Neuroprotective effect of N-acetylcysteine on neuronal apoptosis induced by a synthetic gingerdione compound: Involvement of ERK and p38 phosphorylation
Abstract
Besides being used as a spice, ginger has been applied in oriental medicine to ameliorate symptoms such as inflammatory, rheumatic disorders, and gastrointestinal discomforts. The effects of ginger on neuronal cells, however, have not been explored. We investigate the effect of 1-(3,4-dimethoxyphenyl)-3,5-dodecenedione (I6), a derivative of gingerdione, on cultured cortical neurons. After a 5-day maturation period in vitro, cortical neurons were treated with I6 for 24 hr and cell viability was assessed using MTT assay. I6 induced neuronal death in a concentration-dependent manner. Hoechst 33342, propidium iodide (PI), and TUNEL staining confirmed that the reduced cell viability by I6 was due to apoptosis. Pre-treatment of cell with N-acetylcysteine (NAC) prevented cell death in a concentration-dependent manner. N-acetylcysteine increased phosphorylated levels of p42 and p44 extracellular signal-regulated kinases (ERKs). In parallel, farnesyltransferase and MEK inhibitors blocked ERK phosphorylation and neuroprotective effect of NAC. Unexpectedly, NAC also increased phosphorylated level of p38 mitogen-activated protein kinase (MAPK) and p38 specific inhibitors dose-dependently attenuated the effect of NAC. Farnesyltransferase and MEK inhibitors completely abolished NAC-induced p38 phosphorylation whereas p38 inhibitor did not influence NAC-induced ERK phosphorylation. These results show that NAC serially activates ERKs and p38 MAPK, and ERKs and p38 work together to mediate the neuroprotective effect of NAC. © 2006 Wiley-Liss, Inc.
Ginger (Zingiber officinale Roscoe, Zingiberaceae) has been widely used as a spice for dietary condiment throughout the world. Besides being used as a spice, ginger has been applied in traditional oriental medicine to ameliorate symptoms such as inflammatory, rheumatic disorders, and gastrointestinal discomforts. Plants of ginger family have been shown to inhibit tumor promotion in mouse skin through inhibition of 12-o-tetradecanoylphorbol-13-acetate (TPA)-induced cyclooxygenase 2 (COX-2) expression (Kim et al., 2005) and suppress proliferation of human promyelocytic leukemia (HL-60) cells through induction of apoptosis (Lee and Surh, 1998). Epidermal growth factor-induced cell transformation was blocked by [6]-gingerol (Bode et al., 2001). However, the effects of ginger on neuronal cells have not been explored.
The major constitutes of ginger include gingerol, zingerone, shagaol, and gingerdione. One of the authors (L.J.H) has selected [6]-gingerdione as a lead compound to synthesize a series of gingerdione derivatives and found that 1-(3,4-dimethoxyphenyl)-3,5-dodecenedione (I6) was very potent in inhibiting cell proliferation in HL-60 cells (Hsu et al., 2005). Using an in vitro model of I6-induced apoptosis, we investigated the mechanisms of neuroprotection exerted by NAC. We found that the effect of NAC required activation of Ras-ERK and p38 MAPK pathways as the effect of NAC was no longer seen in the presence of MEK or p38 MAPK inhibitors. Importantly, we have shown a serial activation of ERKs and p38 MAPK by NAC and ERKs and p38 work together to mediate the neuroprotective effect of NAC.
MATERIALS AND METHODS
Chemicals and Reagents
N-acetylcysteine (NAC) and U0126 were purchased from Sigma (St. Louis, MO). SB303580, SB202190, and FTI 277 were obtained from Calbiochem (La Jolla, CA). Phospho-p38 MAPK, p38 MAPK, phospho-ERK, and ERK antibodies were purchased from Cell Signaling Technology (Beverly, MA). At DIV 4-6, cortical neurons were treated with various concentrations of I6, SB 303580, SB 202190, FTI 277, or U0126. When culture was co-treated with I6 and SB303580, SB202190, FTI277, or U0126, the final concentration of DMSO was <0.6%. We have shown that DMSO at this concentration had no effect on the cell viability.
Primary Cortical Neuron Culture
Primary cortical cells were prepared as described previously (Xia et al., 1996). In brief, cerebral cortices were dissected from postnatal (P0–P2) Sprague-Dawley rats and dissociated in 0.025% trypsin at 37°C for 60 min. Trypsin digestion was stopped by trypsin inhibitor and DNase I. After passage through a Pasteur pipette several times, cortical cells were plated at a density of 5 × 104 per well in 96-well plates for cell viability assay, 1 × 106 per plate in 35 mm plates with coverslips for TUNEL reaction and Hoechst staining, or 3 × 106 per plate in 60 mm plates for Western blotting. All plates and coverslips were coated with poly-l-lysine and laminin. The neurons were cultured in neuro basal medium supplement with 5% FBS, 35 mM glucose, 1 mM l-glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. Cytosine-d-arabinofuranoside (4 μM) was added at Day 2 in vitro (DIV 2) after plating to prevent the proliferation of non-neuronal cell.
TUNEL Reaction
Neurons were washed with PBS, fixed in 4% paraformaldehyde, and permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate. After washing with PBS, neurons were labeled (60 min, 37°C) with a fluorescein TUNEL reagent mixture according to the manufacturer's suggested protocol (Roche Diagnostics, Indianapolis, IN). Neuronal cultures were mounted on slides and examined by fluorescence microscopy.
Hoechst33342, PI, and Double Staining of phosho-p38 MAPK and Hoechst 33342
Apoptotic or necrotic cell death was characterized by use of Hoechst 33342 and propidium iodide (PI; Sigma-Aldrich) double staining. Twenty-four hours after I6 treatment, cells were stained with 10 μg/ml Hoechst 33342 and 10 μg/ml PI for 30 min at 37°C. After being washed with PBS twice, cells were fixed with 4% PFA in PBS for 30 min at 25°C. Cells were mounted with Aqua Poly/Mount (Polysciences Inc., Warrington, PA), and imaged on a digidata camera attached to a fluorescence microscope.
Cortical cells on 8-well Lab-Tek chamber slides were fixed with 4% PFA in PBS for 30 min at 25°C, followed by permeabilization using 100% methanol for 5 min at 25°C. Cells were then rinsed twice with PBS and preincubated for 1 hr at 25°C in blocking buffer (2% bovine serum albumin and 0.1% Triton X-100 in PBS). Cells were incubated overnight at 4°C in blocking buffer containing anti-phospho-p38 MAPK antibody (1:50; Cell Signaling Technology), rinsed in PBS, and incubated for 2 hr at 25°C with fluorescein-con jugated anti-rabbit immunoglobulin (1:200; ICN/Cappel, Aurora, OH). The nucleus was stained with 10 μg/ml Hoechst 33342 and imaged on a digidata camera attached to a fluorescence microscopy.
MTT (3-(4,5-Dimethylthianol-2-yl)-2,5 Diphenyl Tetrazolium Bromide) Assay
The colorimetric MTT reduction assay, that measures cell viability, was carried out as described previously (Mosmann, 1983). This method assesses mitochondrial activity by measuring the ability of cultured cells to convert yellow MTT to the purple formazan dye. Cortical neurons in 96-well plate were incubated with MTT (125 μg/ml) in growth medium (without phenol red) for 4 hr at 37°C. The precipitated formazan was solubilized with SDS (25 mg/ml) and quantified spectrophotometrically at a wavelength of 570 nm. Data were presented as the percentage of survival relative to vehicle-treated control culture.
Western Blot Assay
Cells were lysed in a lysis buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.1% SDS, 1 mM phenyl methyl sulfonyl fluoride, and 100 μg/ml leupeptin. Lysates were centrifuged at 19,720 × g for 10 min. Supernatants were collected, subjected to electrophoresis on 8.5% SDS-polyacrylamide gel, and transferred to a nitrocellulose membrane. The blot was incubated in 5% nonfat dry milk for 60 min, reacted with primary antibodies overnight at 4°C, and then incubated with HRP-conjugated secondary antibodies for 1 hr at room temperature. Immunoreactivity was detected by using the Western blot chemiluminescence reagent system (Perkin-Elmer, Boston, MA). Films were exposed at different time points to ensure the optimum density, but not saturated.
Statistical Analysis
Data were expressed as mean ± SEM. Analysis of variance followed by Newman-Keuls test was used for statistical comparisons. Levels of P < 0.05 were considered to be of statistical significance.
RESULTS
I6 Induces Cell Apoptosis in Cortical Neuronal Cultures
After a 5-day maturation period in vitro, cortical neurons were treated with I6 for 24 hr and cell viability was assessed using the MTT metabolism assay. As shown in Figure 1A, cell survival was inversely correlated with I6 concentrations in the range of 20–180 μM. One-way ANOVA showed a significant effect for the drug treatment (F(6,47) = 258.1, n = 6–8 experiments in each concentration, P < 0.0001). Post-hoc comparison showed the differences between control and 60, 80, 120, and 180 μM (P < 0.001). No significant difference was seen between control and 20 and 40 μM, (P > 0.05). The concentration that caused 50% cell death was approximately 60–80 μM. Therefore, 60–80 μM of I6 was used in the following experiments. The vehicle (0.6% DMSO) alone had no effect on the cell viability. I6-treated neurons displayed cell body shrinkage, neurite retraction, and neural process fragmentation. To determine whether the reduced cell viability was due to apoptosis, cortical neurons were stained with Hoechst 33342 and propidium iodide (PI). Intracellular penetration of hydrophilic PI occurs if cell death is due to membrane disruption or necrosis (Bal-Price and Brown, 2000; Hamabe et al., 2003). In I6-treated neurons, very few neurons are PI positive, but many neurons are intensively stained with Hoechst 33342, indicating that after I6 treatment neurons underwent apoptosis (Fig. 1B). To further confirm I6-induced apoptosis, TUNEL assay was conducted on the sister cultures. As seen in Figures 1C and D, treatment with I6 dose-dependently increased the number of darkly stained TUNEL-positive cells (F(3,16) = 27.69, n = 5 in each concentration, P < 0.0001).
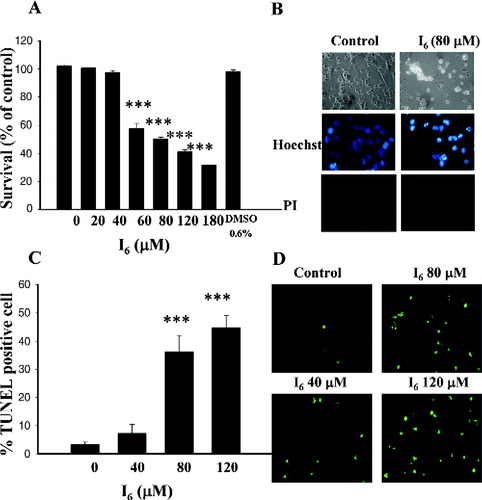
I6 induces cell death in cultured cortical neurons. A: Concentration-dependent effect of I6 on cell viability. Cortical neurons were treated with I6 for 24 hr and survival was assessed using MTT assay. Survival rates were 102.0 ± 1.1% in controls without adding drug, 100.6 ± 0.5%, 97.7 ± 1.2%, 57.7 ± 3.7%, 50.4 ± 0.9%, 41.1 ± 1.5, and 31.5 ± 0.4% in the presence of 20, 40, 60, 80, 120, and 180 μM of I6. Control bar represents survival rates without adding I6. B: Neurons were treated with I6 (80 μM) for 24 hr and morphologic studies were conducted by phase-contrast microscopy, Hoechst 33342, and PI staining. C,D: TUNEL staining showed the concentration-dependent increase in TUNEL-positive cells by I6. ***P < 0.001 vs. control. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To determine whether glial cells would influence the survival of neurons, we tested the effect of I6 on the glials cell viability. We stained our cultured cells with neuronal marker Neu-N and glial cell marker GFAP and the results showed that approximate 10% cells were astrocytes. We made a purified culture of astrocytes and examined the effect of I6 astrocytes. Similar to the action on neurons, I6 induced cell death in astrocytes in a concentration-dependent manner. The concentration that caused 50% cell death was approximately 70 μM (data not shown).
Prevention of I6-Mediated Neuronal Apoptosis by N-Acetylcysteine
We examined the effect of NAC on I6-induced cell death. Cortical neurons were pre-treated with this drug for 30 min before 80 μM I6 was added. The results depicted that NAC was able to prevent cell death caused by I6 (Fig. 2A). A total of 1, 5, and 10 mM of NAC increased the survival rate from 54.8 ± 1.5% to 59.5 ± 2.1%, 71.6 ± 2.0%, and 85.0 ± 2.1% respectively (F(3,60) = 53.72, n = 16 in each concentration, P < 0.0001). We further tested whether NAC rescued cortical neurons from death caused by I6 using TUNEL reaction. Cortical neurons were treated with I6 (80 μM) for 24 hr in the presence of NAC. Figures 2B and C show that NAC reduced TUNEL positive cells in a dose-dependent manner (F(2,12) = 26.5, n = 5 in each concentration, P < 0.0001).
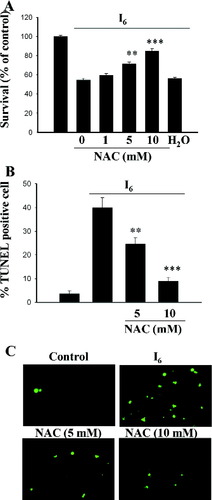
Effects of NAC on I6-induced cell death. A: Cortical neurons were treated with I6 (80 μM) for 24 hr and NAC at different concentrations were added to the medium 30 min before I6. B,C: NAC reduced I6-induced increase in TUNEL-positive cells in a concentration-dependent manner. **P < 0.01, ***P < 0.001 vs. I6. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
NAC Induces ERK Phosphorylation in Cortical Neurons
ERK activation by NAC plays an important role in preventing neuronal death after withdrawal of trophic support (Yan and Greene, 1998). To determine whether ERK pathway was involved in the action of NAC, cortical neurons were treated with NAC (10 mM) for various periods and cellular extract were blotted with antibodies directly against the active form of ERKs. Figure 3A shows that the increase in ERK phosphorylation was transient that peaked at 10 min after application and subsided within 60 min (F(7,16) = 19.57, n = 3 in each time point, P < 0.0001). Newman-Keuls t-tests showed that differences existed between control and 2-, 10-, and 30-min time points. No significant difference was detected between control and 0.5 min or control and 60-, 120-, and 180-min time points (P > 0.05). No change was observed when blotted membrane was reprobed with an antibody that recognizes ERK independently of its phosphorylation state, suggesting that the observed pERK increment was not due to an increase in the total amount of ERK. In addition, as shown in Figure 3B, I6 did not affect pERK by itself (95.7 ± 2.4%, n = 5). As control, an MEK inhibitor U0126 was added to test its effect on ERKs phosphorylation. ERK phosphorylation was decreased to below the baseline by U0126 (59.3 ± 4.0%, n = 5, P < 0.01) (Fig. 3C), suggesting the constitutive activation of ERKs.
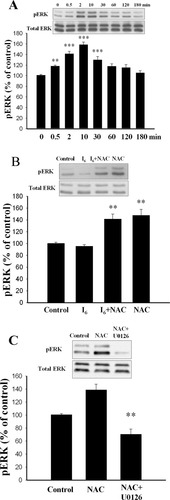
NAC induces ERK phosphorylation in cultured cortical neurons. A: Representative Western blots showing the transient activation of ERK by NAC. Treatment of cortical neurons with NAC (10 μM) at various periods and protein extracts were analyzed by Western blotting with α-dual-P-ERK and α-ERK antibodies. **P < 0.01, ***P < 0.001 vs. control. B: I6 by its own did not affect ERK phosphorylation. Control represents baseline level of p-ERK without adding I6 or NAC. C: Effects of MEK inhibitor U0126 on NAC-induced ERK phosphorylation. U0126 (10 μM) was added to the medium 30 min before the application of NAC. **P < 0.01 vs. NAC.
To determine whether ERK activation was involved in neuroprotective effect of NAC, we pre-treated the neurons with U0126 for 30 min before adding NAC and I6. Figure 4 shows that U0126 attenuated the effect of NAC in a concentration-dependent manner (F(3,28) = 288.7, n = 8 in each concentration, P < 0.0001). Higher concentrations of U0126 (10 and 15 μM) completely abolished the effect of NAC such that there was no difference in survival between neurons treated with I6 and I6 + NAC + U0126 (P = 0.98).
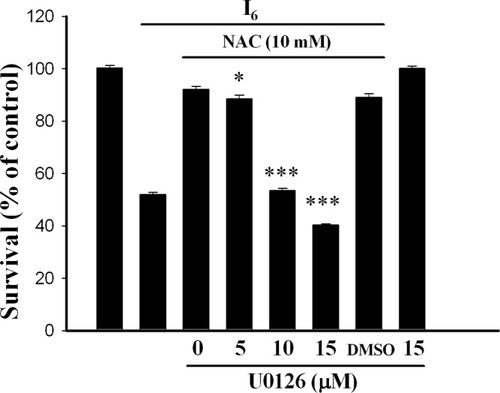
Requirement of ERK activation for the neuroprotective effect of NAC. Application of different concentrations of U0126 at 30 min before NAC attenuated the effect of NAC in a concentration-dependent manner. *P < 0.05, ***P < 0.001 vs. NAC.
In many instances, ERK activation is mediated via the GTP-binding Ras protein (Waskiewicz and Cooper, 1995). To investigate the involvement of Ras-ERK cascade in neuroprotective effect of NAC, a farnesyl transferase inhibitor was applied. Figure 5A shows that NAC increased ERK phosphorylation to 142.5 ± 10.1% of control (n = 5, P < 0.01) and the effect of NAC was blocked by FTI277 (100.8 ± 2.5%, n = 5). Furthermore, FTI277 attenuated the neuroprotective effect of NAC in a concentration-dependent manner (F(3,28) = 43.52, n = 8 in each concentration, P < 0.0001) (Fig. 5B).
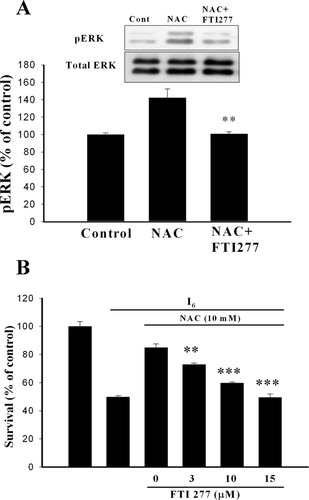
Block of NAC-induced ERK phosphorylation and neuroprotective effect by the farnesyl transferase inhibitor. A: Treatment of cortical neurons with NAC (10 mM) for 10 min increased the phosphorylated levels of ERK and this effect was abolished when FTI277 (10 μM) was added to the medium 30 min before NAC. **P < 0.01 vs. NAC. B: Requirement of Ras activation in the neuroprotective effect of NAC. Administration of different concentrations of FTI277 at 30 min before NAC attenuated the neuroprotective effect of NAC in a concentration-dependent manner. **P < 0.01, ***P < 0.001 vs. NAC.
NAC Induces p38 MAPK Phosphorylation in Cortical Neurons
P38 MAPK is involved in cell death observed in Alzheimer's disease (Zhu et al., 2000), transient forebrain ischemia (Takagi et al., 2000), and apoptosis induced by various stimuli (Kawasaki et al., 1997; De Zutter and Davis, 2001). We examined the role of p38 MAPK in I6-induced cell death. Activation of p38 MAPK was determined by immunoblotting with antibody specific for phosphorylated p38 (Thr180 and Tyr182). Figure 6A shows that pre-treatment of cortical neurons with I6 for 1 hr did not affect the phosphorylated level of p38 (95.6 ± 2.4%, n = 3). Adding NAC (10 mM) to the medium with or without I6 increased significantly phosphorylated level of p38 to 224.4 ± 6.5% (n = 3, P < 0.001) and 234.8 ± 11.0 (n = 3, P < 0.001) of control respectively. Figure 6B shows that the increase in p38 phosphorylation was transient that peaked at 10 min after application and subsided within 180 min (F(7,16) = 52.0, n = 3 in each time points, P < 0.001). Newman-Keuls t-tests showed that differences existed between control and 0.5, 2, 10, 30, 60, and 120 min time points. No significant difference was detected between control and 180 min time points (P > 0.05). No change was observed when blotted membrane was reprobed with an antibody that recognizes p38 independently of its phosphorylation state, suggesting that the observed p38 phosphorylation was not due to an increase in the total amount of ERK. SB203580 is a specific p38 MAPK inhibitor (Lee et al., 1999). Figure 6C shows that NAC-induced p38 phosphorylation was blocked by SB203580.
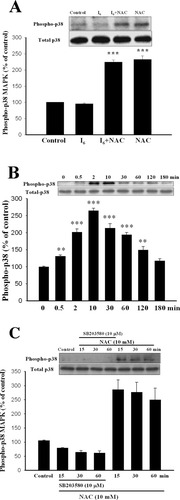
NAC induces p38 MAPK phosphorylation in cortical neurons. A: Treatment of cortical neurons with NAC for 10 min significantly increased the phosphorylated level of p38 MAPK. ***P < 0.001 vs. control. Control represents baseline level of phosphorylated p38 without adding I6 or NAC. B: Representative Western blots showing the transient activation of p38 MAPK by NAC. **P < 0.01, ***P < 0.001 vs. control. C: p38 MAPK inhibitor abolished NAC-induced p38 phosphorylation. Administration of SB253080 (10 μM) at 15, 30, or 60 min before NAC completed blocked NAC-induced p38 phosphorylation.
To determine whether p38 was involved in neuroprotective effect of NAC, we applied the p38 specific inhibitors, SB203580, and SB202190. As shown in Figure 7A, SB203580 alone had no effect on survival at concentrations up to 10 μM but significantly and dose-dependently attenuated neuroprotective effect of NAC. Similar result was observed with SB202190. SB202190 alone had no effect on the survival at concentrations up to 25 μM but significantly and dose-dependently attenuated the effect of NAC (Fig. 7B). To determine whether constitutive activation of p38 provided neuroprotection against I6 toxicity, SB203580 was added in the presence of I6 (40 μM). Cell survival rate was 100.6 ± 0.5% (n = 16) in I6 (40 μM) and was decreased to 94.1 ± 2.9% (n = 16), 77.0 ± 2.6% (n = 16), and 75.7 ± 3.2% (n = 16) respectively after adding 1, 5, or 15 μM of SB203580 to the medium (F(3,60) = 24.26, P < 0.001) (Fig. 7C). Thus, SB203589 dose-dependently enhanced I6-induced cell death, suggesting a neuroprotection against I6 toxicity provided by the constitutive activation of p38.
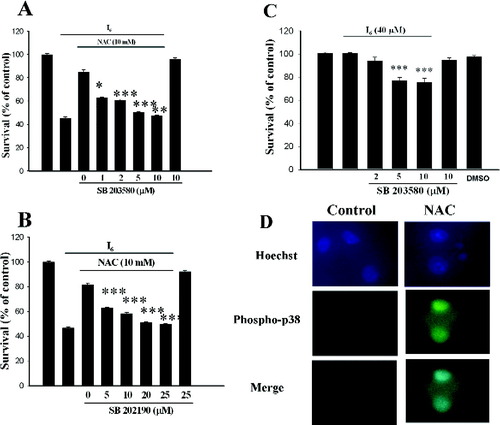
Requirement of p38 activation for the neuroprotective effect of NAC. Administration of different concentrations of SB253080 (A) or SB202190 (B) at 30 min before NAC attenuated the neuroprotective effect of NAC in a concentration-dependent manner. SB253080 or SB202190 themselves had no effect on cell viability. ***P < 0.001 vs. NAC. C: Dose-dependent enhancement of I6-induced cell death by SB203589 suggested that constitutive activation of p38 provided neuroprotection against I6 toxicity. ***P < 0.001 vs. I6. D: Cells were treated with NAC (10 mM) and I6 (80 μM) for 24 hr. NAC and I6 were then washed out and 24 hr later NAC was added to activate p38 MAPK and the cells were stained with anti-phospho-p38 and Hoechst dye. Phospho-p38 positive cells displayed very little nuclear condensation.
Finally, we examined whether cells with phospho-p38 positive have minor risk to undergo nuclear fragmentation. Cells were treated with NAC (10 mM) and I6 (80 μM) for 24 hr. NAC and I6 were then washed out and 24 hr later NAC was added to activate p38 MAPK and the cells were stained with anti-phospho-p38 and Hoechst dye. As shown in Figure 7D, phospho-p38 positive cells displayed very little nuclear condensation.
Cross-Talk Between p38 MAPK and ERK
Apoptosis triggered by KCl-withdrawal in cerebellar granule cells decreased ERK activity but did not activate p38 MAPK (Watson et al., 1998). Ceramide-induced apoptosis in cortical neurons was mediated by an increase in p38 phosphorylation and not by the decrease in ERK activation (Willaime et al., 2001). To our knowledge, this is the first study showing activation of both p38 MAPK and ERK by the neuroprotective concentrations of NAC. Does NAC activate ERK and p38 MAPK sequentially or in parallel? We addressed this issue by treating cortical neurons with NAC (10 mM) to induce p38 phosphorylation and examined whether p38 phosphorylation was affected by Ras or MEK inhibitors. Figure 8A shows that NAC increased p38 phosphorylation to 228.9 ± 27.7% of control and pre-treatment of neurons with FTI277 (10 μM) completely abolished NAC-induced p38 phosphorylation (99.2 ± 11.9%, n = 3). Similarly, NAC increased p38 phosphorylation to 251.9 ± 9.3% of control and pre-treatment of neurons with U0126 (10 μM) completely abolished NAC-induced p38 phosphorylation (117.6 ± 25.2%, n = 3). P38 inhibitor SB 203580 did not influence NAC-induced ERK phosphorylation such that there was no difference in ERK phosphorylation between NAC (140.5 ± 9.3%, n = 5) and NAC + SB 203580 (142.5 ± 8.2%, n = 5). These results suggest that ERK is an upstream regulator of p38 MAPK signaling.
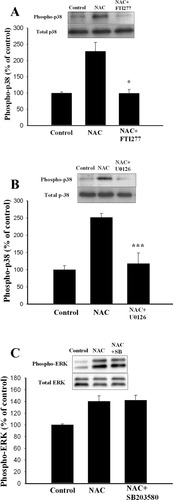
p38 MAPK activation induced by NAC is blocked by MEK inhibitor. A,B: Representative Western blots and densitometric analysis of p38 MAPK activation. Phosphorylated level of p38 MAPK, increased by NAC, was blocked by FTI277 (10 μM) (A) or U0126 (10 μM) (B). *P < 0.05, ***P < 0.001 vs. NAC. C: NAC-induced ERK activation was not affected by SB203580.
I6 Increases Proteolysis of Pro-Caspase-3
Caspases are key mediators of cell death and caspase-3 is an executioner for the death program in cortical neurons in response to various noxious insults (Enari et al., 1998; Gorman et al., 1998; Thornberry and Lazebnik, 1998;). We examined whether I6-induced cell death was dependent on caspase-3 activation. Constitutive expression of the 32-kDa pro-caspase-3 protein was detected in controls. After treatment with I6, the expression of 17-kDa cleaved fragment of caspase-3 gradually increased (Fig. 9). There was no detectable change in relative levels of pro-caspase-3 protein. These results suggest that after treatment with I6 proteolysis of pro-caspase-3 occurs with maintenance of relative pro-caspase-3 protein level.
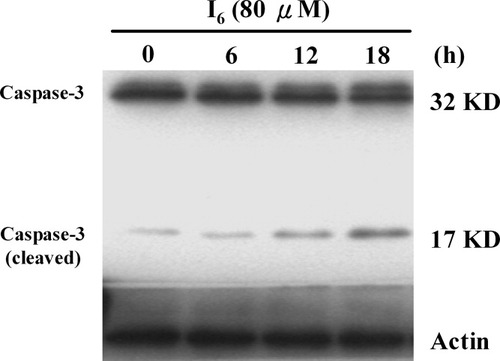
Effect of I6 on caspase-3 protein expression in cultured cortical neurons. Representative Western blot showing 32- and 17-kDa (cleaved) bands of caspase-3. The pro-form caspase-3 is constitutively present in the control cultures. Incubation of I6 progressively increased the p17 cleaved fragment of caspase-3.
DISCUSSION
I6 is a synthetic gingerdione derivative that induces cell death of cultured cortical neurons in a concentration-dependent manner. Neuronal death induced by I6 displayed cell body shrinkage, neurite retraction and neural process fragmentation, the characteristics that resembled apoptosis. Results from Hoechst 33342, PI staining, and TUNEL reaction confirmed that cells treated with I6 were undergoing apoptosis. Furthermore, similar to the action on neurons, I6 concentration-dependently induced cell death in astrocytes. The concentration that caused 50% of astrocytes death was approximately 70 μM. The precise mechanism by which I6 induced neuronal apoptosis is not completely characterized. We found that I6 induced proteolysis of pro-caspase-3 to active form of caspase-3. This result suggests that caspase-3 may mediate I6-induced neuronal apoptosis.
In the present study, we have shown that NAC promoted cell survival. There are several possible mechanisms by which NAC might prevent neuronal cell death. For example, NAC is an antioxidant capable of direct reduction of reactive oxygen species (ROS). In addition, NAC could increase intracellular levels of GSH and serve as a reducing agent. In the absence of trophic support, neurons underwent apoptosis that could be prevented by NAC. N-acetylcysteine also rescued tumor necrosis factor (TNF)- and thrombin-induced neuronal cell death (Talley et al., 1995; Sarker et al., 1999). The neuroprotective effect of NAC required activation of Ras-ERK pathway as the effect of NAC was no longer seen in PC12 MM17-26 cell line that constitutively expressed high levels of Ha-Ras (Asn17), a dominant-negative form of Ras (Yan and Greene, 1998). In accordance with previous studies, our biochemical analysis showed that ERK was activated after application of NAC. MEK inhibitor U0126 blocked NAC-induced ERK phosphorylation as well as its neuroprotective effect. Furthermore, the blockade of NAC-promoted survival and ERK phosphorylation by farnesyltransferase inhibitor indicated a necessary role for Ras.
MAPK signaling pathways are important in regulating a variety of cellular processes (Freeman and Whartenby, 2004). In the nervous system, both the p42/p44 and p38 MAPK pathways have been implicated in neuronal plasticity and apoptosis (Xia et al., 1995; Bolshakov et al., 2000; Zhu et al., 2002). The two parallel pathways in general work in opposition. ERKs (p42/p44) are growth promoting and control synaptic delivery of GluR1-containing AMPA receptors during long-term potentiation (LTP) of synaptic transmission (English and Sweatt, 1997; Zhu et al., 2002), whereas p38 is pro-apoptotic and serves as a signal transduction cascade that participates in the induction of metabotropic glutamate receptor-dependent long-term depression (LTD) (Rush et al., 2002; Thomas and Huganir, 2004). We showed that p38 MAPK was activated by NAC and p38 inhibitors blocked NAC-induced p38 phosphorylation. More importantly, 24 hr after treatment with NAC and I6, doubling staining cells with anti-phospho-p38 and Hoechst dye showed that phospho-p38 positive cells displayed very little nuclear condensation. Furthermore, activation of p38 by NAC was completely blocked by farnesyltransferase and MEK inhibitors suggesting that ERK coupled Ras to p38 activation. Furthermore, NAC-induced activation of ERK was not affected by p38 inhibitor indicating that ERK may be an upstream regulator of p38 MAPK in the NAC-mediated p38 signaling. To our knowledge, this is the first report showing a serial activation of ERK and p38 MAPK by NAC and ERK and p38 work together to mediate the neuroprotective effect of NAC.
The mechanism by which activation of p38 MAPK protects I6-induced cell death is not clear. It has been shown that activation of p38 leads to phosphorylation of myocyte enhancer factor 2 (MEF2) at Ser387 (Han et al., 1997) and cAMP response element binding protein (CREB) at ser133 (Xing et al., 1998). Once activated by p38 MAPK signaling pathway, MEF2, or CREB may regulate the expression of genes that are critical for survival of neurons (Xing et al., 1998; Mao et al., 1999; Okamoto et al., 2000). It will be important to determine whether NAC induction of p38 activity leads to MEF2 phosphorylation and whether the phosphorylation of MEF2 then contributes to neuroprotective effect of NAC. Finally, I6 is a 3,4-dimethoxy substituted dehydro-derivative of gingerol and may pass through blood–brain barrier because of its high lipid solubility. Therefore, it should be cautious when I6 is used to treat inflammation and gastrointestinal discomforts.
Acknowledgements
The authors thank Dr. C. Young for critical comments on the manuscript.




