Upregulation of annexins I, II, and V after traumatic spinal cord injury in adult rats
Abstract
The posttraumatic inflammatory reaction contributes to progressive tissue damage after spinal cord injury (SCI). Annexins, a family of structurally related calcium- and phospholipid-binding proteins, have potent anti-inflammatory effects by inhibiting the activity of phospholipase A2 (PLA2), a key enzyme responsible for inflammation and cytotoxicity. We investigated spatiotemporal expression of annexins I, II, and V after a contusive SCI using the New York University impact device (a 10-g rod, height 12.5 mm) in adult rats. Western blot analysis revealed that annexin I expression increased at 3 days after injury, peaked at 7 days (1.75-fold above the baseline level; P < 0.01), started to decline at 14 days, and returned to the baseline level at and beyond 28 days post-injury. The expression of annexin II started to increase at 3 days, reached its maximal level at 14 days (2.73-fold; P < 0.01), remained at a high level up to 28 days, and then declined to the basal level by 56 days after injury. Annexin V expression started at 3 days, reached its maximal level at 7 days (1.61-fold; P < 0.05) and remained at this level until 56 days after injury. RT-PCR results confirmed expression of all three annexins at the mRNA level after SCI. Immunohistochemistry and immunofluorescence double-labeling analyses revealed that increased annexins I, II, and V were localized in neurons and glial cells. The present study thus revealed increased expression of the three annexin isoforms after moderate contusive SCI. The precise role of annexins in posttraumatic inflammation and neuroprotection after SCI remains to be determined. © 2004 Wiley-Liss, Inc.
There are two mechanisms of damage to the spinal cord after acute spinal cord injury (SCI): the primary mechanical injury and a secondary injury mediated by multiple injury processes. Posttraumatic inflammation plays an important role in the secondary injury process after SCI (Hsu and Dimitrijevic, 1990; Young, 1993; Bartholdi and Schwab, 1995). Annexins are a superfamily of calcium- and phospholipid-binding proteins characterized by the presence of four or eight highly conserved homologous repeats of approximately 70 amino acids, which are responsible for the calcium- and phospholipid-binding properties of the molecule (Flower and Rothwell, 1994; Perretti, 1994; Raynal and Pollard, 1994). Each family member is distinguished by a unique N-terminal of variable length (Raynal and Pollard, 1994; Morgan and Fernandez, 1997). Originally, annexins evoked interest as mediators of the anti-inflammatory actions of glucocorticoids (Flower and Rothwell, 1994; Perretti, 1994). Subsequent research has shown that annexins are implicated in multiple biologic functions ranging from intracellular events like signal transduction to extracellular events where they affect inflammation (Flower and Rothwell, 1994; Perretti, 1994; Raynal and Pollard, 1994). The annexin superfamily consists of at least 94 distinct proteins (Morgan and Fernandez, 1997). It has been shown that three members of this family, annexins I, II, and V, exert potent anti-inflammatory effects (Pepinsky et al., 1988; Cirino et al., 1989; Errasfa and Russo-Marie, 1989; Perretti et al., 1993). It is believed that these annexins exert anti-inflammatory actions through the inhibition of PLA2 activity (Flower, 1990; Raynal and Pollard, 1994), a key enzyme responsible for inflammation and cytotoxicity (Bonventre, 1996; Farooqui et al., 1997). Central injections of annexin I 1-188 markedly inhibited neuronal death (up to 70%) and edema induced by focal cerebral ischemia in rats whereas injection of annexin I antiserum significantly exacerbated the damage (Relton et al., 1991). In addition, annexin I protected against N-methyl-D-aspartate (NMDA)-induced brain damage (Black et al., 1992) and annexins I and V exerted neurotrophic effects on cultured neurons (Takei et al., 1994; Mizuno et al., 1998).
Annexins I, II, and V were found to distribute in the rat and human brain and spinal cord, and were localized to neurons and glia cells (Johnson et al., 1989; Strijbos et al., 1991; McKanna, 1993; Eberhard et al., 1994; Go et al., 1994; Mullens et al., 1994; Naciff et al., 1996; McKanna and Zhang, 1997 Voermans et al., 1997; Young et al., 1999). The expression of annexins was upregulated in pathologic conditions, such as in kainic acid-lesioned cerebellum (Young et al., 1999), multiple sclerosis (Elderfield et al., 1992), experimental allergic encephalomyelitis (Bolton et al., 1990; Elderfield et al., 1993), and hypoxic-ischemic injury (Eberhard et al., 1994). It remains unclear, however, whether SCI induces expression of annexins and, if so, the cellular sources of their expressions.
In the present study, we investigated spatiotemporal expressions of annexins I, II, and V at both protein and mRNA level, as well as identified their cellular sources in a widely accepted contusion SCI model in adult rats. Results of this study have appeared previously in abstract form (Liu and Xu, Program No. 393.13, Soc Neurosci Abs., 2002).
MATERIALS AND METHODS
In total, 90 adult female Sprague-Dawley rats (Harlan, Indianapolis, IN), weighing 200–250 g, were used in this study (Table I). These included animals used for Western blot (n = 54), RT-PCR (n = 18), and immunofluorescence double labeling (n = 18) analyses.
| Groups | Normal | Sham | 8 hr | 1 d | 3 d | 7 d | 14 d | 28 d | 56 d |
|---|---|---|---|---|---|---|---|---|---|
| Western blot | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Immunohistochemistry | — | 6a | — | — | — | 6a | 6a | — | — |
| RT-PCR | — | 6 | — | — | — | 6 | 6 | — | — |
- a Half of the rats were used for immunohistochemistry and half for immunofluorescence double labeling.
Spinal Cord Injury
Spinal cord impact injury was produced using a weight-drop device developed at New York University (Gruner, 1992) and a protocol developed by a multicenter consortium (Basso et al., 1996). Briefly, rats were anesthetized with pentobarbital (50 mg/kg, intraperitoneally), and a laminectomy was carried out at the T9–T10 level. After the spinous processes of T8 and T11 were clamped to stabilize the spine, the exposed dorsal surface of the cord was subjected to weight-drop impact using a 10-g rod (2.5 mm in diameter) dropped from a height of 12.5 mm. After the injury, the muscles and skin were closed in layers, and rats were placed in a temperature- and humidity-controlled chamber overnight. Manual bladder expression was carried out at least three times daily until reflex bladder emptying was established. For sham-operated controls, animals underwent a T10 laminectomy without weight-drop injury. Normal rats that received no injury served as additional controls. All surgical interventions and postoperative animal care were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and the Guidelines of the University of Louisville Institutional Animal Care and Use Committee.
Western Blotting
Western blotting followed procedures described previously (Xu et al., 1998; Yan et al., 1999, 2003) with modification. Briefly, a 10-mm spinal cord segment containing the injury epicenter was dissected after intracardial perfusion of the rat with 200 ml of saline under anesthesia. The cord segment was homogenized in 0.4 ml of RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM Na3VO, 1 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 100 μg/ml phenylmethylsulfonyl fluoride [PMSF], 30 μl/ml aprotinin, and 4 μg/ml leupeptin, pH 7.5) and centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was removed and centrifuged again, and then 40 μg of proteins from the supernatant of each sample was loaded onto 12% polyacrylamide gel, separated by SDS-PAGE, and transferred to a polyvinylidene difluoride membrane by electrophoresis. The membrane was blocked in TBST buffer (20 mM Tris-HCl, 5% nonfat milk, 500 mM NaCl, and 0.1% Tween 20, pH 7.5) for 1 hr at room temperature. The primary rabbit polyclonal anti-annexin I (1:250; Santa Cruz Biotechnology, Santa Cruz, CA), anti-annexin II (1:500; Santa Cruz), or anti-annexin V (1:250; Santa Cruz) was added to the membrane and incubated for 1 hr at room temperature. The membrane was washed once for 15 min and twice for 5 min with TBST at room temperature, incubated with a secondary horseradish peroxidase (HRP)-conjugated donkey anti-rabbit immunoglobulin (Ig)G antibody (1:5,000; Amersham Pharmacia, Piscataway, NJ) for 1 hr, and then washed once for 15 min and twice for 5 min with TBST. The Western blot was visualized using the enhanced chemiluminescence (ECL) plus detection system as described in the manufacturer's instructions. For the negative control, the primary antibody was omitted. A-431 whole cell lysates (Santa Cruz) was used as a positive control for annexin I, and HeLa whole cell lysate (Santa Cruz) was used as a positive control for annexins II and V.
RT-PCR
Total RNA from 10 mm-long spinal cord segments containing the injury epicenter was extracted with RNA START-60 kit (Tel-TEST, Inc.) according to the manufacturer's instructions. RNA pellets from 80–100 mg tissues were suspended in 80–120 μl diethylpyrocarbonate (DEPC)-treated water, and yield and purity were checked by spectrophotometric determination at 260 and 280 nm. Integrity of RNA was determined by the presence of 28S and 18S ribosomal RNA by electrophoresis of samples through 1.0% agarose gels. Initial dilution and cycle series experiments were carried out to determine the range of total RNA input and PCR cycles for which the PCR amplification is linear for PCR products. For annexins I, II, and V, linear product was apparent from PCR cycle 24 to 36. The primer sequences are summarized in Table II.
| Gene | Primer | Sequence | Size (bp) | Reference |
|---|---|---|---|---|
| Annexin I | Sense | 5′GCC CCT ACC CTT CCT TCA AT3′ | 300 | Mizuno et al., 1997 |
| Antisense | 5′GAG TGT CTT CAT CTG TTC CA3′ | |||
| Annexin II | Sense | 5′CAT TCT GAC TAA CCG CAG CA3′ | 261 | Matsuda et al., 1999 |
| Antisense | 5′CGG TTA ATC TCC TGC AGC TC3′ | |||
| Annexin V | Sense | 5′ATG GCT CTC AGA GGC ACC GT3′ | 289 | Kawaminami et al., 2002 |
| Antisense | 5′CGT GTT TCA GCT CGT AGG CG3′ | |||
| Cyclophilin | Sense | 5′ATG GTC AAC CCC ACC GTG T3′ | 205 | Xu et al., 1997 |
| Antisense | 5′CGT GTG AAG TCA CCA CCC T3′ |
RT-PCR was carried out with Access RT-PCR system (Promega, Madison, WI), consisting of a 45-min reverse transcription at 48°C, 2 min of inactivation of AMV reverse transcriptase at 94°C, 32 cycles of denaturing at 95°C (30 sec), annealing at 60°C (1 min), extension at 68°C (2 min), and final extension at 68°C (7 min). Primers for cyclophilin, one of the constitutively expressed housekeeping genes, were used for amplification of cyclophilin mRNA as an internal control. RT-PCR experiments were repeated three times each. Ten microliters of PCR products were separated on 1.5% agarose gels with 0.1% ethidium bromide. The amplified DNA bands were laser scanned and the relative densities of DNA products were quantified using a Jandel Scientific Software program. All mRNA levels were normalized relative to the level of cyclophilin to correct for potential differences in the amount of RNA used and the DNA amplified.
Immunohistochemistry
Spinal cord injured and age-matched sham operated (receiving a T10 laminectomy) rats were sacrificed at 7 and 14 days post-injury by transcardial perfusion with 100 ml of 0.9% saline followed by 500 ml of 4% paraformaldehyde. After perfusion, the spinal cord was carefully removed and a 14-mm long segment containing the injury epicenter was blocked and post-fixed for an additional 2 hr in the same fixation solution. The specimen was transferred to a solution containing 30% sucrose in 0.01 M phosphate buffer (PB; pH 7.4) overnight at 4°C. On the second day, the spinal cord segment was embedded in tissue-freezing medium (Sakura Finetek, Torrance, CA), cut transversely and serially at 20 μm on a cryostat, and mounted on Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA) in five identical sets. Before incubation with primary antibodies, the sections were permeabilized and blocked with 0.3% Triton X-100/10% normal goat serum in 0.01 M phosphate-buffered saline (PBS) for 30 min. The sections were then incubated with primary polyclonal rabbit anti-annexin I (1:100), anti-annexin II (1:100), or anti-annexin V (1:100) antibodies (Santa Cruz Biotechnology) overnight at 4°C. On the second day, sections were incubated with secondary biotinylated goat anti-rabbit IgG antibody (1:400; Vector Laboratories) for 1 hr at room temperature. The reaction product was revealed by incubation for 5 min with 0.02% DAB and 0.003% H2O2 in 0.05 M Tris-HCl (pH 7.6). After reaction, the sections were dehydrated, cleared, and coverslipped. Slides were examined using an Olympus BX60 light microscope. Primary antibody omission controls were used to confirm further the specificity of the immunohistochemical labeling.
Immunofluorescence Double Labeling
The immunofluorescence double-labeling method was employed to identify specific cell types that express the three annexin isoforms after SCI. This method has been described previously (Yan et al., 1999, 2001). Briefly, spinal cord segments from sham-operated or injured animals were embedded in tissue freezing medium, cut horizontally at 20 μm on a cryostat, and mounted on gelatin-coated slides. Before primary antibody incubation, sections were permeabilized and blocked with 0.3% Triton X-100/10% normal goat serum in 0.01 M PBS for 15 min. A mixture of rabbit polyclonal anti-annexin I (1:50), anti-annexin II (1:100), or anti-annexin V (1:100), all purchased from Santa Cruz Biotechnology, and a cell-specific monoclonal antibody was applied to the sections overnight at 4°C. The cell-specific monoclonal antibodies included mouse anti-MAP-2 antibody (1:100; Sigma, St. Louis, MO) to identify neurons, mouse anti-glial fibrillary acidic protein (GFAP) antibody (1:100, Sigma) to identify astrocytes, mouse anti-RIP antibody (1:20; a gift from Dr. S.R. Whittemore, University of Louisville) to identify oligodendrocytes, and mouse anti-OX42 antibody (1:20; Harlan Sera-Lab, Sussex, UK) to recognize microglial cells. On the next day, sections were incubated with fluorescein-conjugated goat anti-rabbit (1:100; ICN Biochemicals, Aurora, OH) and rhodamine-conjugated rabbit anti-mouse (1:100; ICN Biochemicals) antibodies. Primary antibody omission controls and normal mouse, rabbit, and goat serum controls were used to confirm further the specificity of the immunofluorescence double labeling. Slides were washed, mounted, and examined with an Olympus Optical Fluoview confocal microscope.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM) values. One-way analysis of variance (ANOVA) with post hoc Tukey t-test was used to determine statistical significance. A P value of <0.05 was considered statistically significant.
RESULTS
Western Blot Analysis
Western blot analysis revealed the presence of basal level expressions of annexins I, II, and V in normal rat spinal cords (Fig. 1). Increased expressions of all three annexin subtypes were found after SCI (Fig. 1). Specifically, annexin I expression increased at 3 days post-injury, peaked at 7 days (1.75-fold above the baseline level), started to decline at 14 days, and returned to the baseline level at and beyond 28 days post-injury (Fig. 1A). Similarly, the expression of annexin II started to increase at 3 days, reached its maximal level at 14 days (2.73-fold), remained at a high level up to 28 days, and then declined to the basal level by 56 days post-injury (Fig. 1B). Annexin V expression started to increase at 3 days, reached its maximal level at 7 days (1.61-fold) and maintained at the maximal level until 56 days after SCI (Fig. 1C).
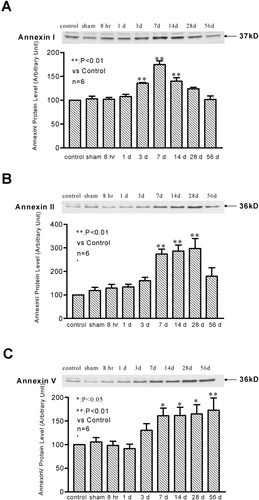
Western blot analysis showing annexin I (A), II (B), and V (C) protein expression at varying time points after SCI. Bar graphs indicate the mean ± SEM (n = 6 rats/group) for each time point. *P < 0.05, **P < 0.01 (compared to non-injured and sham-operated controls).
RT-PCR
To confirm the expression of annexins at the mRNA level, RT-PCR was used to examine expression of all three annexins in sham-operated and in spinal cord-injured rats. Rats were sacrificed at 7 or 14 days post-SCI based on the peak expression point determined by Western blot analysis. Amplified DNA products increased proportionally with increasing concentrations of template RNA (0.2–1.2 μg) and increased proportionally from 24 to 36 cycles (data not shown). These results indicate that RT-PCR used in this study is sensitive and accurate to detect changes in annexin I, II, and V mRNA levels post-injury. RT-PCR of total RNA isolated from spinal cord tissues of sham-operated controls and spinal cord-injured rats resulted in a single band of the expected size of 300, 261, or 289 base pairs (bp), respectively, after using an annexin I-, II-, or V-specific primer pair (Table II). Analysis of densitometric signals revealed that the steady-state level of annexin I mRNA increased by 1.6-fold in the injured spinal cord at 7 days post-SCI compared to that in the sham-operated control (Fig. 2A). Likewise, annexin II and V mRNA levels increased by 2.1- and 1.58-fold, respectively, at 14 days post-SCI, (Fig. 2B,C). The RT-PCR study, along with the findings revealed by the Western blot analysis, thus collectively demonstrated increased expression of the three annexins at both protein and mRNA levels after SCI.
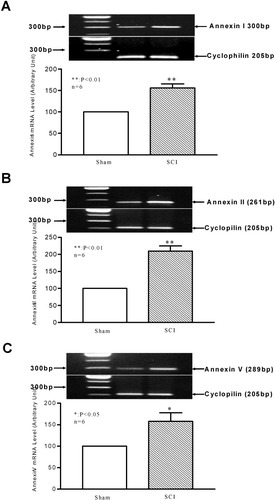
Expression of annexin I (A), II (B), and V (C) mRNAs in the injured spinal cord at 7 or 14 days after SCI. RT-PCR was carried out using specific primers designed for rat annexins I, II, and V as well as cyclophilin (0.5 μg total RNA and 26–28 amplification cycles). Bar graphs indicate the mean ± SEM (n = 6 rats/group) for each time point. *P < 0.05, **P < 0.01 (compared to sham-operated controls).
Immunohistochemistry
To confirm the Western blot and RT-PCR results and determine the spatial distribution of annexins after SCI, immunohistochemistry for the three annexin isoforms was carried out at 7 or 14 days post-injury, based on the maximal expression of a particular annexin. Because similar patterns of annexin immunoreactivity (IR) were observed among three annexins, we used annexin I as an example to demonstrate its distribution at 7 days after SCI (Fig. 3). In a sham-operated control (Fig. 3; left column), annexin I-IR was detectable at low level in both the gray and white matter. Cells that were immunopositive for annexin I were morphologically characteristic of neurons in the ventral horn gray matter and glial cells in the white matter (Fig. 3 A,C,E). In contrast, annexin I-IR was increased markedly in both neurons and glial cells of the injured cord at 7 days post-injury (Fig. 3; right column) as compared to those found in the sham-operated controls. Although the labeling was the strongest at the lesion epicenter, increased annexin I-IR was found throughout the entire length of the specimen (10 mm) examined (Fig. 3B,F). In sections where the primary antibody was omitted, no labeling was found, further confirming the specificity of the antibody (Fig. 3G,H). Spatial distribution of increased annexin I expression in the injured spinal cord at its peak expression was thus confirmed immunohistochemically. These results were consistent with those obtained from the Western blotting and RT-PCR.
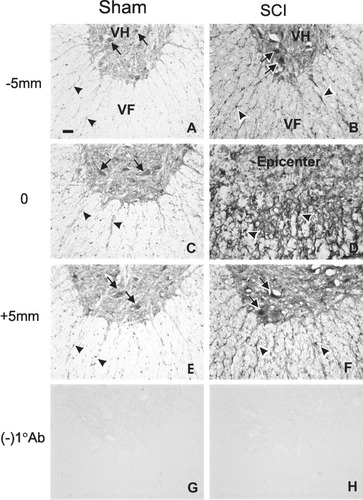
Distribution of annexin I immunoreactivity (IR) in the spinal cords of sham-operated and spinal cord injured rats. Left: In the spinal cord of sham-operated rats, a basal level of annexin I IR was found in ventral horn neurons (arrows) and white matter glial cells (arrowheads). Right: In the spinal cord at 7 days post-injury, a marked increase in annexin I-IR was found in neurons (arrows) and glial cells (arrowheads) at the injury epicenter (0 mm) as well as at 5 mm rostral (−5 mm; B) and caudal (+5 mm; F) to the injury. (G,H) No primary antibody controls. VH, ventral horn; VF, ventral funiculus. Scale bar = 50 μm (A–H).
Immunofluorescence Double Labeling
Although immunohistochemistry confirmed increased expression of three annexin isoforms in the injured spinal cord at their peak expressions after SCI, it did not allow the identification of specific cell types that express these annexins. We thus carried out an immunofluorescence double-labeling experiment to localize specific cell types that express annexins I, II, and V at the same post-injury time points, as compared to sham-operated controls. Because immunohistochemistry data showed that increased annexin expression was found within 5 mm of the injury epicenter (Fig. 3), we searched cell types that express each of the three annexins in an area 4–5 mm rostral to the injury epicenter. Coexistence of annexin I, II, or V and MAP-2, a neuronal marker, was observed in many neurons in the spinal cord gray matter at 7 or 14 days after SCI (Fig. 4A–C, Fig. 5A–C, and Fig. 6A–C). Annexin I-, II-, or V-IR was detected also in astrocytes (GFAP-IR; Fig. 4D–F, Fig. 5D–F, and Fig. 6D–F), oligodendrocytes (RIP-IR; Fig. 4G–I, Fig. 5G–I, and Fig. 6G–I) and microglia (OX-42; Fig. 4J–L, Fig. 5J–L, and Fig. 6J–L). In control rats, similar patterns of cellular localization of annexin isoforms were observed with relatively low labeling intensity (data not shown), confirming the immunocytochemical results shown in Figure 3. The immunofluorescent double-labeling study, along with the immunohistochemical results described above, thus revealed that all three annexin subtypes were increasingly expressed qualitatively in neurons and glial cells of the spinal cord in areas close to a contusive SCI, as compared to the sham-operated controls.
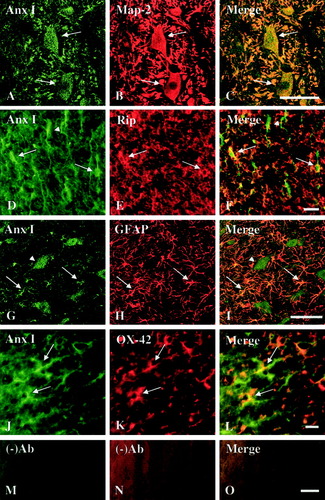
Cellular localization of annexin I (Anx I) expression in the injured spinal cord. Immunofluorescence double labeling shows colocalization of Anx I-IR (A,D,G,J) and cell-specific markers (B,E,H,K) in the injured spinal cord on Day 7 after SCI. A–C: Anx I-IR (A, arrow) was localized to neurons with MAP-2-IR (B, arrow) and can be appreciated in the merged image (C, arrow). D–F: Anx I-IR (D, arrow) was localized in oligodendrocyte with RIP-IR (E, arrow) as seen in the merged image (F, arrow). G–I: Anx I-IR (G, arrow) was colocalized in astrocytes (GFAP-IR; H, arrow), as seen in the mergence (I, arrow). J–L: Anx I-IR (J, arrow) was colocalized in microglia (OX-42-IR; K, arrow). M–O: No primary antibody control. Note that the morphologically identified neuron in the ventral horn was positive for Anx I (G and I, no-tail arrow) but negative for GFAP (H). In addition, (A) and (G) were taken from the gray matter whereas (D) and (J) were taken from the white matter. Scale bar = 50 μm.
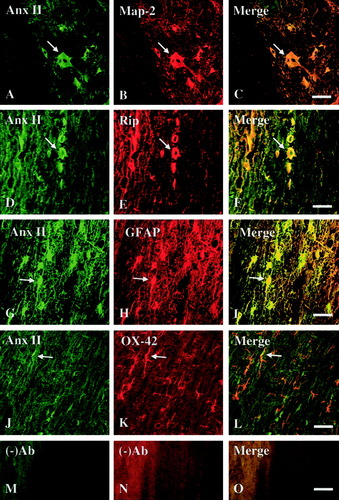
Cellular localization of annexin II (Anx II) expression in the injured spinal cord. Immunofluorescence double labeling shows colocalization of Anx II-IR (A,D,G,J) and cell-specific markers (B,E,H,K) in the injured spinal cord on Day 14 after SCI. A–C: Anx II-IR (A, arrow) was localized to neurons with MAP-2-IR (B, arrow) and can be appreciated in the mergence (C, arrow). D–F: Anx II-IR (D, arrow) was localized in oligodendrocytes with RIP-IR (E, arrow) as seen in the mergence (F, arrow). G–I: Anx II-IR (G, arrow) was localized in astrocytes (GFAP-IR; H, arrow) as seen in the mergence (I, arrow). J–L: Anx II-IR (J, arrow) was localized in microglia (OX-42-IR; K, arrow). M–O: No primary antibody control. Note that (A) was taken from the gray matter whereas (D), (G), and (J) were taken from the white matter. Scale bar = 50 μm.
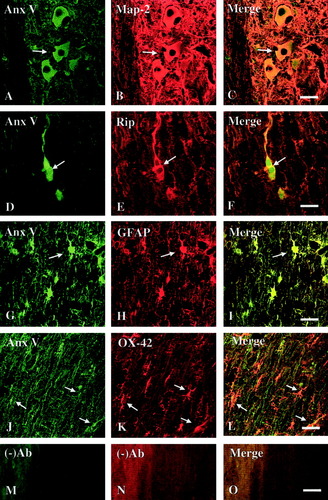
Cellular localization of annexin V (Anx V) expression in the injured spinal cord. Immunofluorescence double labeling shows colocalization of Anx V-IR (A,D,G,J) and cell-specific markers (B,E,H,K) in the injured spinal cord on Day 14 after SCI. A–C: Anx V-IR (A, arrow) was localized to neurons with MAP-2-IR (B, arrow) and can be seen in the mergence (C, arrow). D–F: Anx V-IR (D, arrow) was localized in oligodendrocyte with RIP-IR (E, arrow) as seen in the mergence (F, arrow). G–I: Anx V-IR (G, arrow) was localized in astrocytes (GFAP-IR; H, arrow) as seen in the mergence (I, arrow). J–L: Anx V-IR (J, arrow) was localized in microglia (OX-42-IR; K, arrow). M–O: No primary antibody control. Note that (A) was taken from the gray matter whereas (D), (G), and (J) were taken from the white matter. Scale bar = 50 μm.
DISCUSSION
To our knowledge, this is the first study demonstrating temporal and spatial patterns of annexin expression and their cellular localization after traumatic SCI in adult rats. We demonstrated that all three annexin subtypes were increasingly expressed after SCI, as compared to that in the sham-operated controls. Peak expression of each annexin isoform, however, was somewhat different. Although the expression of annexins I and V peaked at 7 days, annexin II reached its maximal level at 14 days. Interestingly, both annexins II and V remained at their elevated levels for a long duration (annexin II for at least 28 days; annexin V for at least 56 days) as compared to a relatively short elevated period for annexin I (3–14 days). RT-PCR analysis demonstrated that expression of all three annexin isoforms was increased at the mRNA level in the injured spinal cord, which correlated well with increased expression of these annexin isoforms at the protein level after the injury. These data suggest that changes in annexin protein expression were a result of changes in gene expression of annexins.
Cellular Localization of Annexins
Our immunohistochemistry data demonstrated an increase in expression of all three annexin isoforms at and near the site of injury as compared to expression in sham-operated controls. The immunofluorescence double-labeling study further revealed that all three annexins were localized in neurons, oligodendrocytes, astrocytes, and microglia in both the sham-operated and spinal cord- injured rats. These results are in contrast to the studies in which annexin I was not detected in neurons and glial cells in the normal spinal cord (Fava et al., 1989; Elderfield et al., 1993). Our results are in agreement, however, with other reports demonstrating the presence of annexins I, II, and V in neurons and glial cells in the normal rat spinal cord (Naciff et al., 1996). In addition, annexin I was localized in neurons, astrocytes, oligodendrocytes, and microglia in the brain (McKanna, 1993; Eberhard et al., 1994; Go et al., 1994; Mullens et al., 1994; McKanna and Zhang, 1997; Voermans et al., 1997; Young et al., 1999) and annexin V was detected in cerebellar glial cells (Woolgar et al., 1990; Spreca et al., 1992). Furthermore, elevated annexin II immunoreactivity was found in neurons and astrocytes of the acute anoxic/ischemic brain (de la Monte et al., 1995). In the present study, localization of annexins I, II, and V in neurons and glial cells in sham-operated and SCI rats thus not only confirmed basal level expression of these molecules in the normal spinal cord, but also demonstrated their increase in expression after SCI.
Possible Actions of Annexins
The present study shows that expression of annexins I, II, and V was significantly increased in the spinal cord after injury; however, the pathophysiologic relevance of the elevated levels of these molecules after traumatic SCI remains to be determined. Increased annexin expression may represent an inherent protective mechanism that protects tissue from further damage caused by secondary injury. It has been suggested that annexins I, II, and V are potent anti-inflammatory agents (Perretti, 1994; Raynal and Pollard, 1994) and administration of annexins in various animal models exerted significant neuroprotective effects. Neurotrophic effects of annexins I and V on cultured neurons have also been demonstrated in vitro (Takei et al., 1994; Mizuno et al., 1998). Recently, the anti-inflammatory effects of annexin I have also been demonstrated in a gene knockout mouse model (Roviezzo et al., 2002; Hannon et al., 2003). The elevated levels of annexin expression observed in the current study may thus indicate induction of an inherent neuroprotective mechanism after SCI that could be beneficial for tissue repair. Because increased expression of annexins in the present study occurred relatively late after SCI, the pathophysiologic significance of annexin expression in SCI remains unclear. The inflammation is a protective process that is time dependent and regulated by endogenous mediators (Perretti and Gavins, 2003). These endogenous mediators appear at different phases of inflammation and are involved in controlling inflammatory reaction from potential damage (Perretti and Gavins, 2003). These endogenous mediators including annexins function at various phases of inflammation to ensure the timing and extent of inflammation reaction after a particular insult. In general, at early phases of inflammation, proinflammatory mediators such as cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-1β play a major role whereas at late phases of inflammation, anti-inflammatory mediators such as annexins start to function (Perretti and Gavins, 2003). The relatively later expression of the annexins in the present study may thus play an anti-inflammatory role that controls the extent of inflammation and protects tissue from further damage at later phases of SCI.
Possible Mechanisms Underlying Annexin-Mediated Actions
Although annexins have been indicated as strong anti-inflammatory and neuroprotective mediators, mechanisms underlying their functions remain to be fully understood. Recent studies have highlighted the following four underlying mechanisms.
First, annexins may exert anti-inflammatory effects by inhibiting PLA2 activity. A series of experiments demonstrated that all three annexins (I, II, and V) inhibited PLA2 activity (Flower, 1990; Raynal and Pollard, 1994). PLA2 is a diverse family of enzymes that hydrolyze the acyl bond at the sn-2 position of glycerophospholipids to produce free fatty acids and lysophospholipids. These products are precursors of bioactive eicosanoids and platelet-activating factor (PAF), which lead to the concomitant production of reactive oxygen intermediates. The eicosanoids, PAF, and reactive oxygen intermediates are well-known mediators of tissue injury and inflammatory processes and have been implicated in pathologic states of the central nervous system (CNS) including SCI (Bonventre, 1996; Farooqui et al., 1997). In addition, activation of PLA2 results in cleavage of membrane phospholipids that, in turn, leads to cell damage (Klein, 2000).
Second, annexins may downregulate leukocyte/monocyte activation (Maridonneau-Parini et al., 1989; Sudlow et al., 1996; Euzger et al., 1999). A hallmark of inflammation is the mobilization of blood-borne leukocytes across microvessels to kill and remove the invading pathogen and damaged tissue (Perretti and Gavins, 2003). Secreted annexin I was shown to inhibit neutrophil and monocyte infiltration in experimental models of inflammation (Perretti et al., 1993; Perretti and Flower, 1993; Harris et al., 1995; Getting et al., 1997; Lim et al., 1998). In addition, annexin I protected against ischemia/reperfusion injury by affecting neutrophil migration (Cuzzocrea et al., 1997). In contrast to the inhibition of PLA2 activation by multiple annexin isoforms, neutrophil infiltration was inhibited specifically by annexin I. Other annexin isoforms such as annexins II, IV, and V were not found to have inhibitory effects on neutrophil infiltration (Walther et al., 2000).
Third, annexins may inhibit the expression and release of proinflammatory cytokines such as TNF-α and IL-1β. For example, annexin I suppressed the release of both TNF-α and prostaglandin E2 (PGE2) from stimulated human peripheral mononuclear cells (Sudlow et al., 1996). Human recombinant annexin I inhibited TNF-α release and reduced circulating TNF-α and IL-1β levels in endotoxemic IL-6 knockout mice (de Coupade et al., 2001). Also, upregulation of annexin I inhibited TNF-induced apoptosis (Wu et al., 2000).
Finally, annexins may exert anti-inflammatory effects by binding directly to lipid inflammatory mediators, thereby inhibiting their interactions with cellular receptors or accessory binding proteins. It has been shown that annexin V is a regulator of phosphatidylserine (PS)-catalyzed inflammation and coagulation during apoptosis (Reutelingsperger and van Heerde, 1997). Annexin V binds with high affinity to negatively charged phospholipids such as PS in the presence of Ca2+. Once PS is exposed at the cell surface, it exhibits procoagulant and proinflammatory activities. Annexin V binds to the PS-exposing apoptotic cell and can thereby inhibit procoagulant and proinflammatory activities of the dying cell (Reutelingsperger and van Heerde, 1997). This property of annexin V that selectively binds to PS on the cell surface is the basis for annexin V use as a tool to monitor cell apoptosis.
In conclusion, our study demonstrates that SCI induced upregulation of annexins I, II, and V and that their expression was localized in neurons and glial cells. To elucidate the therapeutic effects of annexins on SCI, additional studies are required to investigate the potent effects of exogenously delivered annexins or their blocking antibodies in vitro and in vivo. Furthermore, the molecular mechanisms underlying the action of annexins and their regulations after SCI need to be elucidated. Experiments along these lines are currently in progress in our laboratory.
Acknowledgements
We thank Dr. K. Zhang for his expert help with confocal imaging. We also thank Norton Healthcare and University of Louisville through the James R. Petersdorf Endowment.




