Establishment of graded spinal cord injury model in a nonhuman primate: The common marmoset
Abstract
Most previous studies on spinal cord injury (SCI) have used rodent models. Direct extrapolation of the results obtained in rodents to clinical cases is difficult, however, because of neurofunctional and anatomic differences between rodents and primates. In the present study, the development of histopathologic changes and functional deficits were assessed quantitatively after mild, moderate, and severe spinal cord contusive injuries in common marmosets. Contusive SCI was induced by dropping one of three different weights (15, 17, or 20 g) at the C5 level from a height of 50 mm. Serial magnetic resonance images showed significant differences in the intramedullary T1 low signal and T2 high signal areas among the three groups. Quantitative histologic analyses revealed that the number of motor neurons, the myelinated areas, and the amounts of corticospinal tract fibers decreased significantly as the injury increased in severity. Motor functions were evaluated using the following tests: original behavioral scoring scale, measurements of spontaneous motor activity, bar grip test, and cage-climbing test. Significant differences in all test results were observed among the three groups. Spontaneous motor activities at 10 weeks after injury were closely correlated with the residual myelinated area at the lesion epicenter. The establishment of a reliable nonhuman primate model for SCI with objective functional evaluation methods should become an essential tool for future SCI treatment studies. Quantitative behavioral and histopathologic analyses enabled three distinct grades of injury severity (15-g, 17-g, and 20-g groups) to be characterized with heavier weights producing more serious injuries, and relatively constant behavioral and histopathologic outcomes. © 2005 Wiley-Liss, Inc.
Most previous animal studies of spinal cord injury (SCI) have been conducted using rodent models (Wrathall et al., 1985; Bresnahan et al., 1991; Young, 2002). The results of these studies cannot be applied directly to patients with SCI, however, because the neurofunctional and anatomic features of rodents and human beings are different. For example, the corticospinal tract (CST) is located in the posterior funiculus of rodents; in humans, a major portion of this structure is present in the lateral funiculus with the remaining in the anterior funiculus (Qiu et al., 1991; Lemon et al., 2004). In monkeys, however, the CST is present in the lateral funiculus, as in human beings. Distinct differences in the termination of the CST are also present between rodents and primates (Lemon et al., 2004). If a SCI model could be established in monkeys, human SCI might therefore be reproduced more accurately. Animal models using macaques and common marmosets (Callithrix jacchus) have been established already for diseases like parkinsonism (Gnanalingham et al., 1993; Roeling et al., 1995) and multiple sclerosis (Massacesi et al., 1995), contributing greatly to advancing research in these fields. Hemisection SCI models using the bonnet monkey Macaca radiata (Babu et al., 2000) and the common marmoset (Liu et al., 2001) have also been reported. The establishment of a contusive SCI model in primates remains necessary for preclinical trials, however, because the pathophysiology of contusion injuries is more similar to that of human SCI. The objective of the present study was to establish a graded contusive SCI model in common marmosets.
MATERIALS AND METHODS
Contusive SCI in Common Marmosets
After an intramuscular injection of ketamine (50 mg/kg; Sankyo Co., Ltd., Tokyo, Japan) and xylazine (5 mg/kg; Bayer AG, Leverkusen, Germany) to induce anesthesia, graded contusive SCIs were induced in adult female common marmosets using a modified NYU device (3.5 mm in diameter). Briefly, one of three weights (15, 17, or 20 g) was dropped from a height of 50 mm onto the exposed dura mater at the C5 level in the mild, moderate, and severe injury groups (n = 6 for each group). In the sham group, only a laminectomy was carried out without any injury to the spinal cord (n = 2). The animals were placed in a temperature-controlled chamber until thermoregulation was reestablished. Manual bladder expression was carried out twice a day until voiding reflexes were reestablished. Paralyzed animals were given adequate amounts of food and water until they recovered their ability to ingest food and water without assistance. For 1 week after the injury, ampicillin (Meiji Seika Kaisha, Ltd., Tokyo, Japan) was administered intramuscularly to each of the animals at a dose of 100 mg/kg. The Keio University Ethics Committee and the Animal Experimentation Committee of the Central Institute for Experimental Animals approved all animal procedures, which were in accordance with the NIH (National Institutes of Health) Guide for the Care and Use of Laboratory Animals, before the start of the study.
Magnetic Resonance Imaging of the Injured Spinal Cord
Using a 1.5-Tesla superconducting imager (Sigma, Milwaukee, WI) fitted with a phased-array volume coil, magnetic resonance imaging (MRI) of the injured spinal cord was conducted 3 days, 10 days, 3 weeks, and 8 weeks after injury under the following conditions: (1) sagittal; and (2) axial T2-weighted (T2W) fast spin-echo with a repetition/echo time number (TR/TE number) averaging 4,000 msec/100 msec/15, a field-of-view of 9 cm, a matrix of 256 × 256, and a section thickness of 1.7 mm; and (3) sagittal T1-weighted (T1W) spin-echo with a TR/TE number averaging 400 msec/ 12 msec/15 (all other parameters the same as above). Altered signal intensity at the lesion epicenter on T1W sagittal, T2W sagittal, and T2W axial images was measured using NIH Image software.
Behavioral Analyses
Original behavioral scoring test.
Previous studies of SCI in rodents utilized two-dimensional (2D) functional evaluations (Rivlin and Tator, 1977; Smith et al., 1995; Basso et al., 1996; Mikami et al., 2002). Because most common marmoset movements are three-dimensional (3D; e.g., jumping and climbing the cage), a new functional evaluation protocol was needed. In the present study, each common marmoset was observed for 5 min to assess its ability to perform the basic motions (Table I). Each animal was observed by three observers (using a blind protocol) once a day every day after injury and was given one point for each motion that it could perform.
| Item | Behavior |
|---|---|
| 1 | Changing from a supine to a prone position. |
| 2 | Grasping the cage with its forelimbs. |
| 3 | Grasping the cage with its hindlimbs. |
| 4 | Walking, with weight bearing, on its forelimbs. |
| 5 | Walking, with weight bearing, on its hindlimbs. |
| 6 | A single jump. |
| 7 | Multiple jumps. |
| 8 | Sitting or standing for more than 3 seconds. |
| 9 | Smooth movements without falling through the gaps between the cage bars. |
- * One point was given for each representative basic motion that was accomplished successfully (maximum score = 9).
Measurements of spontaneous motor activity.
Spontaneous motor activity is difficult to evaluate in common marmosets because of their 3D movements. We used cages (350-mm wide, 500-mm deep, and 500-mm high) equipped with infrared sensors (Murata Manufacturing Corp., Nagaokakyo, Kyoto, Japan) on the ceiling to continually record the 3D motion of the common marmosets (Fig. 1A). Our system utilized a passive thermographic infrared sensor to monitor the heat emitted from the animals. The 3D localization of the heat source was monitored, and a change in this localization was recorded as movement. Each animal's data was recorded and monitored on a computer on an hourly basis, and the activity count after the SCI was calculated as a percentage relative to that before the injury.
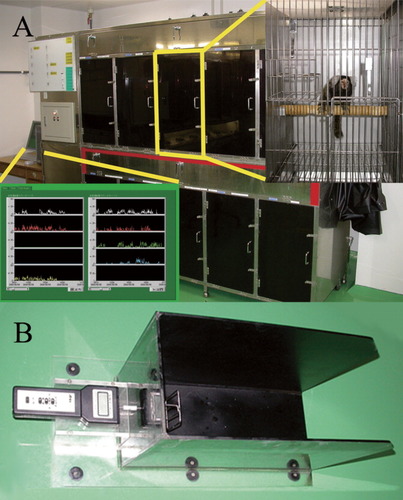
A: Infrared monitoring of spontaneous motor activity. Spontaneous motor activity was measured by infrared sensors equipped on the ceiling of the cage (cage size: 350-mm wide × 500-mm deep × 500-mm high). B: Device used for the bar grip test. The motor function of the upper extremities was evaluated using the bar grip test to estimate grip power. The device consisted of a Newton meter to measure grip power and a holding bar (bar diameter of 2.5 mm in a 1 × 3 [70 mm × 100 mm] grid pattern).
Bar grip test.
The motor function of the upper extremities was evaluated using the bar grip test, which tests the animal's gripping reflex (the motion undertaken when attempting to grasp an object placed before the animal). The device (220-mm wide, 500-mm deep, and 400-mm high with a bar diameter of 2.5 mm in a 1 × 3 [70 mm × 100 mm] grid pattern) used in the present study was based on a device used previously for mice (Smith et al., 1995) (Fig. 1B). The test was carried out three times a day. The percentage of the maximal grip strength relative to that before the injury was calculated on each day after the injury.
Cage-climbing test.
The cage-climbing test was developed to evaluate the coordination of the fore- and hindlimbs. If a common marmoset is placed upside down on the side of a cage (400-mm wide, 600-mm deep, and 750-mm high), it tends to hold on to the cage, reverse its position, and escape upwards. We used a seven-point scale (from 0 to 6; see Table II) to evaluate the ability of each common marmoset to perform the above behavior within 3 min. The evaluations were conducted twice a day from immediately before the injury until 10 weeks after the injury.
| Score | Behavior |
|---|---|
| 0 | Immediately fell without having grasped the cage. |
| 1 | Grasped cage with its hindlimbs, but could not pull its body upwards. |
| 2 | Grasped cage and pulled its body upwards using hindlimbs, but could not turn its body around. |
| 3 | Reversed its position, but could not climb upwards. |
| 4 | Reversed its position and climbed, but could not reach the top of the cage. |
| 5 | Reversed its position and climbed to the top of the cage using mainly hindlimbs. |
| 6 | Reversed its position and climbed to the top of the cage using fore- and hindlimbs equally. |
- * The cage-climbing test was used to evaluate the coordination of the animal's fore- and hindlimbs. Ability was rated using a seven-point scale (score 0–6).
Histologic Analyses
At 10 weeks after injury, each animal was perfused intracardially with 4% paraformaldehyde (PFA; pH 7.4). The injured spinal cord tissues were removed, postfixed in 4% PFA, and immersed overnight in 10% sucrose followed by 30% sucrose. Frozen section blocks were prepared and cut using a cryostat into 20-μm thick axial sections. These sections were stained with hematoxylin-eosin (HE) for general histologic examinations and Luxol fast blue (LFB) for evaluations of the myelinated area after SCI. The myelinated area of the axial sections at the lesion epicenter were measured by MicroComputer Imaging Device (MCID; Amersham Bioscience Corp., Piscataway, NJ) and compared among the three groups (McTigue et al., 1998). Immunostaining with anti-glial fibrillary acidic protein (GFAP) antibody (primary antibody, diluted 1:600, rabbit polyclonal; Dako Cytomation, Glostrup, Denmark; secondary antibody, a horseradish peroxidase [HRP]-labeled goat anti-rabbit IgG for diaminobenzidine [DAB; Sigma] staining) to evaluate the glial scars and with anti-calmodulin-dependent protein kinase IIα (CaMKIIα) antibody (primary antibody, diluted 1:100, mouse monoclonal; Zymed, CA; secondary antibody: an HRP-labeled goat anti-mouse IgG for DAB staining) to examine the CST was also carried out. To quantify the number of motor neurons, axial sections (every 10th section within 1.5 mm of the lesion epicenter and every 50th section more than 1.5 mm distant from the lesion epicenter) were immunostained with anti-choline acetyltransferase (ChAT) antibody (primary antibody, diluted 1:50, goat polyclonal; Chemicon International, Temecula, CA; secondary antibody, a biotin-labeled donkey anti-goat IgG for DAB staining), and the number of ChAT-positive cells located at the ventral horn (putatively at the lamina IX) that were larger than 30 μm was quantified and compared among the three groups.
Statistics
A nonparametric Kruskal-Wallis one-way analysis of variance (ANOVA) was applied to the data for cavity size, myelinated area, motor neuron counts, and motor functions to determine the overall differences among the three groups at each time point after SCI. Data were shown in all graphs as the mean ± standard error of the mean (SEM) with P < 0.05 regarded as statistically significant.
RESULTS
MRI of Spinal Cord after Graded-Contusion Injury
In all injury groups, an iso-signal intensity area on T1W images (T1WI) and a diffuse high signal intensity area on T2W images (T2WI) were observed within the spinal cord at 3 days after injury (Fig. 2A). Although there were significant differences in the size of the high signal intensity area on T2WI among the three groups (*P < 0.05; Fig. 2B), no changes in signal intensity on T1WI were detectable in any of the injury groups at 3 and 10 days after SCI (Fig. 2A). In the mild injury group, the intramedullary high signal intensity on T2WI decreased 3 weeks after injury. In contrast, the distinct high signal intensity on T2WI and the low signal intensity on T1WI was observed in the moderate and severe injury groups, but not in the mild injury group at 3 weeks after injury. There were significant differences in the areas of both the low signal intensity on T1WI and the high signal intensity on T2WI among the three groups at 8 weeks after injury (*P < 0.05; Fig. 2B).
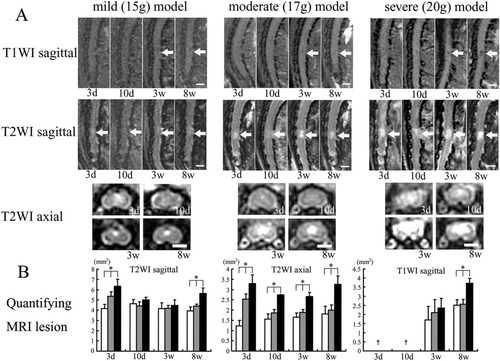
A: MRI of the spinal cord carried out 3 days, 10 days, 3 weeks, and 8 weeks after injury are shown for the mild, moderate, and severe injury groups. At 3 days after injury, a diffuse high signal intensity area is visible on T2WI (arrow); 8 weeks after injury, the lesion is depicted as a low signal intensity area on T1WI and a high signal intensity area on T2WI (arrow). As the severity of the injury increases, the area of low signal intensity on T1WI and the area of high signal intensity on T2WI increases in size. All images of the spinal cord of each group were taken with the same animal. Scale bar = 2.5 mm. B: Quantitative analyses of intramedullary signal intensity. The areas of the low and high signal intensity in the mild, moderate, and severe injury groups were measured using NIH Image software. †No obvious signal changes on T2WI at 3 and 10 days after injury. Statistical analysis by Kruskal-Wallis test revealed significant differences among the three groups at 3 days after injury on T2W sagittal images and T2W axial images. At 8 weeks after injury, significant differences among the three groups are visible on T1W sagittal images, the T2W sagittal images, and the T2W axial images. *P < 0.05, **P < 0.01; same in following figures).
Histologic Findings
As the severity of the injury increased, the cavity size (Fig. 3A), GFAP immunoreactivity (Fig. 3B), and demyelinated area (Fig. 3C) surrounding the cavity also increased. The demyelinated area as assessed by a lack of LFB staining revealed limited demyelination near the gray matter in the mild injury group, further demyelination into the white matter in the moderate injury group, and almost complete demyelination in the severe injury group (Fig. 3C). Quantification of the LFB-positive area (Fig. 3D) revealed significant differences in the mild, moderate, and severe injury groups, with values of approximately 36, 23, and 8%, respectively (**P < 0.01, relative to the value of the sham group; Fig. 3E).
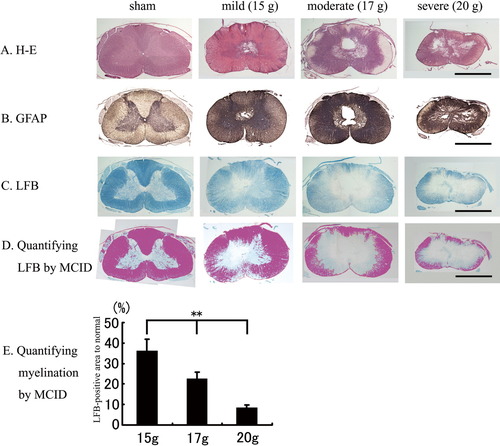
Histopathologic examination 1. A: Representative HE-stained specimens from the sham, mild, moderate, and severe injury groups. As the severity of the injury increases, the size of the cavity also increases. In the severe injury group, marked atrophy of the spinal cord is also visible. All histopathologic images presented were prepared from tissue specimens obtained from the same animals used for the MRI studies. Scale bar = 2.5 mm. B: Representative anti-GFAP-immunostained specimens from the sham, mild, moderate, and severe injury groups. GFAP immunoreactivity is visible around the cavity. As the severity of the injury increases, the GFAP-positive area also increases. Scale bar = 2.5 mm. LFB staining (C) and quantified spared myelination (D, E). Representative LFB-stained specimens from the sham, mild, moderate and severe injury groups. As the severity of the injury increases, the demyelinated area in and around the cavity also becomes larger. Scale bar = 2.5 mm. The percentage of the LFB-positive area after injury relative to the corresponding area in the sham group was calculated using the MCID to be approximately 36, 23, and 8% in the mild, moderate, and severe injury groups, respectively. *P < 0.05, **P < 0.01.
In the sham group, CaMKIIα-positive CST fibers (Terashima et al., 1994) were observed in the lateral funiculus but were not seen in the anterior funiculus. In the injured spinal cord, the loss of CaMKIIα-positive CST fibers was proportional to the severity of the injury, with little loss of CST fibers in the mild injury group, significant loss in the moderate injury group, and almost complete loss of CST fibers in the severe injury group (Fig. 4A,B). The loss of CaMKIIα-positive CST fibers was not localized to the lesion epicenter but was spread across a rostral-caudal area (data not shown). Although the number of ChAT-positive motor neurons also decreased with increasing injury severity (Fig. 4C,D), the loss was limited to the lesion epicenter, with sparing of the ChAT-positive motor neurons immediately rostral and caudal to the injury (Fig. 4E).
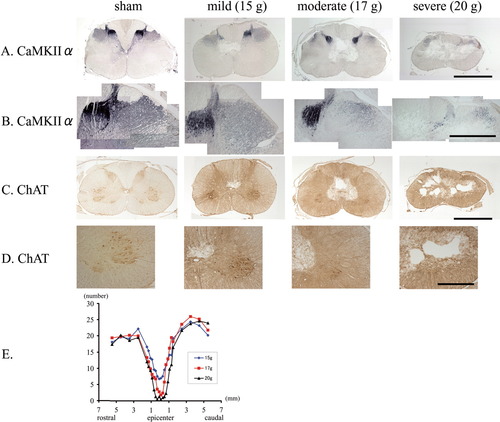
Histopathologic examination 2. A, B: Anti-CaMKIIα immunostaining. Representative anti-CaMKIIα-immunostained specimens from the sham, mild, moderate, and severe injury groups. As the severity of the injury increases, the loss of CST fibers in and around the cavity also becomes larger. Scale bar = 2.5 mm (A); 1 mm (B). C, D: Anti-ChAT immunostaining. Representative anti-ChAT-immunostained specimens from the sham, mild, moderate, and severe injury groups. All three models showed a loss of ChAT-positive neurons in the ventral horn (putatively at the lamina IX). Scale bar = 2.5 mm (C); 1 mm (D). E: Quantitative analysis of the number of ChAT-positive neurons in the ventral horn after SCI. ChAT-positive cells over 30 μm in size were considered to represent neurons in the ventral horn of the normal spinal cord. The number of neurons was counted over a total length of 11 mm (5.5 mm rostral and 5.5 mm caudal to the center of injury). The number of ChAT-positive neurons at the lesion epicenter decreases as the severity of the injury increases.
Evaluation of Motor Function
Original behavioral scoring scale.
In the mild injury group, the score for basic motions decreased to about 4 immediately after injury and increased rapidly thereafter, reaching a plateau of about 8 points by 5 weeks after SCI. In the moderate injury group, the score for basic motions decreased to about 2 immediately after injury and increased gradually thereafter, reaching a plateau of about 7 points by 8 weeks after SCI. In the severe injury group, the score for basic motions dropped to almost 0 immediately after injury and increased more slowly than the other two groups did, reaching a plateau of about 5 points by 8 weeks after SCI. The scores among the three groups differed significantly at all points (Fig. 5A).
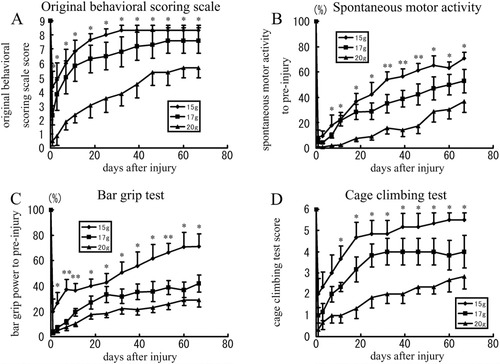
Behavioral analyses. A: Original behavioral scoring scale. Immediately after injury, the score decreased to approximately 4, 2, and 0 in the mild, moderate, and severe injury groups, respectively. By 10 weeks after injury, the score had increased to an approximate plateau of 8.3, 7.6, and 5.7 in the three respective groups. B: Measurements of spontaneous motor activity. Spontaneous motor activity fell to below approximately 10% of the preinjury level in all models immediately after injury but increased subsequently to approximately 70, 50, and 35% of the preinjury level in the mild, moderate, and severe injury groups, respectively, by 10 weeks after injury (the percentage of spontaneous motor activity after injury relative to that before injury is shown by the vertical axis). C: Bar grip test. The bar grip strength decreased in all of the groups immediately after the injury. It recovered gradually, reaching an approximate plateau of 70, 40, and 30% of the preinjury level in the mild, moderate, and severe injury groups, respectively, by 10 weeks after injury (the percentage bar grip strength after injury relative to that before injury is shown by the vertical axis). D: Cage-climbing test. Immediately after injury, the score was approximately 2, 1, and 0 in the mild, moderate, and severe injury groups, respectively. By 10 weeks after injury, the score of the animals from the mild injury group had improved to 5 or 6. In the moderate and severe injury groups, the average score reached an approximate plateau of 4 and 2.8, respectively. *P < 0.05, **P < 0.01.
Measurement of spontaneous motor activity.
Immediately after injury, the spontaneous motor activities decreased below approximately 10% of that before injury and then increased gradually in all injury groups. By 10 weeks after injury, the motor activities had returned to approximately 70, 50, and 35% in the mild, moderate, and severe injury groups, respectively. The motor activities of the three groups differed significantly from that in the second week after injury (Fig. 5B).
Bar grip test.
The percentage of the maximal grip strength after injury relative to that before injury (% grip strength) decreased to approximately 20, 5, and 5% in the mild, moderate, and severe injury groups, respectively, at 1 day after injury. The % grip strength gradually recovered thereafter and reached a plateau of approximately 70, 40, and 30% in the mild, moderate, and severe injury groups, respectively. Significant differences in the values of the % grip strength were observed among the three groups after the seventh day after injury (Fig. 5C).
Cage-climbing test.
Immediately after injury, the cage-climbing test score decreased to about 2, 1, and 0 in the mild, moderate, and severe injury groups, respectively. The scores of the mild and moderate injury groups then recovered rapidly, reaching a plateau of about 5 and 4 points, respectively, by 6 weeks after injury. In the severe injury group the score recovered more slowly, reaching a plateau of about 2.5 points by 8 weeks after injury. All animals from the mild injury group had a cage-climbing test score of 5 or 6 by 9 weeks after injury. The cage-climbing test scores differed significantly among the three groups during the 2nd to 10th week after injury (Fig. 5D).
DISCUSSION
Significance of Contusive SCI Model in the Common Marmoset
Macaque monkeys, which have been used to establish various disease models, are anatomically close to humans and their use may be advantageous for evaluating functions of the neocortex (Hoffman and McNaughton, 2002; Koketsu et al., 2003; Tonchev et al., 2003a, b). There are some problems, however, associated with the use of this species. First, macaques are difficult to handle because they are often medium to large in size. Second, because most macaques are not grown within the animal facility of the institute, the possibilities of infection, unknown history after birth, and inhomogeneous quality present problems. Third, because macaques deliver only one offspring a year, the number of macaques available for experimentation is limited. In contrast, common marmosets are small (300–500 g after maturity), easier to manipulate, and their high breeding efficiency allows for an adequate number of common marmosets to be obtained for use in research experiments. Furthermore, because common marmosets can be bred in experimental colonies, the supply is stable and reliable, with adequate genetic and microbiological control to minimize biases (Lipp, 1980; Massacesi et al., 1995; 't Hart et al., 2000; Schultz-Darken, 2003). Common marmosets have therefore been used to create several kinds of neurodegenerative disease models, such as the experimental autoimmune encephalomyelitis (EAE) model for multiple sclerosis (Massacesi et al., 1995; 't Hart et al., 2004), cerebrovascular disease (Marshall et al., 2000), Alzheimer's disease (Baker et al., 1993; Maclean et al., 2000), delayed dyskinesia (Klintenberg et al., 2002), parkinsonism (Gnanalingham et al., 1993; Roeling et al., 1995), and Huntington's disease (Kendall et al., 1998). Recently, Liu et al. (2001) reported the use of a hemisection SCI model in common marmosets, but this model did not have an objective functional evaluation parameter or a multifaceted analysis. A contusive SCI model therefore may be more suitable for preclinical studies, because contusion injuries are pathophysiologically closer to clinical SCI than are hemisection injuries.
Comparisons Between MRI and Histology of Injured Spinal Cord
MRI is essential for predicting the prognosis and planning the treatment of patients with SCI. Previous clinical studies have demonstrated that patients with intramedullary low signal intensity on T1WI and high signal intensity on T2WI in the chronic stage of SCI have a poor outcome (Kulkarni et al., 1987; Yamashita et al., 1990; Duncan et al., 1992). Our previous study using rodents also demonstrated that T2 low signal intensity of the spinal cord observed early after injury reflects the extent of hemorrhage and may indicate a poor prognosis, whereas T1 low and T2 high signal intensity during the subacute to chronic periods indicate the persistence of paralysis and a limited recovery of function (Ohta et al., 1999). In the present study, MRI of injured spinal cords in common marmosets also showed similar patterns and time courses to those observed in humans (Ramon et al., 1997; Shimada and Tokioka, 1999). The injured spinal cord of common marmosets at 3 days after injury showed diffuse high signal intensity on T2WI, and the area of high signal intensity reflected the severity of the injury. Diffuse T2 high signal intensity during the acute stage of SCI has been attributed to bleeding or inflammation (Kulkarni et al., 1987; Duncan et al., 1992; Ohta et al., 1999). As bleeding and inflammation subside over time, the area of intramedullary high signal intensity on T2WI decreased. During the chronic stage, the injured spinal cords of the common marmosets showed low signal intensity on T1WI and high signal intensity on T2WI, and the areas of these signals reflected the severity of injury (Fig. 2A). Histologic examinations consistently revealed that the size of the cavity and the area of glial scar at the lesion epicenter increased as the severity of the injury increased (Fig. 3A,B). This pattern of intramedullary signal changes was consistent with previous studies showing that T1WI represents the cavity itself, whereas T2WI represents the cavity along with the surrounding glial scar (Yamashita et al., 1990; Ohta et al., 1999). In the present study, a discrepancy between the area of T2 high signal intensity and the size of the cavity at the lesion epicenter was observed in the severe injury group, probably caused by cavity shrinkage after the intracardial perfusion. The staining of adjacent sections with LFB and GFAP revealed that demyelination had occurred in the areas of glial scar formation (Fig. 3). The LFB-positive myelinated area and the CaMKIIα-positive CST area at the lesion site decreased significantly with increasing injury severity. These patterns of glial scar formation and demyelination were also similar to the histology of human cervical SCI (Norenberg et al., 2004). Furthermore, the number of ChAT-positive motor neurons in the ventral horn decreased with increasing injury severity. Taken together, these quantitative examinations of the morphology of the injury site indicated that greater injury severity causes a progressive decrease in residual tissue, such as the myelinated area and the motor neurons.
Comparisons Between Histologic Findings and Functional Recovery After SCI in Common Marmosets
The patterns of motor function recovery after graded contusive SCI differed among the three groups. The mild injury group showed an almost spontaneous recovery from paralysis, whereas the moderate injury group showed residual paralysis (predominantly of the upper extremities). Severe paralysis of the extremities was observed in the severe injury group, making it almost impossible for the animals to walk or bear weight. The original behavioral scoring scale, the cage-climbing test and the monitoring of spontaneous motor activity revealed that the mild and moderate injury groups showed a more rapid recovery from the paralysis after injury and recovered to a higher level of function. In the severe injury group, however, functional recovery occurred more slowly and the final level of function was lower than that observed in the other injury groups. There was a significant correlation between the residual myelinated area at the lesion epicenter and spontaneous motor activity at 10 weeks after SCI (Fig. 6). The bar grip power of the mild injury group recovered more rapidly, compared to that in the other injury groups, reaching a plateau of approximately 80% of the preinjury level. In contrast, the bar grip power of the moderate and severe injury groups recovered to approximately 40% and 20% of the preinjury level, respectively. A compensatory response by the spared motor neurons may have contributed to the partial recovery of bar grip power in the mild injury group, despite the significant loss in the numbers of motor neurons at the lesion epicenter, as reported previously (Nakamura et al., 1996, 1997). In contrast, less recovery of bar grip power was observed in the moderate and severe injury groups because the number of spared motor neurons was too small to compensate for the loss of bar grip power (Nakamura et al., 1996, 1997). Taken together with these findings, the motor dysfunction caused by the damage of long-tract fibers could be assessed broadly by spontaneous motor activity and the cage-climbing test, whereas the motor dysfunction caused by the loss of motor neurons in the ventral horn could be assessed broadly by the bar grip test.
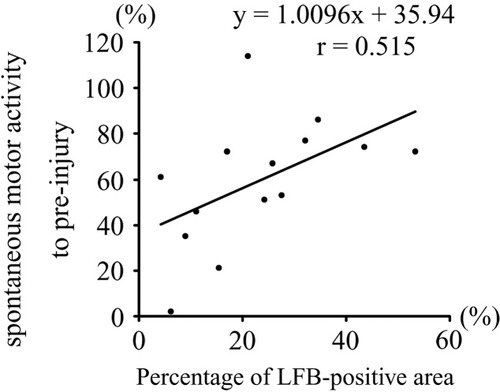
Correlation between spared myelinated area and behavioral analysis results (spontaneous motor activity). Residual LFB-positive area vs. spontaneous motor activity (at 10 weeks). A positive correlation between the spared myelinated area and spontaneous motor activity was observed.
The methods of functional evaluation devised for this study were not uniformly applicable to all severity levels and phases after the injury. For example, the monitoring of spontaneous motor activity and the bar grip test were useful for assessing all severity levels of injury over the long-term, whereas distinguishing differences in cage-climbing test performances in the mild and moderate injury groups were difficult during the chronic phase of injury, even though the test results faithfully reflected differences in all severity levels during the acute phase. The bar grip test therefore is more suitable for evaluating therapeutic methods yielding a relatively rapid recovery from paralysis, regardless of the ultimate level of recovery. The original behavioral scoring scale was useful for evaluating severe injury, although distinguishing differences in scoring were difficult in the mild and moderate injury groups. When selecting a method of functional evaluation, the severity of the injury and the timing of the evaluation thus must be taken into careful consideration. In other words, a more accurate evaluation of motor function will be possible if two or more of these methods are used in combination, instead of a single test.
In conclusion, a reliable, graded, in vivo model of contusive SCI was successfully established in the common marmoset by modifying an existing method of producing injury in the rat (Wrathall et al., 1985; Bresnahan et al., 1991; Young, 2002). MRI-based evaluations, neurologic outcome measures, and morphometric analyses of the injury site were found to accurately discriminate among the three grades of contusion injury severity. This model will facilitate future in vivo preclinical studies for several kinds of SCI treatment (Iwanami et al., 2005). In the present study, we have characterized the histologic findings of the SCI site in detail. Considering the motor function of the animals, however, we wish to characterize the histologic findings on the primary motor area in the brain and pyramidal tract in the future.
Acknowledgements
This work was supported by a project to realize regenerative medicine from the Japanese Ministry of Education, Sports and Culture, the Human Frontier Science Program Organization, Core Research for Evolutional Science and Technology (CREST) from the Japan Science and Technology Corporation (JST), the General Insurance Association of Japan, a National Grant-in-Aid for the Establishment of a High-Tech Research Center in a Private University in Japan, a Keio University special grant-in-aid for innovative collaborative research projects (to H.O.), a grant-in-aid from the 21st Century COE Program of the Ministry of Education, Culture, Sports, Science and Technology, Japan to Keio University, and a Keio University grant-in-aid for the encouragement of young medical scientists (to A.I. and J.Y.).
We thank Mrs. A. Hirayama for her arrangement of the figures. We also thank Mr. K. Ando, Miss F. Toyoda, and Miss M. Kamioka for their care of the common marmosets and their assistance with the operations.




