Dawley rats aged 15 and 21 days were anesthetized by diethyl ether and decapitated. Cerebral cortexes were isolated and meninges removed in cold HBSS. The tissue was mechanically homogenized by trituration and by Dounce homogenization in cold Tissue Protein Extraction Reagent buffer (Pierce Biochemicals, Rockford, IL) and sonicated for 30 sec on ice. Protein samples were centrifuged for 10 min at 12,000g, and the supernatant was used for analysis.
Cell culture.
Purified OLG-lineage cells at various stages of development were washed twice with ice-cold PBS and harvested on ice with a modified RIPA buffer containing 150 mM NaCl, 50 mM Tris HCl (pH 7.4), 0.25% sodium deoxycholate, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS), 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF, 1 mM β-glycerolphosphate, and protease inhibitor cocktail III (Calbiochem, La Jolla, CA). Cell lysates were sonicated three times in 10-sec bursts and centrifuged for 10 min at 12,000g, and the supernatant was collected for biochemical analysis. Protein concentrations were estimated with a BCA assay kit (Pierce Biochemicals), and samples (5–20 μg) were loaded onto 10% or 15% SDS-PAGE gels and separated for 1.5 hr at 120 V. Gels were transferred to Immobilon PVDF (Millipore, Bedford, MA) membranes for 1.5 hr at 110 V, blocked for 30 min at room temperature in 3% BSA in TBS + 0.1% Tween-20, and incubated with primary antibody at 4°C overnight. The primary antibodies were diluted in 3% BSA in TBS + 0.1% Tween-20 overnight at the indicated dilutions: mouse antihistone H1 (1:400), rabbit anti-TS (1:1,000), rabbit anti-TPR (1:400), goat antiactin (1:400), goat anti-COX-1 (1:200), and mouse anti- MBP (1:500). The blots were rinsed three times in TBST and incubated with the appropriate HRP-conjugated secondary antibodies (1:3,000) diluted in 5% nonfat dry milk in TBS + 0.1% Tween-20 for 1 hr at room temperature. Bands were visualized with the Pierce ECL kit (Pierce Biochemicals).
Nuclear Fractionation
For cell fractionation, cytoplasmic contamination was removed from nuclei by sucrose density gradient centrifugation and performed using previously published methods (Greenberg and Bender, 2004). Briefly, cells in 100-mm dishes (∼1.0 × 107 cells/ml) were washed twice with ice-cold PBS and lysed for 5 min on ice in 2 ml sucrose buffer I [0.32 M sucrose, 10 mM Tris-HCl (pH 8.0), 3 mM CaCl2, 2 mM magnesium acetate, 0.1 mM EDTA, 1 mM DTT, and 0.1% Triton X-100]. Complete cell disruption was verified by phase-contrast microscopy. The cell lysate was gently mixed with an equal volume of sucrose buffer II [2 M sucrose, 5 mM magnesium acetate, 0.1 mM EDTA, 10 mM Tris-HCl (pH 8.0), 1 mM dithiothreitol (DTT)]. The resulting cell homogenate was overlaid onto a 2.2-ml cushion of sucrose buffer II and the gradient was topped off by overlaying with sucrose buffer I. The nuclei were sedimented by ultracentrifugation in a Beckman SW41 rotor at 30,000g for 1 hr at 4°C. The supernatant was removed and designated the cytoplasmic/nonnuclear membrane fraction, and the pellet was designated the nuclear fraction. Nuclei were solubilized in buffer containing 50 mM Tris-HCl (pH 7.4), 25 mM KCl, 5 mM MgCl2, 2% Triton X-100 and briefly sonicated on ice. Protein concentrations were measured with a BCA assay kit (Pierce Biotechnology).
Intracellular Ca2+ Measurement
Freshly isolated OPCs were plated onto laminin-2 (10 μg/ml)- plus fibronectin (10 μg/ml)- plus poly-L-lysine (100 μg/ml)-coated 25-mm glass coverslips and grown for 1–2 days or allowed to differentiate for 5–6 days. Prior to the assay, cells were washed twice in HBSS-HEPES and loaded with 1 μM Fura-2AM dye for 20 min at 37°C in the dark. After loading, cells were washed twice in HBSS-HEPES and stimulated in the same buffer. Calcium transients were measured following stimulation with the Attofluor Ratio Vision digital fluorescence microscopy system (Atto Instruments, Rockville, MD) attached to an inverted microscope (Zeiss Axiovert S100) and an F-Fluar ×40 oil- immersion objective. This system allows for the excitation of single cells at 334 nm and 380 nm while measuring emission at 520 nm. A field of cells was visualized, and 30 cells were selected. The change in the 334/380 excitation ratio was measured every 5 sec. The average absolute peak change in ratio 334/380 of selected cells within this field was measured. For the inhibitor studies, Fura-2-loaded cells were preincubated with 1 μM SQ29,548 at room temperature for approximately 10 min prior to stimulation with U46619.
Intracellular cAMP Measurement
Freshly isolated OPCs were plated onto laminin-2 (10 μg/ml) plus fibronectin (10 μg/ml) plus poly-L-lysine (100 μg/ml)-coated mm glass 24-well plates and grown for 1–2 days or allowed to differentiate for 5–6 days. Prior to stimulation with agonist, the cells were washed twice with serum-free DMEM, starved for 30–60 min, and then incubated for 20 min with 200 μM 3-isobutyl-1-methylxanthine (IBMX) to inhibit phosphodiesterase activity. The agonist U46619 (10 μM) or forskolin (5 μM) was added to the cells for 15 min at 37°C in the presence of IBMX. The medium was removed and the cells were lysed in 0.1 M HCl for 1 hr at room temperature. The lysates were collected and centrifuged, and the supernatants were used for the acetylated cAMP assay kit (Assay Designs, Ann Arbor, MI) according to the manufacturer's instructions.
TXB2 measurement
OPCs were plated onto poly-L-lysine-coated 12-well plates and grown for 3–4 days. Cells were washed twice in HBSS and placed in fresh medium. Endogenous TXB2 release was measured in cell culture supernatants over a 24-hr period for day-0, day-2, and day-10 cells. For arachidonic acid stimulation, cells were washed twice in HBSS and placed in N1 medium. Vehicle or 50 μM indomethacin was added for 15 min before treatment with 20 μM arachidonic acid for 10 min. At the end of all time periods, supernatants were collected, and a 100-μl aliquot was used to measure the stable metabolite of TXA2 (TXB2) via colorimetric ELISA (Assay Designs).
Cell Transfection
Proliferating CG-4 cells were seeded onto poly-L-Lysine-coated six-well dishes and grown overnight. Cells were transfected with 1 μg of the rat MBP-Luc construct using the Effectene Transfection reagent (Valencia, CA) for 18–24 hr. Cells were then washed twice and stimulated with vehicle or U46619 for 48 hr in minimal differentiation medium. After the stimulation period, cells were washed twice in cold PBS and harvested in Glo-Lysis Buffer (Promega, Madison, WI). After addition of the Steady Glo-Luciferase Substrate, luminescence in the cell samples was quantified by using the Wallac Victor2 multilabel plate reader (Perkin Elmer, Boston, MA). In these experiments, transfection efficiencies were determined to be <5% as determined by transfection with a pEGFP plasmid and fluorescence microscopy analysis.
Statistical Analysis
Data were expressed as mean ± SEM, and statistical significance was assessed by using a two-tailed paired t-test. Differences were considered statistically significant at P < 0.05.
RESULTS
Expression of TPR, TS, and COX-1 in Differentiating OLGs
We previously showed that TPRs are enriched in the myelinated fibers of the optic tract, striatum, and internal capsule (Borg et al., 1994) and that this labeling pattern derived from OLGs (Blackman et al., 1998). Subsequent studies demonstrated the presence of functional TPRs on cultured OLGs and that these receptors can be purified by ligand affinity chromatography (Blackman et al., 1998, 1999). Furthermore, it was also shown that TPR activation is associated with OPC proliferation and OLG survival (Lin et al., 2005). In the present studies, we further characterized TPR localization and signaling during OLG development. The first experiments investigated the expression of the TXA2 signaling components in OPCs and OLGs, i.e., COX, TXA2 synthase (TS), and TPR. Specifically, rat brain cortical tissue from postnatal ages 15 and 21 days was harvested, and the lysates were prepared and analyzed by immunoblot. These time points correspond approximately to early and late stages of active myelination in the rat CNS. During this developmental period, an increase in the expression of the myelin-specific proteins 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP) and MBP was detected. The results also revealed the presence of the TXA2 signaling cascade components, i.e., COX-1, TS, and TPRs, during this developmental time period (Fig. 1A). The next experiments determined the expression pattern of these signaling components specifically associated with OLG-lineage cells. To this end, OPCs were purified from neonatal rat brain, plated onto tissue culture dishes, and expanded for 2–3 days with the B104 cell-line conditioned medium (B104CM). OPCs at this stage of development were defined as day-0 cells. These cells were then induced to differentiate in differentiation medium (DM) as described in Materials and Methods. The OPCs used in these experiments were >95% pure at day 0 as well as day 10 (as visualized by immunostaining for OLG-lineage markers and GFAP staining for type-1 astrocytes). Cell protein samples were harvested at days 0, 2, and 10 of differentiation. Western blotting revealed the presence of TPR, TS, and COX-1 at each time point (Fig. 1B). It can also be seen that there was a progressive increase in each of their levels during differentiation from immature to mature OLGs. Collectively, these data indicate that the enzymes of the TXA2 synthetic pathway and TPRs are expressed during OLG differentiation in culture and that these expression levels increase as the cells mature. Presumably, a similar increase of these components in whole brain is not apparent, because OLGs make up a very small percentage of the total brain lysate.

Components of the TXA2 synthetic pathway are expressed in developing brain and OLGs. A: Rat brain cortical tissue was harvested at postnatal ages 15 days (P15) and 21 days (P21) and analyzed for the expression of COX-1, TS, TPR, CNP, and MBP. B: Purified neonatal rat OPCs were differentiated in culture; harvested at various time points in development [day 0 (progenitor cells), day 2 (immature OLGs), day 10 (mature OLGs)]; and analyzed for the expression of COX-1, TS, and TPR. Proteins were isolated and immunoblotting was performed as described in Materials and Methods. The expression levels of the enzymes COX-1 and TS, and the TP receptor increased as a function of OLG differentiation.
Cellular Localization of the TPR Signaling Components
Because the cellular distribution of the TXA2 signaling components in OPCs/OLGs is presently unknown, additional studies using confocal immunocytochemical analysis were performed on each of these cell types. Specifically, purified OPCs were plated onto glass coverslips; cultured as described in Materials and Methods; and fixed/ permeabilized at days 0, 2, and 10 of differentiation. A midsection confocal plane of the fixed OPCs revealed that the day-0 cells exhibited a characteristic bipolar morphology and stained positively for the OPC marker A2B5 (Fig. 2A). It can also be seen that the OPCs expressed the enzymes TS (Fig. 2B) and COX-1 (Fig. 2C). These images demonstrate that both of these enzymes are localized predominantly in the cytoplasmic compartment and in perinuclear sites, which is consistent with their localization in other cell types (Morita et al., 1995). A similar TS and COX-1 distribution pattern was obtained with day-2 (O4 positive; Fig. 2D–F) and day-10 (MBP positive; Fig. 2G–I) OLGs. Furthermore, it was observed that COX-1 and TS stained both major and minor processes in the immature and mature OLGs.
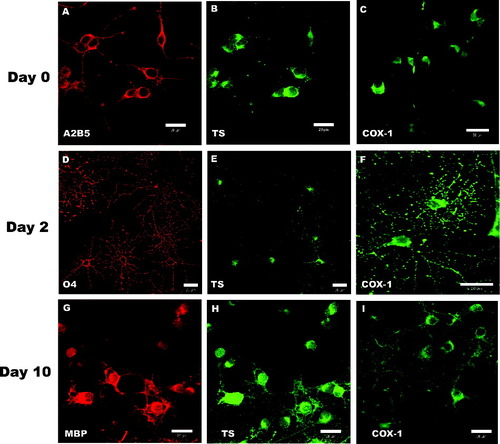
Intracellular localization of TS and COX-1 in OPCs and OLGs. OPCs were plated onto coverslips, induced to differentiate, fixed and stained at days 0, 2, and 10 of differentiation, and analyzed via confocal microscopy. The antibodies to the OLG lineage-specific markers A2B5 (A; red), O4 (D; red), and MBP (G, red) were used to identify cells at each time point. Each row represents cells at the same stage of differentiation. Cells were colabeled with anti-TS (B,E,H; green) and COX-1 (C,F,I; green) at each developmental stage. Scale bars = 20 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The next series of experiments examined the distribution pattern of TPRs in both OPCs and OLGs. Figure 3 illustrates a midsection confocal plane of the fixed and permeabilized OPCs. It was found that TPRs were localized on the cell surface (Fig. 3A), as might be expected for a G-protein-coupled receptor (GPCR), as well as in the perinuclear (endoplasmic reticulum/Golgi) compartments and in the cell processes. We next examined the TPR distribution pattern in immature OLGs on days 1–2 of differentiation. Confocal midsection images of these cells (and Z-stack analysis of these sections) showed staining for the O4 antigen (Fig. 3B). Figure 3B also illustrates that there is a cell surface, cytoplasmic, and perinuclear distribution of TPRs similar to that observed for bipolar OPCs.
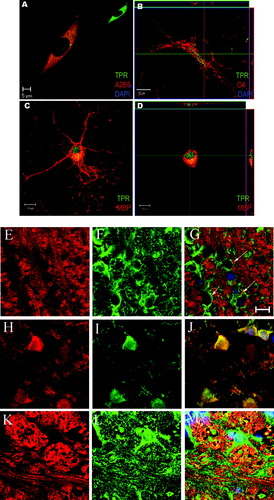
Intracellular localization of TPRs in developing OLGs. OPCs/OLGs were analyzed via confocal microscopy at days 0, 2, and 10 of differentiation for TPR expression and localization. A: OPCs label positive for A2B5 (red) and TPRs (green). The nucleus is indicated by DAPI (dark blue) staining. Inset shows TPR labeling alone. B: Early immature OLGs are immunoreactive for O4 (red), TPR (green), and DAPI (dark blue). Z-axis confocal sectioning of X-Z plane (horizontal inset) and Y-Z plane (vertical inset) reveals that TPRs localize predominantly to the cytoplasmic, perinuclear compartment. C: Mature MBP-positive (red) cells show TPR (green) localization in cytoplasmic and nuclear compartments. Colocalization of cytoplasmic MBP and TPRs is indicated by orange staining. D: Z-axis confocal sectioning of the cell in C indicates TPR localization in the nucleus (horizontal and vertical insets) and cytoplasm (vertical inset). E–M: Rat brain cryostat sections from 22-day-old rat cerebellum (E–J) and 33-day-old rat medulla (K–M) were immunostained and analyzed via confocal microscopy. E shows staining in the cerebellum for MBP (red), and F shows coimmunostaining for TPR (green). The overlay of the two along with DAPI (blue) staining for the nucleus is shown in G. Arrows indicate strings of OLGs staining for TPR within the myelin fibers. H shows staining for OLG cell body marker, CNPase (red) coimmunostained for TPR (I), and the colocalization of the two is shown in J. K shows staining in medulla for MBP (red), and L shows coimmunostaining for TPR (green). The overlay of the two along with DAPI (blue) staining for the nucleus is shown in M. Colocalization of the green and blue staining (indicated by arrows) shows TPR localization in the nucleus of OLGs. Scale bars = 10 μm unless otherwise indicated. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
However, the immunostaining pattern of mature OLGs (day 10) was substantially different. Specifically, TPR immunoreactivity was found not only on the cell surface and in the perinuclear region but also in the nuclear compartment (Fig. 3C). The nuclear localization of TPRs was confirmed by performing a Z-stack analysis of the confocal sections (Fig. 3D). This analysis also revealed that, whereas TPRs localize in the nucleus, MBP staining is limited to the extranuclear compartments. To determine whether a similar OLG staining pattern for TPRs and MBP is observed in vivo, immunohistochemical analysis of OLGs in cryostat sections from developing rat brain cerebellum and medulla was performed. As expected, TPRs were present in the myelinated tracts of the white matter as well as in certain areas outside these tracts (Borg et al., 1994). Figure 3E illustrates the MBP immunostaining of myelinated fiber tracts in the cerebellum. Figure 3F shows the coimmunostaining of TPR in the same field as Figure 3E. It can be seen that TPRs were associated with cells that exhibit the classic “string of pearls” orientation along myelin fiber tracts (Fig. 3F,G), characteristic of OLGs in the myelinated tracts of the CNS (Peters et al., 1991). The identification of these TPR-expressing cell bodies as OLGs was confirmed by immunostaining with the OLG cell body marker CNPase (Fig. 3H). Coimmunostaining with TPR antibody revealed intense TPR immunoreactivity in the CNPase-positive cells (Fig. 3I,J).
Figure 3K illustrates the MBP staining pattern in the large medial longitudinal myelin fiber tracts in the medulla. Costaining of these sections with TPR antibody again revealed selective OLG staining (Fig. 3L). In this case, however, there appeared to be significant TPR localization in the nuclear compartment (arrows in Fig. 3M), indicative of a more mature OLG stage. Thus, TPRs are associated with OPCs and OLGs both in vitro and in vivo, and the TPR distribution pattern appears to shift toward a more nuclear localization as OLGs mature.
To confirm that this nuclear shift in TPR localization occurs as a function of OLG maturation, cell fractionation and biochemical analysis were performed. To this end, cultured rat brain OPCs (day 0) and mature OLGs (day 10) were lysed, and total cellular protein was fractionated by sucrose density gradient centrifugation to separate the nonnuclear fractions (cytoplasm plus ER/Golgi) from the nuclear fraction (Greenberg and Bender, 2004). These fractions were then probed by Western blotting for the presence of TPRs and a known nuclear marker, histone H1 protein, and a nonnuclear marker, MBP. It can be seen that, although TPRs are associated primarily with the nonnuclear compartment in OPCs (Fig. 4, left), there is substantial TPR localization in the nuclear fraction in OLGs (Fig. 4, right). Consistent with previous results, TPRs appear to migrate at two separate molecular sizes in the 55-kDa region (Borg et al., 1994). The fractionation results, therefore, confirm the confocal imaging data and provide additional evidence that TPRs become associated with the nuclear compartment as OLGs mature.
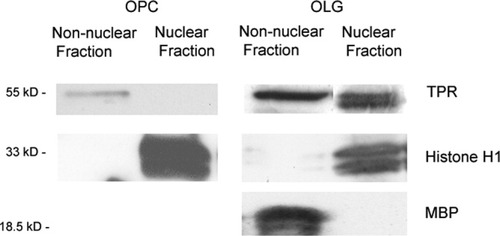
Nuclear localization of TPRs developing OLGs. OPCs or mature OLGs were fractionated by sucrose density gradient centrifugation into nonnuclear and nuclear fractions. Each fraction was blotted with anti-TPR, antihistone H1, or anti-MBP. In OPCs, TPRs localized to the nonnuclear fraction. In OLGs, TPRs colocalized to the nuclear faction with histone H1, whereas MBP localized to the nonnuclear fraction. Similar results were obtained from fractionation of two separate OLG cultures.
Production of TXA2 By Developing OLGs
Whereas the previous studies demonstrated the expression of COX-1, TS, and TPRs both in vivo and in culture, the next series of experiments examined whether immature and/or mature OLGs have the capacity to synthesize TXA2 during their differentiation. Initially, cells at day 0 (OPCs) and day 10 (OLGs) of differentiation were stimulated with exogenous arachidonic acid, and the production of TXA2 was monitored by measurement of its stable metabolite TXB2. As shown in Figure 5A, both OPCs and mature OLGs metabolized exogenously added arachidonic acid to TXA2, an effect that was inhibited 75–80% by preincubation with the COX inhibitor indomethacin. Additional studies determined the relative ability of OPCs and OLGs to synthesize TXA2 endogenously under basal conditions. Specifically, OPCs were grown for 24 hr in mitogen-containing B104 CM, and cell-derived TXA2 production was measured. As can be seen in Figure 5B, OPCs metabolize endogenous arachidonic acid to TXA2 during their proliferative phase. In separate experiments, OPCs were cultured in differentiation medium for 1 or 10 days, and the amount of endogenous TXA2 produced during a 24 hr period was measured at each time point. It was found that, similarly to OPCs, immature and mature OLGs also produce significant amounts (approximately 1 nM) of TXA2. Taken together, these results (Fig. 5) coupled with the immunoblotting data (Fig. 1) demonstrate that OPCs and OLGs, at all stages of maturation, are capable of synthesizing TXA2 and possess the signaling components required to respond to its production.
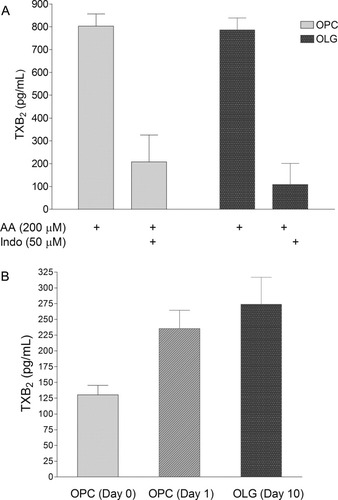
TXA2 production in developing OLGs. OPCs or OLGs were plated onto 12-well dishes, and TXB2 levels were measured in culture medium supernatants. A: After a 10-min incubation with arachidonic acid (200 μM), OPCs (gray bars) and OLGs (black bars) synthesize considerable amounts of TXB2. This was attenuated by a 10-min preincubation with 50 μM of the COX inhibitor indomethacin. B: Proliferating OPCs (day 0), differentiation-induced OPCs (day 1), and mature OLGs (day 10) produce TXB2 under endogenous cell culture conditions as measured over a 24-hr culture period. Results represent the means ± SEM of measurements from three separate cultures.
Effect of TPR Activation on Intracellular Ca2+ in Developing OLGs
We next investigated potential TPR second messenger signaling intermediates (Ca2+ and cAMP) in OPCs and OLGs. In this connection, previous work showed that TPR activation of a human neurofibrosarcoma (T265) cell line by the well-characterized agonist U46619 (10 μM) stimulates increases in intracellular calcium, whereas TPR activation of primary rat Schwann cells causes an increase in intracellular cAMP levels (Muja et al., 2001). We measured, based on this signaling divergence, TPR-stimulated Ca2+ and cAMP levels at two different stages of OLG development and investigated whether these signaling pathways change during the developmental process. For the calcium measurements, OPCs were plated onto 25-mm coverslips and loaded with fluorescent calcium indicator Fura-2AM. It was found that treatment of OPCs with U46619 (10 μM) resulted in a rapid increase in intracellular Ca2+ levels (Fig. 6A). In separate studies, primary OPCs were allowed to differentiate to mature OLGs for 5–6 days, and the TPR-mediated calcium response was again measured. Similarly to OPCs, stimulation of mature OLGs with 10 μM U46619 produced a substantial Ca2+ response (Fig. 6C). In both OPCs and OLGs, the increase in intracellular Ca2+ levels was blocked by pretreatment with the TPR antagonist SQ29,548 (1 μM), whereas carbachol-mediated Ca2+ increases were unafffected, demonstrating the TP receptor specificity of this pathway (Fig. 6B,D).
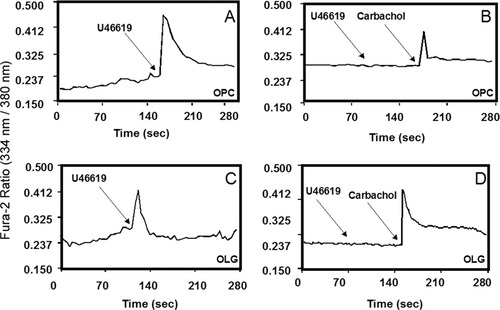
Effect of TPR stimulation on intracellular calcium levels in OPCs and OLGs. OPCs (A,B) or OLGs (C,D) were grown on fibronectin plus laminin-2 plus poly-L-lysine-coated coverslips. Cells were loaded with 1 μM Fura-2AM indicator dye in HBSS for 30 min and stimulated with 10 μM U46619. Increase in intracellular Ca2+ was visualized by a rise in ratio of 334 nm/380 nm emission. U46619 induced a rapid increase in Ca2+ concentrations in both OPCs and OLGs (A,C), which was blocked by SQ29,548 (B,D). Carbachol-mediated calcium responses were unaffected.
An additional series of experiments determined whether TPR activation also affected cAMP levels in developing OPCs. However, the results demonstrated that U46619 did not produce a significant increase in cAMP levels in either OPCs (90% of control levels) or mature OLGS (104% of control levels). Forskolin was used as a positive control to test the functionality of the cAMP assay. Collectively, these results indicate that TPRs in OPCs and OLGs signal through changes in intracellular Ca2+ levels but do not appear to affect cellular cAMP levels.
Effect of TPR Stimulation During OLG Development
Because the above results indicated a functional TPR signaling pathway in OPCs and OLGs, the next series of experiments determined the effects of such signaling during OLG maturation. In these studies, OPCs were induced to differentiate by thyroid hormone-containing medium and stimulated with varying doses of U46619 for 2 days. At the end of this period, the differentiating OLGs were harvested, and MBP expression was analyzed by immunoblotting. It was found that U46619 caused a dose-dependent increase in MBP expression (Fig. 7A).
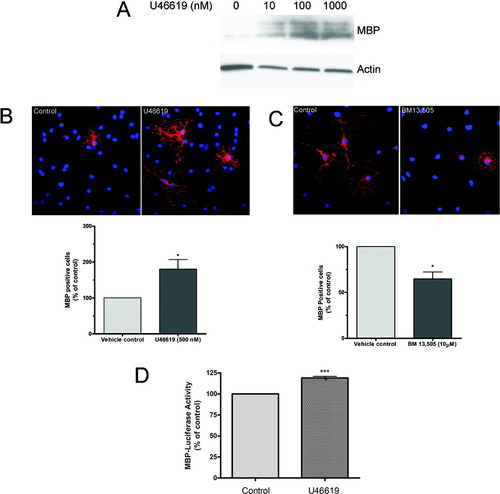
Effect of TPR activation on MBP expression in differentiating OLGs. A: OPCs were induced to differentiate and treated with vehicle (0.0095% ethanol) or U46619 (10–1,000 nM) for 48 hr, and MBP expression was analyzed by immunoblot analysis. Actin is shown as a protein loading control. Blot is representative of three experiments. B: OPCs were induced to differentiate and treated with vehicle (0.0095% ethanol) or U46619 (500 nM) for 48 hr fixed and stained for MBP. Representative images of vehicle control and U46619-treated cells are shown. MBP staining is indicated in red; DAPI nuclear staining (blue) indicates the total number of cells in the field. The graph represents quantification of MBP-positive cells. Data represent the mean ± SEM of three or four independent experiments performed in triplicate and correspond to at least 4,500 cells counted for each treatment condition; *P < 0.05. C: OPCs were induced to differentiate and treated with vehicle or the TPR antagonist BM13,505 (10 μM) for 4 days and stained for MBP. Representative images of vehicle control and BM13,505 treated cells are shown. The graph represents the quantification of MBP-positive cells. Data represent the mean ± SEM of three or four independent experiments performed in triplicate and correspond to at least 4,500 cells counted for each treatment condition; *P < 0.05. D: CG-4 cells were transfected with the MBP-Luc reporter and induced to differentiate for 48 hr in the presence of vehicle or U46619 (500 nM). Cell lysates were harvested, and luminescence in the samples was measured. Data represent the mean ± SEM of three or four experiments; ***P < 0.0005. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To evaluate TPR-mediated MBP expression further, immunocytochemical staining was used. In these studies, isolated OPCs were plated onto coverslips and treated with vehicle or U46619 for 48 hr under conditions that favor OLG differentiation. At the end of this period, the cells were fixed and stained for MBP, the marker of OLG differentiation. It was found that treatment of cells with U46619 produced a 1.8–2-fold increase in the percentage of MBP-positive cells (Fig. 7B). These results are in agreement with the quantification of MBP expression as measured by immunoblot (Fig. 7A).
We next assessed whether endogenous TPR activation plays a role in MBP expression. In these studies, OPCs were treated with either vehicle or the metabolically stable TPR antagonist BM13,505 (10 μM) for 4 days. This time period was chosen to allow a portion of the OPCs to mature to the MBP-positive stage. It was found that treatment with BM13,505 produced a 35% reduction in the number of MBP-positive cells (Fig. 7C). Thus, it appears that MBP expression can be increased by either exogenous or endogenous TPR activation.
Finally, the mechanism of TPR-mediated myelin gene expression was examined in a luciferase gene reporter assay. In these experiments, the CG-4 progenitor cell line was transiently transfected with the rat MBP-Luc construct, which contains the rat MBP promoter and which drives expression of the firefly luciferase gene. Luciferase gene expression and enzymatic activity were then measured and used as an index of transcription from the MBP promoter. Transfected CG-4 cells were induced to differentiate and stimulated with U46619 (500 nM), and luciferase activity was measured after 48 hr. It was found that TPR activation stimulated a significant increase in gene transcription from the MBP promoter as measured by luciferase activity (Fig. 7D). Collectively, these data suggest that the TPR-mediated increase in MBP expression involves increased transcription from the MBP promoter.
DISCUSSION
Prostaglandins (PGD2, PGE2, PGF2α, and PGI2) have been shown to be involved in cell growth and differentiation in many cell types, e.g., osteoclasts, chondrocytes, adipocytes, skeletal muscle, and smooth muscle (Miller et al., 1996; Negrel, 1999; Suzawa et al., 2000; Horsley and Pavlath, 2003; Li et al., 2004). Other eicosanoids such as TXA2 have also been shown to modulate cell development; e.g., TXA2 inhibits VEGF-induced endothelial cell differentiation and promotes megakaryocytopoiesis (Ashton and Ware, 2004; Tanaka et al., 2004). Separate studies with site-specific drugs or knockout mice for COX-1, COX-2, PG receptors, and TP receptors have revealed the importance of prostaglandins and TXA2 in hemostasis, renal physiology, immune function, inflammation, parturition, and fertility (Narumiya and FitzGerald, 2001; Williams et al., 1999). However, despite such knowledge for multiple cell types, much less is known regarding the role of PGs and TXA2 in nervous system development and function. Nevertheless, the presence of TPRs has been reported in astrocytes, oligodendrocytes, and Schwann cells (Nakahata et al., 1992; Kitanaka et al., 1996; Blackman et al., 1998; Muja et al., 2001). In addition, TPR mRNA has been reported to be present in some neurons (Wacker et al., 2005). TPR-mediated MAPK activation has been reported in 1321N1 astrocytoma cells (Kobayashi et al., 2000). TPRs have also been reported to enhance the interleakin-6 (IL-6) biosynthesis via the PKA p38 MAPK/CREB pathway in 1321N1 cells (Obara et al., 2005). In spite of these findings, the precise function of TPR signaling in the neural cell types is not well defined.
Recently, however, our studies showed that stimulation of TPRs promotes proliferation of OPCs and prolongs survival of mature OLGs. These studies also demonstrated that TPR signaling in OPCs, OLGs, and Schwann cells involves the activation of ERK/CREB (Muja et al., 2001; Lin et al., 2005). Taken together, these findings suggest a role for TXA2 in the modulation of myelinating cell function. The present studies sought to characterize the TPR signaling cascade during OLG development as well as identify a potential role for TPR signaling in the expression of MBP.
In these experiments, we first determined the expression and distribution patterns of TPRs and the TXA2 biosynthetic enzymes (COX-1 and TS) during OLG differentiation. Western blot analysis demonstrated that TXA2 signaling components are expressed during myelination in the developing rat brain (Fig. 1A). Furthermore, in cultured cells, both COX-1 and TS are up-regulated as the bipolar OPCs differentiated into mature multipolar OLGs (Fig. 1B). To gain further insight into the function of this signaling pathway during development, the cellular distribution pattern of these components was visualized by confocal immunostaining in cultured cells. It was found that COX-1, TS, and TPR coexpressed with stage-specific OLG markers (A2B5-, O4-, and MBP-positive cells; Fig. 2). This presence of the TPR signaling components (COX-1, TS, and TPR) raised the possibility that, similarly to other cell types, e.g., platelets, OLGs may possess a self-contained TPR signaling pathway.
The results from subsequent experiments support this possibility; it has been found that OLGs at all stages of development produced significant amounts of TXA2 (Fig. 5). This was found to be the case under basal conditions and under conditions in which the COX-1 substrate, arachidonic acid, was provided. Thus, TXA2 is endogenously produced by OLGs, and this production can be enhanced by extracellular sources of arachidonate.
Interestingly, we also observed an increase in TXA2 production when OPCs were induced to differentiate (Fig. 5). This finding may suggest an up-regulation of phospholipase activity commensurate with the increased expression of TXA2 synthase and COX-1 enzymes during the differentiation process. Indeed, a previous report has provided evidence for such an increase in lipid metabolism during OLG differentiation and myelinogenesis (Vartanian et al., 1992). Alternatively, during OLG development in vivo, TXA2 may also be derived from other CNS cell types, e.g., microglia or neurons (Giulian et al., 1996). In either case, it appears that OPCs/OLGs possess both the synthetic machinery to elaborate TXA2 and the capacity to signal in response to its synthesis, in a manner analogous to its action in blood platelets.
With regard to TXA2 signaling mechanisms, previous studies have established that this eicosanoid elicits its biological functions through its GPCR (Hirata et al., 1991). These receptors couple to at least two G-proteins (Gq, G13) and signal to multiple downstream kinases and transcription factors (Shenker et al., 1991; Knezevic et al., 1993; Offermanns et al., 1994; Ushikubi et al., 1994; Djellas et al., 1999; Huang et al., 2004). Thus, a hallmark of classical TPR activation is signal transduction originating at the plasma membrane. However, a particularly provocative finding of the present study was an increase in TPR localization to the nuclear compartment during OLG differentiation (Fig. 3). Interestingly, immunoblotting of this fraction revealed the presence of TPRs with two apparent molecular weights in the 55-kDa region (Fig. 4). This finding is consistent with our previous results from whole rat brain and indicates the possible existence of TPR isoforms in OLGs (Borg et al., 1994). Taken together, these results represent the first demonstration of nuclear GPCR localization in OLGs and suggest the possible involvement of TPRs in direct OLG nuclear signaling. In this connection, our previous studies have shown that TPR stimulation in OPCs and OLGs promotes the activation of MAPK, CREB, and c-fos, which are known to be involved in various gene transcription events. Consequently, the presence of nuclear TPRs may suggest the existence of an additional mechanism for TPR signaling in OLGs. Similar nuclear localization of GPCRs has been described in other cell types, suggesting the possibility of nuclear GPCR signaling in this cell type (Helliwell et al., 2004; Marrache et al., 2005).
The present work also demonstrates that TPR signaling increases MBP protein expression, as measured by immunoblot (Fig. 7A), immunofluorescence (Fig. 7B,C), and a luciferase reporter (Fig. 7D). Although the underlying mechanism for this stimulation of MBP expression is presently unknown, different possibilities exist. For example, intracellular Ca2+ and numerous kinases (capable of activating gene expression) are recruited downstream of TPR signaling and may be involved in the stimulation of the MBP promoter by direct or indirect interactions (Umemori et al., 1999; Miskimins and Miskimins, 2001; Clark et al., 2002). Clearly, additional studies are required to elucidate these interesting possibilities.
In summary, the current studies demonstrate 1) that TXA2 signaling components are expressed in vivo during OLG development and that this expression increases as a function of maturation in cultured OPC and OLG cells; 2) that both OPCs and OLGs have the capacity to synthesize TXA2 from either endogenous or exogenous arachidonic acid; 3) that the distribution of TPR localize in multiple compartments, including the cell surface, the perinuclear region, and the cell processes in OPCs; 4) that TPR localization shifts to the nuclear compartment during OLG maturation; and 5) that TPR stimulation of developing OLGs promoters increases activity of the MBP promoter and increased expression of MBP. Collectively, these results suggest a potential role for TXA2 and TPRs in the process of OLG maturation and myelin synthesis.
Acknowledgements
We are grateful to John R. Gadient for assistance in primary cell culture preparation. We also thank Drs. Richard D. Minshall and Ayesha N. Shajahan for their help with the confocal imaging studies; Fadi T. Khasawneh for assistance in the calcium measurements; and Dr. Jin-Sheng Huang for critical reading of the manuscript. This work was supported in part by a grant from the National Multiple Sclerosis Society (RG 3054B2/1) awarded to G.C.L.




