Prevention of spinal motor neuron death by insulin-like growth factor-1 associating with the signal transduction systems in SODG93A transgenic mice
Abstract
The role of insulin-like growth factor-1 (IGF-1) in amyotrophic lateral sclerosis (ALS) and its mechanism of action are important from both pathogenic and therapeutic points of view. The present study investigated the changes of IGF-1Rβ and the key intracellular downstream protein insulin receptor substrate-1 (IRS-1) by using SOD1G93A transgenic mice with continuous intrathecal IGF-1 treatment. The number of lumbar spinal motor neurons was preserved with IGF-1 treatment in a dose-dependent manner. The numbers of immunopositive motor neurons for IGF-1Rβ and IRS-1 were not significantly different between wild-type and Tg mice with vehicle treatment, whereas treatment of Tg mice with IGF-1 decreased the numbers of immunopositive motor neurons in a dose-dependent manner. On the other hand, the ratio of immunopositive motor neurons per total living motor neurons in vehicle-treated mice was greatly increased in Tg mice with vehicle treatment compared with wild-type mice. With IGF-1 treatment, the ratio was dramatically decreased in a dose-dependent manner. These results suggest that IGF-1 treatment prevents motor neuron loss by affecting the signal transduction system through IGF-1R and the main downstream signal, IRS-1. © 2005 Wiley-Liss, Inc.
Amyotrophic lateral sclerosis (ALS) is a progressive, fatal neurodegenerative disease that is characterized by selective loss of central and peripheral motor neurons (Rowland and Shneider, 2001). Familial ALS (FALS) accounts for under 10% of diagnosed cases, one-fourth of which are associated with missence mutations in the antioxidant enzyme copper/zinc superoxide dismutase (Rowland and Shneider, 2001). There are many hypotheses about the underlying cause of this disease; one theory is that motor neurons lack crucially needed trophic factors, resulting in neuronal degeneration, cell death, and atrophy of target muscles.
Insulin-like growth factor-1 (IGF-1) is a 70-amino-acid protein that is structurally similar to insulin and acts as a major neurotrophic factor and a neuroprotective survival factor (Dore et al., 1997; Rotwein et al., 1998; Werther et al., 1998). The biological functions of IGF-1 are mediated by the IGF-1 receptor (IGF-1R). The IGF-1R consists of alpha and beta subunits; the alpha subunit has an IGF-1 binding domain, and the beta subunit has a tyrosine kinase domain (Ullich et al., 1986). Because IGF-1Rβ is a membrane-penetrated subunit that plays an important role in signal transduction, binding of IGF-1 to the extracellular domain of IGF-1R activates an intracellular downstream protein [insulin receptor substrate-1 (IRS-1)] with phosphorylation (Giorgetti et al., 1993). IRS-1 further transmits the IGF-1 signal through two downstream signaling cascades for cell survival: the mitogen-activated protein kinase (MAPK) kinase (MEK)/extracellular-signal-regulated kinase (ERK) and the phosphotidylinositol 3-kinase (PI3-K)/Akt pathways (Dudek et al., 1997).
In ALS patients, serum concentrations of IGF-1 are either normal or slightly decreased (Braunstein and Reviczky, 1987; Denys et al., 1988). Although the expression of IGF-1 in spinal cords of ALS patients was found to be normal, spinal cords of ALS patients had more IGF-1R than spinal cords without this disease (Adem et al., 1994). On the other hand, Kasper et al. (2003) reported that IGF-1 delivered by adeno-associated virus retrogradely to spinal motor neurons prolongs life and delays disease progression in the same mouse model. Thus, the role of IGF-1 in ALS and the mechanism of its action are important from both pathogenic and therapeutic points of view. In the present study, therefore, we investigated the changes of IGF-1Rβ and the important intracellular downstream protein IRS-1 by using SOD1G93A transgenic mice with IGF-1 treatment.
MATERIALS AND METHODS
All experimental procedures were approved by the Animal Care and Use Committee of Okayama University. The Tg mouse line with the G93A human SOD1 mutation was obtained from Jackson Laboratories (Bar Harbor, ME; Gurney et al., 1994), and the line was maintained as hemizygotes by mating Tg males with C57BL/6J females according to the supplier's recommendation. The offspring were genotyped by a PCR assay of DNA obtained from tail tissue. They are the low-expressing SOD1 mutant mice that display disease onset at 220–230 days and die approximately 40 days later. There is a difference in disease onset between male and female Tg mice, so only female Tg mice were used in the present study (Veldink et al., 2003).
Recombinant human IGF-1 (Fujisawa Pharmaceuticals, Osaka, Japan) was dissolved in sterile artificial cerebrospinal fluid (aCSF; NaCl 122 mM, KCl 3.1 mM, NaHCO3 5 mM, KH2PO4 0.4 mM, CaCl2 1.3 mM, MgSO4 1.0 mM, D-glucose 10 mM, pH 7.4) to give 1 mg/kg of body weight/day as a high-dose treatment (n = 4) or 100 μg/kg of weight/day as a low-dose treatment (n = 4). Three mice were treated with vehicle (aCSF) as controls. For continuous intrathecal infusion into the lumbar spinal cord region, an osmotic minipump (Alzet minipump, model 2004) with a cannula attachment was implanted into the back of each animal at 140 days of age. The minipump was designed for 0.25 μl/hr of nominal infusion rate and was attached to sterile polyethylene tubing (Becton Dickinson and Company, Sparks, MD). With animals under anesthesia with isoflurane (Abbott Laboratories, Abbott Park, IL) and nitrous oxide, the minipump was placed subcutaneously at a lumbar site. A rostrally directed cannula was threaded through the muscle close to the exposed region of the spinal column, and the tip of the cannula was placed in the spinal subarachnoid space at the level of L6 to S1. The minipump was replaced with a new one every 4 weeks, and the treatment was continued until the animal reached the end stage of the disease.
For the histological studies, non-Tg littermates (n = 3) and Tg mice treated with vehicle (n = 3) or low-dose or high-dose IGF-1 (n = 4) were deeply anesthetized at 240 days of age and transcardially perfused with phosphate-buffered saline (PBS; pH 7.4), followed by 4% paraformaldehyde in 0.1 M phosphate buffer. The region of the spinal cord spanning L4–L5 was fixed by immersion in the same fixative and frozen after cryoprotection. For immunohistochemistry, frozen transverse sections through the L4–L5 segments were treated with 0.3% hydrogen peroxide for 30 min. After treatment with PBS, the sections were incubated with 5% bovine serum albumin to block the nonspecific binding of antibodies. Sections were reacted with primary antibodies and stained for immunoreactivity by the avidin-biotin peroxidase complex (ABC) method, by using ABC kits (Vector Laboratories, Burlingame, CA). Polyclonal antibody against IGF-1Rβ was purchased from Santa Cruz Biotechnology (sc-713; Santa Cruz, CA) and diluted 1:500 with PBS (pH 7.4); polyclonal antibody against insulin receptor substrate 1 (IRS-1) was from Upstate Biotechnology (06-248; Lake Placid, NY) and also was diluted 1:500 with PBS. Immunoreactivity was visualized with 3,3′-diaminobenzidine tetrahydrochloride. A set of sections was treated without the primary antibody to ascertain the specific detection by the antibodies used.
On microscopic examination, only the profiles of motor neurons with nucleoli in both the anterior horn areas were counted by investigators blinded to the condition of the treatments as described previously (Oppenheim, 1986). Only cells with a diameter above 15 μm and bearing clear nucleoli were counted by investigators blinded to the condition of the treatments. In addition, each mouse section was immunostained for choline acetyltransferase (ChAT goat antiserum; Chemicon, Temecula, CA) using Vector ABC kit (Vector Laboratories, Burlingame, CA) to confirm the results of motor neuron counting.
Multiple group comparisons of the differences in quantitative measurements were made by analysis of variance (ANOVA), followed by Fisher's least protected significant difference test. Statistical significance was accepted at the P < 0.05 level.
RESULTS
Our previous study showed that G93A mice treated with vehicle showed onset of clinical signs at 223.6 ± 7.1 days (mean ± SD) of age. The low-dose IGF-1 treatment delayed the disease onset by 21.4 days (P < 0.01), and the high-dose treatment delayed it by 33.7 days (P < 0.01). The mean survival of the mice increased from 260.6 ± 5.5 days to 281.7 ± 4.0 days with low-dose IGF-1 (P < 0.01) and 289.2 ± 11.5 days with high-dose IGF-1 (P < 0.01). The survival period was extended by 21.1 days with low-dose IGF-1 and 28.6 days with high-dose IGF-1, and the number of motor neurons was 1,215.0 ± 97.2 (/mm thickness, mean ± SD) in wild-type mice, which was decreased in Tg mice with vehicle treatment (281.5 ± 36.4). Treatment with IGF-1 prevented such motor neuron loss in Tg mice in a dose-dependent manner, with the low dose (715.0 ± 46.5, +P < 0.05 vs. vehicle treatment) and the high dose (900.0 ± 346.7, +P < 0.05). However, there was no significant differennce between low- and high-dose treatment. Immunoreactivities of p-Akt, p-ERK, and bcl-2 were observed in the motor neurons, interneurons, and glial cells of the non-Tg spinal cords, whereas the motor neurons and other cell types in vehicle-treated Tg mice gave only slight immunoreactivities for these proteins. In contrast, the motor neurons and other cells in the IGF-1-treated mice retained their immunoreactivity for p-Akt in a dose-dependent manner, showing that IGF-1 induced survival signal-related proteins in a dose-dependent manner. P-ERK and bcl-2 were generally expressed at higher levels in spinal cord tissue treated with high-dose IGF-1 than low-dose IGF-1 or vehicle (Nagano et al., 2005).
In this study, the numbers of immunopositive motor neurons in both anterior horn areas for IGF-1Rβ were not significantly different between wild-type mice (150.0 ± 38.8/mm thickness) and Tg mice with vehicle treatment (194.8 ± 6.8). However, treatment of Tg mice with IGF-1 decreased the number of immunopositive motor neurons in a dose-dependent manner, with the low dose (74.0 ± 9.2, **P < 0.01 vs. wild type, ++P < 0.01 vs. vehicle treatment) and the high dose (20.0 ± 1.6, **P < 0.01, ++P < 0.01). On the other hand, the percentage of immunopositive motor neurons per total living motor neurons in vehicle-treated mice (69.3 ± 11.6%, **P < 0.01) was greatly increased compared with wild-type mice (12.3% ± 3.5%). With IGF-1 treatment, the ratios were dramatically decreased in a dose-dependent manner, with the low dose (10.3% ± 1.4%, ++P < 0.01 vs. vehicle treatment) and the high dose (2.2% ± 1.0%, ++P < 0.01 vs. vehicle treatment, **P < 0.01 vs. wild type; Fig. 1).
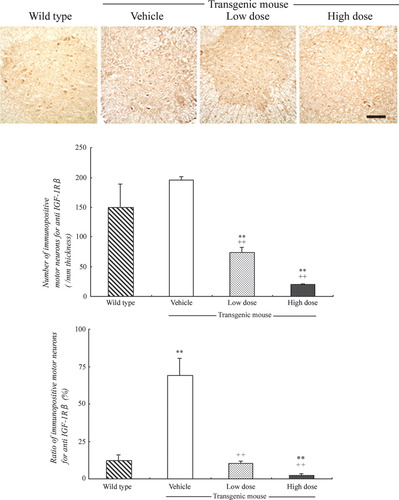
Immunohistochemistry for IGF-1Rβ in the anterior horn of wild-type and SOD1G93A mice (upper panel), numbers of immunopositive motor neurons (middle panel), and ratios (bottom panel). Note the similar numbers of immunopositive motor neurons between wild-type and Tg mice with vehicle treatment and the dose-dependent decrease with IGF-1 treatment (upper and middle panels). Also note the increase of the ratio in Tg mice with vehicle treatment and the dose-dependent decrease with IGF-1 treatment (bottom panel). See text for details. Scale bar = 100 μm.
The number of immunopositive motor neurons in both anterior horn areas for IRS-1 was 196.5 ± 25.9/mm thickness in wild type, which did not change in Tg mice with vehicle treatment (217.5 ± 12.1). In contrast, treatment with IGF-1 decreased the number of immunopositive motor neurons of Tg mice in a dose-dependent manner with the low dose (52.8 ± 15.3, **P < 0.01 vs. wild type, ++P < 0.01 vs. vehicle treatment) and the high dose (45.0 ± 7.2, **P < 0.01, ++P < 0.01). The ratio of immunopositive motor neurons per total number of living motor neurons for IRS-1 in wild-type mice was 16.7% ± 2.6%, which greatly increased to 74.6% ± 13.1% (**P < 0.01 vs. wild type) in Tg mice with vehicle treatment. With IGF-1 treatment, the ratios of immunopositive motor neurons were again dramatically decreased in a dose-dependent manner with the low dose (7.3% ± 2.2%, ++P < 0.01 vs. vehicle treatment) and the high dose (5.0% ± 2.4%, ++P < 0.01 vs. vehicle treatment, **P < 0.01 vs. wild type; Fig. 2). The sections without the primary antibody had no staining (data not shown).
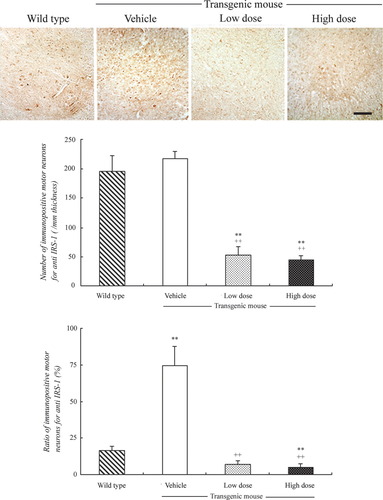
Immunohistochemistry for IRS-1 in the anterior horn of wild-type and SOD1G93A mice (upper panel), numbers of immunopositive motor neurons (middle panel), and ratios (bottom panel). Note the similar numbers of immunopositive motor neurons between wild-type and Tg mice with vehicle treatment and the dose-dependent decrease with IGF-1 treatment (upper and middle panels). Also note the increase of the ratio in Tg mice with vehicle treatment and the dose-dependent decrease with IGF-1 treatment (bottom panel). See text for details. Scale bar = 100 μm.
DISCUSSION
In the present study, we found that 1) the percentage of immunopositive motor neurons for IGF-1Rβ in anterior horn areas of Tg mice was increased with vehicle treatment compared with wild type, but was decreased with IGF-1 treatment, and 2) the percentage of immunopositive motor neurons for IRS-1 in the anterior horn areas of Tg mice was increased with vehicle treatment compared with wild type, but was again decreased with IGF-1 treatment. In a previous study, we observed that continuous intrathecal injection of IGF-1 delayed disease onset and prolonged survival in the ALS model mice (Nagano et al., 2005). We observed that the low-dose treatment with IGF-1 delayed the onset of disease by 21 days, and the high-dose treatment delayed it by 33 days. With the same protocol, IGF-1 treatment dose-dependently prevented the loss of motor neurons in anterior horn areas compared with vehicle treatment. Nagano et al. observed that the protective effects of IGF-1 were mediated by activating phosphorylated forms of the survival signals Akt and ERK, and bcl-2 (Nagano et al., 2005).
In ALS patients, serum concentration of IGF-1 was found to be either normal or slightly decreased (Braunstein and Reviczky, 1987; Denys et al., 1988). Although expression of IGF-1 in the spinal cords of ALS patients is normal, expression of the receptor IGF-1R is increased (Adem et al., 1994). Wilczak et al. (2003) reported that the level of free IGF-1 was 53% lower in ALS patients than controls in the spinal cords, although total IGF-1 was the same level in the ventral anterior horns of both groups. This could be because of specific increases of IGF binding proteins (IGFBP) 2, 5, and 6 in the spinal cords (Wilczak et al., 2003). Among six IGFBPs, IGFBP 2, 5, and 6 bind IGF-1 with high affinity and prevent the binding of IGF-1 to IGF-1R (Wilczak et al., 2003). Combined with previous reports, the present results suggest that intrathecal injection of IGF-1 increases the level of free IGF-1 in CSF of Tg mice and probably also increases the level in spinal cords. Excessive amounts of free IGF-1 could down-regulate IGF-1R levels in the spinal cord (Fig. 1).
IGF-1R consists of alpha and beta subunits (IGF-1Rα, IGF-1Rβ), which are assembled with disulfide bonds. IGF-1Rα is an extracellular protein and has an IGF-1 binding domain. IGF-1Rβ is a membrane-penetrated domain and has a tyrosine kinase domain (Ullich et al., 1986). The present study showed that the number of immunopositive motor neurons for IGF-1Rβ in spinal cords of Tg mice was almost the same as in wild type (Fig. 1, middle panel). However, the percentage of immunopositive motor neurons for IGF-1Rβ was greatly increased in Tg mice vs. wild type (Fig. 1, bottom panel). This result suggests that IGF-1Rβ is up-regulated in most surviving motor neurons of Tg mice, although the numbers of motor neurons were decreased. The number of immunopositive motor neurons for IGF-1Rβ of Tg mice was decreased with IGF-1 treatment in a dose-dependent manner (Fig. 1, middle panel). The percentage of immunopositive motor neurons for IGF-1Rβ of Tg mice was also decreased with IGF-1 treatment in a dose-dependent manner (Fig. 1, bottom panel). These results also suggest that the IGF-1 treatment down-regulated IGF-1R expression in spinal cords.
IRS-1 is an intracellular protein activated by IGF-1 or insulin, and the activation further promotes the downstream survival signals MEK/ERK and PI3-K/Akt pathways (Dudek et al., 1997). In the present study, the number of immunopositive motor neurons for IRS-1 in spinal cords of Tg mice was almost the same as in wild type (Fig. 2, middle panel). However, the percentage of immunopositive motor neurons for IRS-1 was much higher in Tg mice than in wild type (Fig. 2, bottom panel). This result suggests that IRS-1 is up-regulated in most surviving motor neurons of Tg mice, although the number of motor neurons is decreased. The number of immunopositive motor neurons for IRS-1 of Tg mice was decreased with IGF-1 treatment in a dose-dependent manner. On the other hand, the percentage of immunopositive motor neurons for IRS-1 of Tg mice was also decreased with IGF-1 treatment in a dose-dependent manner. These results show that IRS-1 is up-regulated in motor neurons of ALS model mice and is down-regulated with IGF-1 treatment (Fig. 2).
In a previous study, we showed that IGF-1 treatment induced the survival signal-related proteins (p-Akt and p-ERK) in a dose-dependent manner (Nagano et al., 2005). In this study, we showed that IGF-1 treatment decreased IGF-1Rβ and IRS-1.
In summary, we have investigated signal transduction systems in SODG93A Tg mice that received continuous intrathecal injection of IGF-1 and observed significant increases of percentage of IGF-1Rβ- and IRS-1-immunopositive motor neurons in Tg mice with vehicle treatment as well as a significant decrease with IGF-1 treatment. These results suggest that IGF-1 treatment prevents motor neuron loss by affecting the signal transduction system through IGF-1R and the main downstream signal, IRS-1.
Acknowledgements
This work was partially supported by Grant-in-Aid for Scientific Research (B) 15390273 and (Hoga) 15659338 and the National Project on Protein Structural and Functional Analyses from the Ministry of Education, Science, Culture and Sports of Japan and by grants (to Y. Itoyama, I. Kimura, and S. Kuzuhara) from the Ministry of Health, Welfare and Labor of Japan.




