Role of LRP in TGFβ2-mediated neuronal uptake of Aβ and effects on memory
Abstract
There is increasing evidence that soluble amyloid-β peptide (Aβ) uptake into neurons is an early event in the pathogenesis of Alzheimer's disease (AD). Identification of the early events leading to neuronal dysfunction is key to developing therapeutic strategies, but relative roles of receptors and factors modulating uptake are poorly understood. Studies have shown that transforming growth factor β (TGFβ), particularly TGFβ2, can influence the targeting of Aβ to cells in vitro. TGFβ2 can target Aβ to neurons in organotypic hippocampal slice cultures (OHSC). We examine a specific mechanism for TGFβ2-mediated targeting of Aβ to neurons. The receptor-associated protein (RAP), a low-density lipoprotein receptor-related protein (LRP) antagonist, can attenuate the cellular targeting of Aβ both in vitro and in vivo and prevent Aβ/TGFβ2-induced memory retention deficits. Using both in vitro and in vivo methods, we identify LRP as playing a role in TGFβ2-mediated Aβ uptake, neurodegeneration, and spatial memory impairment. © 2004 Wiley-Liss, Inc.
The low-density lipoprotein receptor-related protein (LRP) is a multifunctional receptor mediating internalization and degradation of lipoproteins and protease/protease inhibitor complexes (Strickland et al., 1995), α2-macroglobulin (A2M) (Borth, 1992), Apolipoprotein E (ApoE) (Herz et al., 1988), and the amyloid precursor protein (APP) (Kounnas et al., 1995). Interestingly, A2M, ApoE, and APP are all genetically associated with Alzheimer's disease (AD) (Strittmatter et al., 1993; Blacker et al., 1998) and found in senile plaques (Rebeck et al., 1995). Evidence for a role for LRP in AD comes from genetic studies (Kang et al., 1997) and confirmation by five other laboratories (Baum et al., 1998; Hollenbach et al., 1998; Kamboh et al., 1998; Lambert et al., 1998; Wavrant-DeVrieze et al., 1999). The impact of LRP polymorphisms is unclear, however, and levels of LRP protein do not always correlate with cognitive decline in AD (McIlroy et al., 2001; Sanchez-Guerra et al., 2001; Causevic et al., 2003). The mechanisms by which LRP and its ligands may contribute to AD pathogenesis are unknown but it is suggested to play a role in the balance between amyloid-β peptide (Aβ) synthesis and clearance (Arelin et al., 2002).
Generation and deposition of Aβ in the brain are believed to be crucial events in AD pathogenesis (Selkoe, 1994), whereas sequestration and clearance of Aβ limits amyloid deposition (Ladu et al., 1994; Rebeck et al., 1995; Kang et al., 1997). For example, A2M can complex with Aβ, leading to Aβ uptake and degradation through the LRP-mediated pathway (Du et al., 1997; Narita et al., 1997). Because LRP is highly expressed in the central nervous system (CNS) (Wolf et al., 1992), internalization of ApoE-enriched lipoprotein particles by way of the LRP may impact neuronal membrane remodeling (Holtzman et al., 1995). Interactions between ApoE and Aβ are also thought to be critical in AD pathogenesis and Aβ deposition (Rebeck et al., 1993; Ladu et al., 1994). This interaction seems to involve LRP, because ApoE-enhanced soluble Aβ uptake into synaptosomes is inhibited by the LRP antagonist receptor-associated protein (RAP) (Gylys et al., 2003).
Recent studies have revealed a potential link between LRP and TGFβs (Hussaini et al., 1996; Schneider and Nimpf, 2003), and LRP-1 has been shown recently to be identical to TGFβ receptor V (Huang et al., 2003). TGFβ expression is altered in AD (Chao et al., 1994; Flanders et al., 1995; Vawter et al., 1996; Lesne et al., 2003) and TGFβ affects Aβ accumulation (Frautschy et al., 1996; Wyss-Coray et al., 1997) and Aβ clearance by activated microglia (Wyss-Coray et al., 2001). TGFβ isoforms 1, 2, and 3 are expressed within neurons, astrocytes, and microglia (Constam et al., 1992; Krieglstein et al., 1995); TGFβ isoforms and receptors have a different regional distribution in the brain (Pelton et al., 1991) and they are considered to be independent regulatory molecules (Sanford et al., 1997). TGFβs are expressed in a number of CNS insults, including traumatic injury (Klempt et al., 1992; Lindholm et al., 1992; Logan et al., 1992), hypoxic injury (del Rio-Hortega, 1932,) and neurodegenerative diseases such as Parkinson's disease (Vawter et al., 1996) and AD (Chao et al., 1994; Flanders et al., 1995; Lippa et al., 1995; Peress and Perillo, 1995; Vawter et al., 1996). Recent studies show that the presence of ApoE and TGFβ1 can trigger vascular β-amyloidosis by increasing intracellular formation of stable deposits of Aβ/ApoE (Mazur-Kolecka et al., 2003).
Although much of the focus in TGFβ research has been on isoform 1 (Van der Wal et al., 1993; Frautschy et al., 1996), recent studies on isoform 2 have led to some intriguing findings that define specific roles for TGFβ2. The TGFβ2 isoform is likely most relevant to AD pathogenesis because both protein and mRNA are elevated. TGFβ2 is localized in neurofibrillary tangle (NFT)-bearing neurons, astrocytes (Flanders et al., 1995), and plaque neurites (Peress and Perillo, 1995). Interestingly, TGFβ2 is a binding protein for soluble APP derivatives (Bodmer et al., 1990) and both TGFβ1 and 2 can bind Aβ and accelerate oligomerization (Mousseau et al., 2003). This is consistent with the many forms of amyloid-producing diseases, including AD and prion neurodegenerative diseases, that exhibit early neuronal loading of TGFβ2 (Tashiro et al., 1998). Previous results from our lab demonstrate important functional differences among the TGFβ isoforms in their ability to alter cellular distribution and degradation of Aβ (Harris-White et al., 1998). TGFβ exacerbation of Aβ neurotoxicity has been previously reported to occur in the absence of TGFβ receptors (Mousseau et al., 2003). As the role of TGFβ2 and its receptors in Aβ-dependent toxicity in vitro and in vivo remain unclear, the current study examines a mechanism for altered uptake of Aβ by TGFβ2 into neurons through the LRP pathway, and effects on neurodegeneration and cognition in vivo.
MATERIALS AND METHODS
Animals
Surgical and animal procedures were carried out with strict adherence to the current guidelines set out in the NIH Guide for the Care and Use of Laboratory Animals. Accordingly, all experiments involving animals were approved by the appropriate UCLA and VA institutional committees. ICR mice (Jackson ImmunoResearch) were used for organotypic hippocampal slice culture (OHSC) studies and C57/bl6 mice (Jackson ImmunoResearch) were used for in vivo studies.
Organotypic Hippocampal Slice Culture
Hippocampal slice cultures were prepared according to the method of Stoppini et al. (1991) with some modification. Briefly, slice cultures were prepared from 6–7-day-old ICR mouse pups. Slices were cut at 400 μm on a Stoelting tissue chopper and transferred to Costar membrane inserts (0.4 μm). Initially, slice culture media consisted of minimal essential medium (MEM) + HEPES (50%; Gibco, Grand Island, NY), heat-inactivated horse serum (25%; Sigma, St. Louis, MO), and Hank's balanced salt solution (25%; Sigma) containing a total of 6.5 mg/ml glucose and penicillin-streptomycin (50 U/ml-0.05 mg/ml). After the first 4 days in culture, slice media was replaced gradually with a serum-free media prepared by replacing the horse serum with the supplement TCM (final concentration 2%; ICN Pharmaceuticals, Costa Mesa, CA). The exchange of serum-containing media with serum-free media was as follows: (1) 75% serum media/25% serum-free media on Day 4 in vitro; (2) 50% serum media/50% serum-free media on Day 6 in vitro; and (3) 100% serum-free media on Day 7. Experimental treatment of OHSC began on Day 7. Final concentrations of reagents were: 10 μg/ml Aβ 40 (2.2 nM); 1 μg/ml Aβ 42 (220 pM); 10 ng/ml TGFβ2 (400 pM); and 250 nM RAP. Treatment remained on OHSCs for 4 days and media was changed as normal (no additional treatment added) for 7 additional days. Slices were either fixed or frozen 11 days from the start of treatment.
Aβ Preparation
Aβ oligomers were prepared by monomerizing lyophilized Aβ with hexafluoroisopropanal (HFIP), followed by evaporation and reconstitution in 4 mM HEPES buffer. After 24-hr incubation at 4°C to enhance aggregation of monomer, Aβ42 oligomers were diluted to final pump concentration (for ICV infusion) with 4 mM HEPES buffer, pH 8.0 (5 μg/100 μL) or diluted in culture media (in vitro studies). Aβ was obtained from C. Glabe (University of California, Irvine) and TGFβ2 from R & D Systems (Minneapolis, MN).
Surgery
Aβ pump solution (100 μL) was infused into the right ventricle through a stainless steel cannula (coordinates to Bregma in mm were: lateral: +0.1 posterior: −0.05, and ventral: −0.25; ventral coordinate from dura mater: −0.2) of female C57/bl6 mice using mini-osmotic Alzet pumps (1002; Durect Corporation, Cupertino, CA) over a 2-week period. Stainless steel cannulas were cut to a custom length (2.7 mm; Plastics One, Roanoke, VA). Per hour delivery was 12.5 ng Aβ42 (5 μg total), 0.058 ng RAP (0.0195 μg total), and 0.3 ng TGFβ2 (10 ng total) in 4 mM HEPES, pH 8.0. The pump released 0.25 μL/hr into a ventricular volume of 35 μL (140-fold dilution), and 18 μl of new cerebrospinal fluid (CSF) was produced hourly, resulting in projected Aβ concentrations of 80 nM in CSF during infusion. After ventricular penetration into neuropil and normal catabolism, soluble Aβ levels of about 5 μg/g protein were maintained in circumventricular brain areas. We were attempting to mimic soluble brain parenchyma Aβ levels known to correlate well with cognitive decline and neurodegeneration in both AD brain and animal models. This produces low levels of soluble Aβ that enable assessment of factors that enhance or modulate toxicity without amyloid plaques.
Immunocytochemistry and Image Analysis
At the conclusion of the OHSC experiments, slices were submersion-fixed in 4% paraformaldehyde for 1 hr followed by three rinses in Tris-buffered saline (TBS). Slices were then cryopreserved in increasing concentrations of sucrose (10, 15, and 20%), sectioned at 10 μm on a crysostat (Microm Instruments Model HM505E) and mounted onto gelatin-coated slides. At the conclusion of the in vivo studies, animals were perfused with cold HEPES buffer containing protease inhibitors. Half of the brain was dissected and snap frozen in liquid nitrogen for biochemistry. The other half brain was immersion fixed in 4% paraformaldehyde and paraffin embedded; 10-μm sections were cut on a microtome (Microm Instruments Model HM335E), and then mounted onto poly-L-lysine/gelatin-coated glass slides.
For Aβ immunohistochemistry of OHSC (10G4 antibody against Aβ 5-13; Yang et al., 1994), slices were pretreated with 70% trichloroacetic acid (6 min) and rinsed with TBS. Endogenous peroxidase activity was suppressed using a peroxidase suppressor buffer (Vector Labs) for 30 min at room temperature and nonspecific binding sites were blocked with Superblock Blocking Buffer (Pierce, Rockford, IL) for 1 hr at room temperature. The 10G4 antibody was applied (overnight at 4°C) to slices at a 1:500 dilution in TBS containing 0.1% Tween 20, 3% bovine serum albumin (BSA), and 8 mM sodium azide. Slices were then rinsed and incubated with a biotinylated anti-mouse secondary antibody (1:500 in TBS plus Tween 20; 1 hr at room temperature). Slices were rinsed and incubated with ABC solution (Elite ABC kit; Vector Labs) for 45 min at room temperature. Diaminobenzidine-metal enhanced chromogen (Pierce) was used to reveal 10G4 antibody binding, and the specificity of Aβ staining was verified by preincubating 10G4 antibody in the presence of Aβ1–40 peptide before immunostaining. Preabsorption of 10G4 antibody was carried out by adding 1 μL of antibody to 50 μL of 3% BSA in TBS containing 30 μg Aβ40 peptide. The solution was incubated overnight at 4°C, brought up to 800 μL with 3% BSA in TBS, and centrifuged at 14,000 × g for 30 min at 4°C. The resulting supernatant was used for immunocytochemistry. To identify Aβ in mouse brain sections, sections were incubated overnight at 4°C (1:100) with DAE polyclonal antibody (anti-Aβ1–13) made against synthetic peptides Aβ1–13 and named after the first three amino acids of the Aβ peptide, Asp-Ala-Glu (Lim et al., 2000), or with anti-Aβ42 antibody (1:50; BioSource International., Camarillo, CA). For fluoro-Aβ studies, the fluorescein signal was amplified with tyramide signal amplification (TSA) Direct system (NEN, Boston, MA).
All histologic and immunohistochemical images were acquired with a Nikon E400 Eclipse microscope equipped with a SPOT digital camera. Images were analyzed on a PC computer using Image Pro software. Throughout the image analysis process, all sections for all treatments were carried out with identical microscope light and condenser settings. Each capture was exposed to the same computer subroutine to minimize biases of the microscopist. Most importantly, the density slice thresholding was maintained constant throughout analysis. Data was exported from Image Pro to Microsoft Excel for further analysis.
Fluoro-Jade
To quantitate degenerating cells, we stained brain sections with the degeneration stain Fluoro-Jade B (Histo-Chem, Jefferson, AR) (Schmued et al., 1997; Schmued and Hopkins, 2000).
ApoE Enzyme-Linked Immunosorbent Assay
OHSC were prepared from transgenic mice expressing human ApoE alleles E3 and E4 (Mahley et al., 1995) and treated with 10 ng/ml TGFβ2 (R & D) for 2 days. Human ApoE was measured from collected media by sandwich enzyme-linked immunosorbent assay (ELISA) using 2E1 monoclonal antibody to ApoE (Boehringer-Mannheim, , Indianapolis, IN) to capture and goat anti-human ApoE antibody to detect with alkaline phosphatase as reporter, as described previously (Gracia et al., 1994).
Western Blot
Frozen brain tissue was weighed and homogenized in 15× volume of TBS with protease inhibitors (0.05 mM fenvalerate, 0.05 mM cantharidin, 1 mM sodium vanadate, 1 mM sodium pyrophosphate, 0.02 mM phosphoramidone, 1 mM EGTA, 0.1 mM EDTA, 0.02 mM leupeptin, 0.0015 mM aprotinin, and 0.5 mM AEBSF) and phosphatase inhibitors (50 mM sodium fluoride and 0.25 mM phenylmethylsulfonylfluoride [PMSF]). Samples were sonicated on ice 3× 10 sec, transferred to centrifuge tubes, and centrifuged at 100,000 × g for 30 min at 4°C. Supernatant fractions were loaded onto a 10% Tris-glycine gel to evaluate NR2B levels (NMDAR2B; Chemicon, Temecula, CA) and the 25-kDa synaptosomal-associated protein (SNAP25; Sternberger Monoclonals Inc., Lutherville, MD). Protein content was determined to equalize protein concentrations across samples. Bands were visualized using ECL techniques (Amersham, Piscataway, NJ) with subsaturating exposures and quantified using a BioRad scanning densitometer.
Morris Water Maze
Mice were subjected to the Morris water maze in a paradigm similar to that described for Aβ-infused rats (Winkler et al., 1994; Frautschy et al., 2001) with the following modifications for mouse. A 1.525-m diameter white tank with nontoxic white paint was used for contrast with black mice, with a platform diameter of 12.5 cm and water temperature was maintained at 25°C. Distal stationary cues were placed around the walls of the room, and no proximal, mobile, or auditory cues were present during trials. Before the first trial of each day, mice were placed on the platform for 60 sec for spatial orientation. Mice were then removed from the platform, and their heads covered with a drape until they were placed in a random start position facing the tank wall. Initially, mice were trained for 1.5 days in a visible platform test (four trials/block, one block in the morning and another block in the afternoon; three blocks total) for elimination of animals with motor or visual deficits. Acquisition of spatial memory was evaluated subsequently in six blocks of a hidden platform test over three days (four trials/block, 60 sec maximum, two blocks/day). After a 3-day delay after the last acquisition testing, retention was evaluated in a probe test with platform removed from the tank and swim paths evaluated for 20 sec. Normal mice swim to and persistently cross the correct former location of the platform to an extent assumed to be proportional to the strength of their memory, whereas animals with hippocampal dysfunction show a weaker tendency to swim in the correct quadrant with no spatial bias to the quadrant of the pool to which they had been trained (i.e., NE or SW). We allowed a 3-day delay in probe trial, because preliminary studies showed Aβ had little impact on retention unless probe trials were delayed (unpublished observations). Percent time or path in quadrants, path lengths, and acquisition and swim speeds were determined by HVS Image software and video tracking system (HVS Image Ltd., Buckingham, UK).
Statistical Analysis
Stat View statistical analysis software was used to carry out analysis of variance (ANOVA), post hoc analyses, and t-testing.
RESULTS
In Vitro: OHSC
Using fluorescein isothiocyanate (FITC)-labeled Aβ42 to treat OHSC, we confirmed our previous observation that TGFβ2 targets exogenous Aβ to cells in the CA1 subfield of the OHSC. Compared to treatment with FITC-Aβ42 alone, which resulted in relatively more diffuse fluorescence throughout the OHSC, the presence of TGFβ2 drove a rapid (24-hr) uptake of exogenous FITC-Aβ42 into CA1 neurons (data not shown).
Extending these findings, we explored a mechanism for TGFβ2-mediated targeting of Aβ to neurons via induction of ApoE and uptake by the LRP receptor. After 4 days of exposure, TGFβ2 nearly doubled ApoE release from OHSC (100 ± 8.1% for control vs. 182.2 ± 24.1% for TGFβ2-treated OHSC; P = 0.03, t-test). Using OHSC, we show that the LRP receptor antagonist RAP (250 nM) (Bu, 1998) can effectively block TGFβ2-mediated targeting of Aβ to CA1 hippocampal neurons. Figure 1 shows a Western blot of the Aβ42 preparation 2 weeks and 4 weeks after it was prepared. The blot shows predominately monomer and dimer in the 4–8 kDa range at 2 weeks. Figure 2 shows representative micrographs for immunolocalization of Aβ in Aβ + TGFβ2-treated slices and attenuation of staining by RAP. To quantitate this effect, we counted Aβ-immunoreactive cells in the CA1 pyramidal layer (800 × 100 μm area) and show that RAP does significantly attenuate TGFβ2-mediated targeting of Aβ to neurons (Fig. 2B; P = 0.004, t-test). Furthermore, RAP can block Aβ + TGFβ2-induced neurodegeneration in the slice cultures (Fig. 3), as indexed by Fluoro-Jade-positive cell counts in the CA1 region of the OHSC (800 × 100 μm area; treatment ANOVA P = 0.0058, for homogeneity of variance, data was square root transformed). No Fluoro-Jade-positive cells were found in the group treated only with TGFβ2 (not shown on graph). Fluoro-Jade is a dead neuron-specific probe that correlates well with propidium iodide (Schmued et al., 1997) and can be used to either count dead neurons or estimate total death per area. We also quantitated SNAP25 by Western blot analysis. There was a trend for protection of SNAP25 levels by RAP but the results did not reach significance (data not shown).
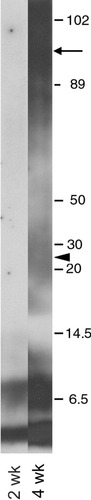
Western blot analysis of Aβ42 preparation. Aβ was monomerized then incubated for 24 hr at 4°C in 4 mM HEPES (pH 8). To evaluate stability of soluble Aβ in pump, we incubated samples at 37°C for 2 weeks (infusion period) and 4 weeks and ran samples on a tricine gel for Western blot analysis After 2-week incubation (the infusion period), all Aβ immunoreactivity (ir) was in the 4–8 kDa range with the low salt, high pH buffer preventing Aβ42 aggregates. After the infusion period, however, soluble Aβ preparation would not have remained stable, because longer incubations (4 weeks) at 37°C resulted in some aggregates (arrow) and higher molecular weight oligomer formation (arrowhead).

Attenuation of TGFβ2-mediated uptake of Aβ by RAP. Murine OHSC were treated with Aβ (40 + 42) (2.2 nM and 220 pM, respectively), and TGFβ2 (400 pM) either with or without 250 nM RAP. Sections (10 μm) were stained with the Aβ antibody 10G4. A: Arrows in upper panel indicate Aβ immunoreactivity (Aβir) in neurons in the CA1 region of Aβ + TGFβ2-treated OHSCs. Lower panel indicates that addition of RAP (to Aβ + TGFβ2-treated OHSCs) eliminated Aβ labeling of CA1; the only remaining Aβ staining was diffuse and associated with microglia-like cells (arrows). Scale bar = 50 μm. B: Aβir neuron counts in CA1 region of OHSC. RAP co-treatment reduces the number of neurons that are Aβir after Aβ + TGFβ2 treatment. *P = 0.004, t-test. Bars are mean ± standard error of the mean (SEM); n = 6–9 slices per group.
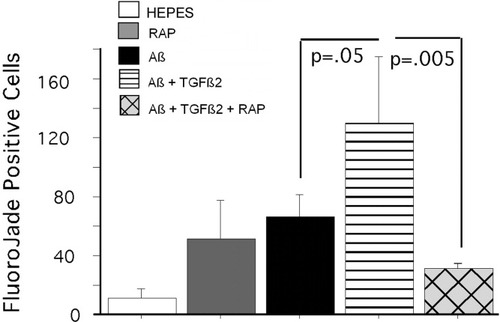
Fluoro-Jade staining in OHSC. Sections (10 μm) from murine OHSC were stained with Fluoro-Jade as an indicator of neurodegeneration. Image analysis of CA1 layer showed that TGFβ2 (400 pM) exacerbated Aβ toxicity in the OHSC (P = 0.05, Aβ vs. Aβ + TGFβ2) and RAP (250 nM) attenuated the neurotoxicity associated with Aβ + TGFβ2 treatment (P = 0.03, Aβ + TGFβ2 vs. Aβ + TGFβ2 + RAP). Bars are mean ± SEM; n = 5–6 slices per group.
In Vivo: ICV Infusion Model
ICV infusion of Aβ with TGFβ2 resulted in widespread parenchymal plaque-like deposition and neuronal labeling of Aβ. Figure 4A–F shows Aβ immunostaining (left, anti-DAE) and cresyl violet (right) staining of the temporal cortex from representative brain sections from these mice. Figure 4A/B and 4C/D are representative micrographs from two mice infused with Aβ + TGFβ2. Figure 4E/F show the same region in a mouse infused with Aβ + TGFβ2 + RAP. In Figure 4A and 4C, neuronal labeling of neurons in layer III/IV of the temporal cortex is easily visible. Cresyl violet staining of adjacent tissue sections reveals darkly stained and abnormally shaped cells in the same region as the Aβ staining. We quantitated cell-associated DAE immunoreactivity (DAEir) in the same region shown in Figure 4A–F (Fig. 4G). ANOVA of DAEir revealed a significant treatment effect (P = 0.0023 for square root-transformed values). DAEir was increased in mice infused with Aβ + TGFβ2 (P = 0.022 vs. Aβ alone) and coinfusion of RAP with Aβ + TGFβ2 attenuated this increase (P = 0.014 vs. Aβ + TGFβ2). We did not observe CA1 hippocampal pyramidal cell Aβ loading using the DAE antibody, but we did observe neuron staining using an Aβ42-specific antibody (BioSource International). Aβ was infused into the lateral ventricle at a low dose. Where Aβ ends up in the brain can be influenced by proximity to the infusion cannula, direction and volume of CSF flow, and other clearance mechanisms at work in the ventricular space. The hippocampal Aβ42 staining was located in the CA2/3 area close to the ventricle. As in the cortex, there were many darkly stained and abnormally shaped cells stained with cresyl violet in the hippocampus.
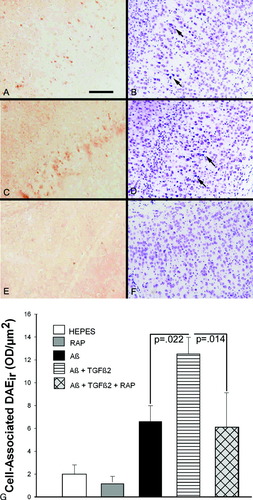
Targeting of Aβ to neurons in vivo. Mice were chronically infused with Aβ, TGFβ2 or RAP (or a combination) into the lateral ventricle using a 2-week mini-osmotic pump, and sacrificed at 14 weeks post-infusion. A–D: Brains of two separate mice infused with Aβ + TGFβ2. A and C, 10 μm sections stained with the polyclonal Aβ antibody DAE; B and D, adjacent sections stained with cresyl violet (CV). Aβir was apparent in neurons of cortical layers III/IV of the Aβ + TGFβ2-treated mice. CV staining of adjacent sections revealed degenerating cells in the same regions as the Aβir (see arrows on B and D). E, F: Mouse infused with Aβ + TGFβ2 + RAP. RAP attenuated the Aβir in the cortical neurons (E) and the degeneration (F). G: Quantitation of cell-associated Aβir in the ICV-infused mice. AB treatment alone increased DAEir over HEPES infusion (P = 0.03 HEPES vs. Aβ). Aβ + TGFβ2-infused mice had more cell-associated Aβir than Aβ treatment alone (P = 0.022, Aβ vs. Aβ + TGFβ2) and RAP attenuated this increase (P = 0.014, Aβ + TGFβ2 vs. Aβ + TGFβ2 + RAP). Scale bar = 50 μm. Bars are mean ± SEM; n = 6–11 mice per group.
Preliminary and published data (Frautschy et al., 2001; Nakamura et al., 2001) demonstrate delayed effects of Aβ on cognition. We therefore waited 12 weeks from the start of ICV infusion before testing mice for cognitive deficits. Figure 5 shows the schedule (Fig. 5A) and results of Morris water maze testing in the mouse ICV infusion study. Latencies to find a visible platform were similar across all treatment groups with no significant differences found between groups (Fig. 5B). Two mice were removed from the study (one mouse from the HEPES group and one from the TGFβ2 group) after failing to find the platform after three blocks, due to visual or motor deficits. After exclusion of visible platform failures (1% of animals), the group sizes were as follows: HEPES, n = 7; RAP, n = 11; TGFβ2, n = 8; Aβ, n = 8; Aβ + TGFβ2, n = 6, Aβ + RAP, n = 10; and Aβ + TFβ2 + RAP, n = 8). Regression analysis of acquisition data showed significant learning in all ICV-infused groups of mice (Fig. 5C). Figure 5D shows the interaction bars for combined blocks 5 and 6 of the acquisition training. By the end of the training, there were no significant differences in latencies between groups (P = 0.28). A 3-day delay followed by a 20-sec probe trial (removal of the platform) revealed striking treatment differences in retention memory. Probe trial data was analyzed for percent of time in the target quadrant (Fig. 5E) and percent of path in the target quadrant (Fig. 5F). ANOVA for probe trial data revealed a significant treatment effect for both % time in target quadrant (data log transformed for analysis; P = 0.05) and % path in target quadrant (P = 0.04). These data demonstrated that compared to Aβ42 treatment alone, Aβ plus TGFβ2 infused mice showed deficits in retention memory (P = 0.01 for % time and P = 0.04 for % path) and these deficits were prevented by coinfusion with RAP (P = 0.001 for time and P = 0.002 for path).
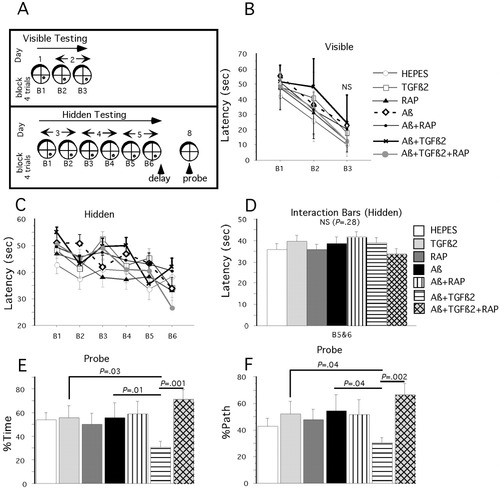
Retention memory deficits in Aβ + TGFβ2 infused mice. Morris water maze results from ICV-infused mice 12 weeks from start of infusion. A: Schematic outlining the course of Morris water maze testing over 8 days. B: Visible platform testing did not reveal any significant difference between treatment groups. C: Hidden platform (acquisition) testing. Regression analysis of acquisition data showed significant learning in all ICV-infused groups of mice. D: Block by treatment interaction bars from hidden platform testing demonstrated that by the end of the acquisition phase, there were no significant differences between treatment groups and all groups were performing similarly. E, F: Twenty-second probe (retention) trial carried out at the end of the acquisition testing. ANOVA for probe trial data revealed a significant treatment effect for both % time in target quadrant (P = 0.05) (E) and % path in target quadrant (P = 0.04) (F). Compared to Aβ42 treatment alone, Aβ + TGFβ2 infused mice showed deficits in retention memory (P = 0.01 for % time and P = 0.04 for % path) and these deficits were prevented by coinfusion with RAP (P = 0.001 for time and P = 0.002 for path). Bars are mean ± SEM; n = 6–11 mice per group.
After completion of behavior testing (14 weeks from start of ICV infusion), mice were perfused with cold HEPES buffer with protease inhibitors. The left side of the brain was dissected into regions and flash frozen in liquid nitrogen for biochemical analysis of synaptic markers. Spatial memory in rodents involves postsynaptic factors associated with NMDA receptor function and deficits in spatial memory tasks have been correlated with decreases in NMDA receptor subunit expression, particularly subunit NR2B (Clayton et al., 2002). Figure 6 shows the Western blot quantitation of NR2B levels in the entorhinal cortex of infused mice. ANOVA of NR2B data revealed a significant treatment effect (P = 0.04, data square root transformed). Infusion with Aβ + TGFβ2 reduced NR2B levels and coinfusion with RAP attenuated this effect (P = 0.024, Aβ + TGFβ2 vs. RAP + Aβ + TGFβ2).
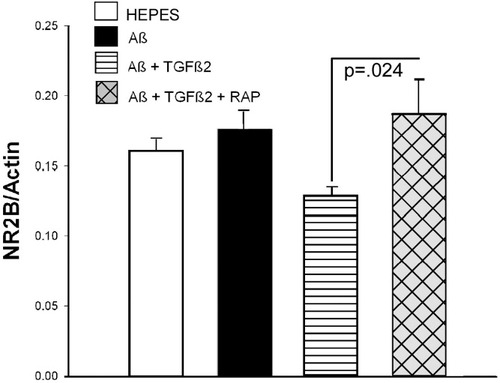
Loss of NR2B in Aβ + TGFβ2-infused mice. NR2B levels were quantitated by Western blot analysis. Mice infused with Aβ + TGFβ2 show a loss of NR2B levels in the entorhinal cortex. TGFβ2 exacerbated Aβ-induced NR2B loss (P = 0.03, Aβ vs. Aβ + TGFβ2) and RAP coinfusion prevented this loss (P = 0.024, Aβ + TGFβ2 vs. Aβ + TGFβ2 + RAP). Bars are mean ± SEM; n = 6–9 mice per group.
DISCUSSION
These data argue for a crucial role for TGFβ2 in driving neuronal Aβ uptake and targeting and increasing neurodegeneration of Aβ both in vitro and in vivo. Data support the hypothesis that TGFβ2 is driving extracellular uptake of Aβ and increasing intracellular Aβ. That targeting and neurotoxicity are blocked by RAP demonstrate mediation of this effect through an LRP-like receptor. These results and our observed TGFβ2 induction of ApoE levels in OHSC media suggest that LRP receptor function or numbers could be altered. These data are consistent with observations in AD brain that LRP and ApoE are associated with senile plaques in AD (Rebeck et al., 1995). These data are also consistent with in vitro studies showing that high-density lipoprotein (HDL)-like particles containing ApoE or ApoJ can transport Aβ to cells, particularly neurons (Beffert et al., 1998), where they bind and are internalized by lipoprotein receptors such as LRP.
Our results show that ICV infusion of Aβ or Aβ + TGFβ2 did not affect acquisition of spatial information because all groups of mice learned to locate a submerged platform in the Morris water maze. Retention of spatial learning, however, was impaired in the Aβ + TGFβ2-infused mice. Because retention of spatial memory learning is impaired after a 3-day interval without impairment of acquisition, TGFβ2 alters Aβ effects on the processing of spatial memory learning. Previous studies using Morris water maze testing have found similar results with acquisition and retention trials (Minetti et al., 1996). It is not uncommon that acquisition deficits are missed but probe deficits are detected, and this may result from acquisition tests being confounded by many variables such as mice not using spatial cues exclusively (e.g., egocentric and scanning strategies) to learn platform position (van Groen et al., 2002; Shukitt-Hale et al., 2004). Larger retention deficits in probe trials may be due also to the sensitivity of this test to detect pure spatial memory deficits. Alternatively, the probe test may be sensitive to detecting Aβ-specific deficits. For example, Westerman et al. (2002) showed that although acquisition impairments remained constant from middle age to old age in APP Swedish mutation mice accumulating Aβ, the probe retention deficits worsened.
In the present study, the first trial of each block in the acquisition phase was carried out immediately after mouse orientation on the platform, and the retention test was carried out after a 3-day interval. This may imply that TGFβ2 alters Aβ effects on the processing of spatial memory learning, having no impact on short-term spatial memory, but impairing retrieval or late stages of consolidation. Further characterization of the severity of spatial deficits will require tests with enhanced sensitivity such as multiple probe trial maze testing (Gallagher et al., 1993).
The factors and circumstances that induce neuron dysfunction and death in the AD brain are unclear. There is controversy as to whether Aβ in the form of plaques is neurotoxic or whether more soluble or intracellular Aβ correlates with severity of dementia. Aβ is present in intracellular compartments and evidence is rapidly growing in favor of intracellular accumulation of Aβ as an early event in AD pathology (Kim et al., 2003; Shie et al., 2003). D'Andrea et al. (2001) showed in AD brain that Aβ42 first accumulates in the perikaryon of pyramidal neurons as discrete granules that are cathepsin D (lysosome) positive. Lysis of these cells resulted in local, radial dispersion of their cytoplasmic contents in the extracellular space. Additional studies have confirmed Aβ accumulation in lysosomal compartments (Frautschy et al., 1998), and that loss of lysosomal membrane integrity is an early step in Aβ pathogenesis and may explain alteration in compartmentalizing extracellular and cytoplasmic components in the AD brain (Yang et al., 1998). Aβ binds avidly to LRP receptor ligands such as A2M and ApoE, and LRP-mediated clearance of Aβ can lead to Aβ in the lysosome. Furthermore, neuronal cell death in AD has been shown to correlate with ApoE uptake and intracellular Aβ stabilization (LaFerla et al., 1997).
ApoE likely serves multiple roles in the CNS, one of which may be to clear Aβ from the extracellular space through receptors like LRP. What is unclear is whether this function is neurotoxic or neuroprotective and in humans, the answer may depend on the isoform of ApoE. It has been suggested that ApoE4 promotes depolymerization of microtubules and thus ApoE may participate in maintaining integrity of the cytoskeleton (Mahley et al., 1995; Nathan et al., 1995). In studies looking at the different human isoforms of ApoE, there seems to be a differential accumulation of ApoE3 and E4 in neurons. Both isoforms are retained intact but ApoE3 shows greater cellular accumulation (Ji et al., 1998). The intracellular fate of ApoE is unknown but retention of ApoE within the cell is due most likely to association with the slow endosome to lysosome transport and may involve heparan sulfate proteoglycans (HSPGs) as chaperones (Ji et al., 1998). Previous studies have implicated the failure to degrade Aβ in the late endosome or secondary lysosome as a mechanism for the accumulation of Aβ in AD (Yang et al., 1998). Uptake through the LRP/ApoE pathway may indeed be a normal pathway for clearance of Aβ in normal human brain (Kang et al., 2000). Alterations in ApoE levels or LRP receptor function/number by factors such as TGFβ2, however, could cause this system to fail with neurotoxic results. In this study, we have shown that TGFβ2 increased ApoE release into OHSC media, yet the mechanism for this increase is unclear. Little is known about TGFβ2 in the CNS but previous studies have shown that TGFβ1 can increase ApoE mRNA in macrophages (Zuckerman et al., 1993) but does not increase ApoE mRNA or transcription in neurons and astrocytes (Laping et al., 1994).
Neuronal immunoreactivity for TGFβ2 seems specific for NFT neurons in Alzheimer's disease (Flanders et al., 1995); however, the mechanism of induction and the role of TGFβ2 in AD are unknown. TGFβs have been shown to play a role in synaptic plasticity and dendritic arborization and TGFβ2 is concentrated in the subsynaptic portions of mature muscle fibers (McLennan and Koishi, 1994; McLennan et al., 1998; Murakami et al., 1999) and this distribution is characteristic of proteins involved in synaptic function (Duclert and Changeux, 1995). Synaptic effects may be mediated by crosstalk between the TGFβ signaling pathway and another important signaling pathway, the Wnt pathway (Salinas, 2003). Wnt proteins are a large family of secreted glycoproteins and some of the first evidence that Wnts regulate the synapse came from studies utilizing Wnt-7A mutant mice. Wnt-7A has been shown to regulate microtubule organization and the clustering of synaptic proteins (Salinas, 2003). Wnt-7A signaling can increase microtubule stability through inhibition of glycogen synthase kinase-3β (GSK-3β) (Lucas et al., 1998). TGFβ-mediated MAP kinase activation controls Wnt-7A gene expression and Wnt-mediated signaling (Tuli et al., 2003). In different contexts, Wnts and TGFβ signaling can interact either antagonistically or synergistically (Salinas, 2003). Preliminary data from our lab shows that in addition to inducing morphologic degenerative changes in the OHSC model, Aβ + TGFβ2 can increase AT8 (hyperphosphorylated tau) staining of pyramidal neurons in the CA1–CA2 region. NFT formation has been associated with abnormal tau phosphorylation due to decreased protein phosphatase or increased protein kinase activity, such as GSK-3β, in AD (Mandelkow et al., 1993; Iqbal et al., 1994). TGFβ2, highly localized in NFT-bearing neurons is AD, could destabilize microtubules by inhibiting Wnt-7A signaling. Aβ injected into the dorsal hippocampus has also been shown to induce a loss of Wnt signaling and a deficit in spatial memory (De Ferrari et al., 2003). Precisely how Wnt signaling causes inhibition of GSK-3β is unclear but it seems to involve two members of the LRP receptor family, LRP5 and LRP6 (Herz and Bock, 2002). As suggested by our in vitro and in vivo data, signaling interactions between the LRP and TGFβ pathways could alter normal Aβ clearance pathways.
In addition to Wnt signaling, LRP is known to physically associate with NMDA receptors via the postsynaptic density-95 (PSD95) (Gotthardt et al., 2000). Influx of calcium due to LRP-mediated activation of NMDA receptor channels may affect a variety of downstream signaling cascades and provide a mechanism for changing local synaptic plasticity (Tashiro et al., 1998). Alterations in brain LRP expression or activity may therefore impact neuronal homeostasis, synaptic plasticity, and amyloid deposition and thereby modify the pathogenesis of AD. In the current study, NR2B, a subunit of the NMDA receptor, is reduced in mice infused with Aβ + TGFβ2 and these mice exhibit memory retention deficits. A reduction in NR2B protein expression has been shown to correlate with deficits in Morris water maze performance (Clayton et al., 2002). It was demonstrated recently that LRP levels are reduced in AD brain (Kang et al., 2000), but it remains to be determined how this relates to the progression of AD or if low LRP levels are simply age-related or are an end result of extensive neuron loss.
The results of the current study provide in vitro and in vivo evidence that TGFβ2 can alter uptake of Aβ through the LRP receptor. Our results lead us to postulate that alterations in LRP function or numbers contribute to Aβ pathogenesis by impeding appropriate Aβ degradation. Changes in signaling and structural changes in the synaptic membrane can lead to the demonstrated accumulation of toxic Aβ and deficits in memory retention. More studies are needed to determine the precise interactions of TGFβ2, Aβ, ApoE, and the LRP receptor.
Acknowledgements
This work was supported by National Institutes of Health (grant AG022080 to M.E.H.-W. and grant AG10685 to S.A.F.), Veterans Administration Merit Award (to M.E.H.-W.), and a career development award from the Claude D. Pepper Older Americans Independence Counsel (to M.E.H.-W.). We thank G. Cole for helpful discussions and F. Yang and P. Chen for help with tissue processing. We also thank HVS Image newsgroup and particularly Dr. A. Blokland, Maastricht University, Netherlands, Dr. M.M. Nicolle at Mayo Clinic, FL, and Dr. B. Shukitt-Hale, Tufts University for helpful interpretation of Morris water maze data.




