Calcium signal-mediated expression of the vasoactive intestinal polypeptide gene and its small contribution to activity-dependent survival of mouse cerebellar granule cells
Abstract
We have demonstrated previously in primary cultures of mouse cerebellar granule cells (CGCs) that endogenously synthesized pituitary adenylate cyclase-activating polypeptide (PACAP) contributes at least in part to the activity-dependent survival of CGCs (Tabuchi et al. [2001] Neurosci. Res. 39:85–93). In this study, we have demonstrated that expression of vasoactive intestinal polypeptide (VIP), a member of the same VIP/secretin/glucagon family as PACAP, was activated markedly by Ca2+ influx through L-type voltage-dependent Ca2+ channels (L-VDCCs), which could be induced under the depolarizing condition induced by high concentration of potassium (K+) in the medium. The activation of VIP mRNA expression, different from that of PACAP, was dependent partly on de novo protein synthesis. On the other hand, mRNA expression of secretin and PACAP/VIP receptors (PAC1, VPAC1, and VPAC2) was not activated by the Ca2+ influx; rather, PAC1 mRNA expression was reduced. Exogenously added VIP prevented apoptosis of CGCs under nondepolarizing conditions, the effect of which was mediated specifically through the VPAC1 receptor. Furthermore, the survival of CGCs under depolarizing conditions could be mediated partly through VPAC1, the contribution of which was much less than that of PAC1. These findings indicate that PACAP and VIP genes are coordinately activated by the Ca2+ signals in CGCs, but the contribution of VIP to the activity-dependent survival of CGCs is quite small. © 2004 Wiley-Liss, Inc.
Vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) are members of the VIP/secretin/glucagon family and structurally closely related neuropeptides with widespread distribution in the central and peripheral nervous systems (CNS and PNS, respectively). Both VIP and PACAP have been shown to be potent vasodilators in several species and to have a broad spectrum of biological actions, such as neuronal differentiation, proliferation, survival, and synaptic transmission (Sherwood et al., 2000; Vaudry et al., 2000b; Moody et al., 2003). VIP is a 28-amino acid polypeptide originally identified in porcine small intestine (Said and Mutt, 1970) and PACAP is present in two distinct bioactive forms, PACAP38 and PACAP27, both derived from a 175-amino acid precursor (Li et al., 1999). The effects of PACAP and VIP are mediated by activation of at least three types of receptors, one of which (PAC1) binds PACAP with a 1,000-fold higher affinity than VIP and two of which (VPAC1 and VPAC2) bind PACAP and VIP with an equally high affinity (Hashimoto et al., 1993; Pisegna and Wank, 1993; Spengler et al., 1993). Although PACAP and VIP can function cooperatively through these receptors in the nervous systems (Drahushuk et al., 2002; Hamelink et al., 2002; Ganea and Delgado, 2003), it remains unknown how coordinately or differentially the members of the VIP/secretin/glucagon family are expressed in neurons.
Cultured cerebellar granule cells (CGCs) of rodents provide a good system for studying molecular mechanisms for activity-dependent survival of neurons because membrane depolarization induced by high potassium (K+) in the medium serves to maintain the survival of CGCs, whereas deprivation of membrane depolarization induced by low K+ leads to apoptosis (Gallo et al., 1987; Yan et al., 1994; Galli et al., 1995). In high K+ medium, a series of genes encoding secretary neuropeptides, such as brain-derived neurotrophic factor (BDNF) (Ghosh et al., 1994; Sano et al., 1996; Tabuchi et al., 2000), secretogranin II (Fujita et al., 1999), parathyroid hormone related peptide (PTHrP) (Holt et al., 1996; Ono et al., 1997), and PACAP (Vaudry et al., 2000a, 2002; Tabuchi et al., 2001), are expressed in an activity-dependent manner that accompanies Ca2+ influx into CGCs through L-type voltage-dependent calcium channels (L-VDCC). Ca2+ influx-mediated activation of these genes is thought to be involved not only in activity-dependent survival of CGCs but also in expressing versatile neuronal functions. It remains unknown, however, how cooperatively the expression of these genes is controlled in an activity-dependent manner. We therefore focused particularly on VIP gene expression and the effect of VIP on activity-dependent survival of CGCs, comparing it to that mediated by PACAP (Tabuchi et al., 2001).
Abbreviations
BDNF, brain-derived neurotrophic factor; CGCs, cerebellar granule cells; CHX, cycloheximide; PACAP, pituitary adenylate cyclase-activating polypeptide; VIP, vasoactive intestinal polypeptide.
MATERIALS AND METHODS
Reagents
Cycloheximide and nicardipine were purchased from Sigma. Human PACAP38 and VIP were purchased from Peptide Institute Inc. (Japan). PACAP(6–38) was from American Peptide (Sunnyvale, CA) and acetyl-(Tyr1, D-Phe2)-GRF(1–29) amide (Ac-[Y1, D-F2]-GRF[1–29]NH2) from BACHEM (Torrance, CA).
Cell Culture and Treatment
A primary culture of mouse CGCs was prepared from 1-week-old mice (ICR). The procedure for dissociating the cerebellar granule cells has been described previously (Ichikawa et al., 1998). Briefly, cells were seeded in poly-L-lysine-coated 60-mm dishes (Iwaki, Japan) and cultured in Dulbecco's modified Eagle medium (Nissui, Japan) containing 10% fetal calf serum (Sanko Junyaku Co., Japan) and 25 mM KCl in 10% CO2 and air. To inhibit proliferation of non-neuronal cells, the medium was replaced with a fresh batch supplemented with 10 μM cytosine arabinoside (Sigma) 2 days later. The medium containing 25 mM KCl was replaced with medium containing 5 mM KCl 4 days after the start of culture. After exposure to 5 mM KCl medium for 24 hr, cells were stimulated with 25 mM KCl (high K+) by addition of 40 μl of a 2 M KCl solution to the medium (4 ml), and cultured for a given period. For the control, the corresponding buffer without KCl was added as a vehicle.
RT-PCR Analysis for Detecting Specific Transcripts
Total cellular RNA was extracted from the cultured cells using ISOGEN (Nippon Gene, Japan). Total RNA (1 μg) was reverse-transcribed into cDNA in 20 μl of 1× First Strand buffer containing 0.5 μM oligo (dT)15 (5′-AAGCTTTTTTTTTTV-3′) as a primer, 200 U SuperScript II reverse transcriptase (Gibco BRL), 400 μM of each dNTP, and 10 U RNase inhibitor (Gibco BRL), as recommended by the manufacturer. After reverse transcription, the reaction mixture was treated with 1.1 U RNase H (Gibco BRL) at 37°C for 20 min and used for PCR as the cDNA solution. PCR was carried out in 50 μl of 1× PCR buffer containing 1 μl of the cDNA solution, 1.25 U of AmpliTaq Gold DNA polymerase (Perkin Elmer), 1.5 mM MgCl2, 200 μM of each dNTP, and 0.5 μM of each primer. To analyze the induction of VIP mRNA expression, VIP sense (5′-TCAGCTTGGACAGCAGAGCA-3′) and VIP antisense (5′-CTCACTACAGAAGGTGGTCC-3′) primers were used. For detection of secretin mRNA, Secr-sense (5′-AGGACCCCAAGACACTCAGAC-3′) and Secr-antisense (5′-CATCTGGGTGGCCTGGTTGTT3′) were used. For further detection of receptor mRNA, the following primer pairs were used: PAC1 (sense, 5′-CTTGGAGATCACAGACATGGG-3′; antisense, 5′-CTCGGCATACAAGATCCAGTC-3′); VPAC1 (sense, 5′-TACATACTCATCGGCTGGGGA-3′; antisense, 5′-GGAAAGACCCTACGACGAGTT-3′); and VPAC2 (sense, 5′-CTGCTGCTAATCCCCCTGTTT-3′; antisense, 5′-ACGGTGTATCTGTAGGGCACT-3′). The primer set for PAC1 was designed to detect all isoforms of the transcript. For internal control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA was amplified using the GAPDH primer pair as described previously (Ichikawa et al., 1998). For amplification of VIP, secretin, PAC1, VPAC1, and VPAC2 cDNA, the PCR conditions, after a preheating step at 95°C for 10 min, were as follows: denaturation at 96°C for 45 sec; annealing at 59°C (for secretin) or 57°C (other genes) for 45 sec; and extension at 72°C for 1 min for 31 (for VIP); 30 (for PAC1) or 34 (for secretin, VPAC1 and VPAC2) cycles; and a final extension at 72°C for 10 min. GAPDH amplification was carried out for 31 cycles under the same conditions except for the annealing (at 55°C). PCR products were separated by electrophoresis on 2% agarose gels and the densities of the DNA bands stained with ethidium bromide were analyzed using a Bit-Map loader (ATTO, Japan) and software (NIH Image 1.52). For comparisons between KCl-stimulated and -unstimulated samples, we selected cycle numbers at which the linearity could be identified.
Immunocytochemistry
For immunostaining, CGCs were cultured on poly-L-lysine-coated 35-mm dishes (Iwaki, Japan). Cells were fixed with a 2% glutaraldehyde solution, and washed three times with phosphate-buffered saline (PBS). Cells were permeabilized with PBS containing 3% bovine serum albumin (Sigma), 0.3% Triton X-100, and 5% goat serum (Vector Laboratories) for 1 hr at room temperature, and incubated with 1:1,000 goat anti-mouse VIP (M-19) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. Cells were then washed three times with PBS and incubated with 1:100 rabbit biotinylated anti-goat IgG (Vector Laboratories) for 1 hr at room temperature. Finally, cells were immunostained by the formation of an avidin-biotin-peroxidase complex using a Vectastain Elite ABC kit (Vector Laboratories) and the peroxidase reaction of hydrogen peroxide with diaminobenzidine.
Cytotoxicity Assay
Cytotoxicity was assessed by counting the apoptotic cells, whose nuclei were stained with the DNA dye bisbenzimide, Hoechst 33258 (Hoechst staining). The procedure for Hoechst staining has been described previously (Ichikawa et al., 1998). Briefly, cells seeded on poly-L-lysine-coated 25-mm cover slips were fixed with a 2% glutaraldehyde solution and stained with 1 μg/ml of Hoechst 33258 (Wako Pure Chemicals, Japan). Nuclei were viewed under a fluorescent microscope (BX-50 and BX-FLA; Olympus), and scanned with a CCD camera (CoolSNAP; Olympus). Apoptotic cells were characterized by condensed, cleaved, or highly fluorescent nuclei. The percentage of apoptotic cells was defined as the apoptotic cell number divided by the total cell number (approximately 200 cells).
Statistical Analysis
All values represent the mean ± standard error (SE) from a number of independent experiments, as indicated in figure legends. The same tendency was obtained from at least two independent experiments. Two groups were assessed using Student's t-test followed by the F-test. P < 0.05 was taken as the minimum level of significance.
RESULTS
Induction of VIP mRNA Expression Evoked Via Membrane Depolarization
The mouse VIP gene consists of seven exons (Fig. 1A[b] see Sena et al., 1994) and the secretin gene comprises four exons (Fig. 1A[b]; see Lan et al., 1994). To investigate whether membrane depolarization caused by increasing KCl concentration of the medium from 5 to 25 mM activates expression of both genes, we monitored mRNA expression of these genes after KCl stimulation by RT-PCR. Using primer sets shown in Figure 1A, we detected DNA bands corresponding to the deduced size of amplified VIP cDNA (Fig. 1B[a]). At 3 hr after stimulation of CGCs with 25 mM KCl, an increase in VIP mRNA expression was detected, gradually increased for 24 hr, and finally began to decrease 48 hr later (Fig. 1). By contrast, VIP mRNA expression levels remained constant when the KCl concentration was maintained at 5 mM (Fig. 1B[a]). The level of secretin mRNA expression did not change significantly when the KCl concentration was increased to 25 mM (Fig. 1B[b]). We failed to detect amplified DNA bands for glucagon, a member of the same family as VIP, even though the cycle number for PCR was increased to 45 (data not shown). These findings and our previous study indicate that among the VIP/secretin/glucagon family, expression of PACAP and VIP genes is induced by membrane depolarization but that of the secretin gene is not.
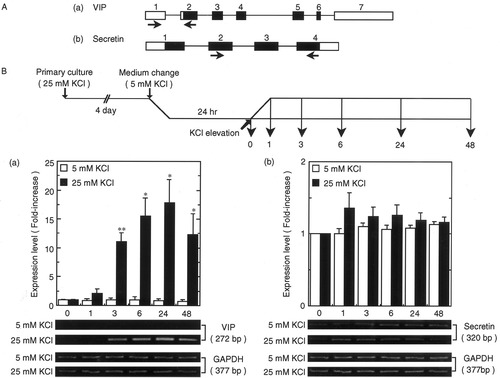
Regulation of VIP and secretin gene expression by membrane depolarization. A: VIP (a) and secretin (b) gene structure. Boxes, exons; open boxes, non-coding regions; solid boxes, coding regions; arrows, primer positions for RT-PCR. B: After CGCs had been cultured for 24 hr in 5 mM KCl, KCl elevation or vehicle treatment was carried out. At times indicated (0, 1, 3, 6, 24, and 48 hr), total RNA was prepared and subjected to RT-PCR. PCR products were separated by 2% agarose gel electrophoresis (VIP, 272 base pairs; secretin, 320 base pairs). Intensity of each amplified band relative to the control (0 hr) shown as fold-increase in the panel. Each value has been normalized with respect to the amount of GAPDH transcript; data represent mean ± SE from at least three independent experiments. *P < 0.05; **P < 0.01 vs. control (0 hr).
Requirement of Activation of L-VDCCs for Induction of VIP Gene Expression and its Dependency on De Novo Protein Synthesis
We next investigated whether membrane depolarization-induced VIP mRNA expression was mediated by Ca2+ influx through L-VDCCs. Treatment of CGCs with 5 μM nicardipine, a potent blocker for L-VDCCs, completely inhibited the increase in VIP mRNA expression induced by 25 mM KCl (Fig. 2A[a]). Furthermore, membrane depolarization increased immunoreactivity to VIP (Fig. 2A[b]; compare KCl at 5 and 25 mM). This increase was observed predominantly in cytoplasm of CGCs and was inhibited completely by nicardipine (Fig. 2A[b]; compare 25 mM KCl to 25 mM KCl + nicardipine).
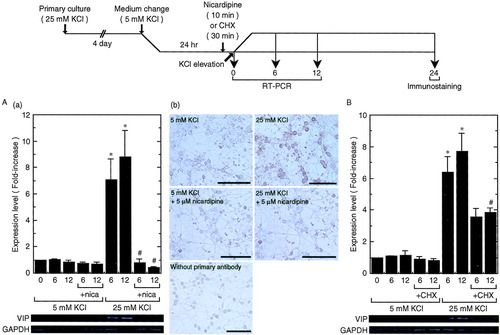
Effect of an L-VDCC blocker or protein synthesis inhibitor on membrane depolarization-induced VIP mRNA expression. A: Experimental procedure was the same as that in Figure 1. (a) Nicardipine was added 10 min before KCl elevation. RT-PCR was carried out as in Figure 1. Data represent the mean ± SE from four independent experiments. *P < 0.05 vs. control (0 hr); #P < 0.05 vs. 25 mM KCl (at the same time). (b) Immunostaining of CGCs with anti-VIP antibody was carried out 24 hr after KCl elevation. Scale bar = 25 μm. B: Experimental procedure and RT-PCR was the same as that in Figure 1. Cycloheximide (CHX) was added 30 min before KCl elevation. Data represent the mean ± SE from three independent experiments. *P < 0.05 vs. 5 mM KCl at the same time; #P < 0.05 vs. 25 mM KCl at the same time.
Previous studies have demonstrated that the increase in expression of prepro-PACAP transcripts was induced without de novo protein synthesis (Tabuchi et al., 2001). We next investigated the effect of a protein synthesis inhibitor, cycloheximide (CHX), on membrane depolarization-induced VIP mRNA expression. As shown in Figure 2B, treatment of CGCs with CHX repressed the increase in VIP mRNA expression by about 50%. These observations indicated that induction of VIP mRNA expression, different from that of the PACAP gene, was dependent partly on de novo protein synthesis.
Effect of Membrane Depolarization on Expression of PACAP/VIP Receptor Genes
We investigated whether expression of PAC1, VPAC1, and VPAC2 receptor mRNA changes upon membrane depolarization. The PAC1 gene consists of 18 exons (Fig. 3A[a]; see Hashimoto et al., 1996) and the VPAC1 and VPAC2 genes, 13 exons (Fig. 3A[b] and [c], respectively; see Mackay et al., 1996; Karacay et al., 2001). Using the primer sets shown in Figure 3A, we examined their mRNA expression after stimulation with 25 mM KCl using RT-PCR. Amplified DNA bands were detected for all receptors but the number of cycles required for detection of PAC1, VPAC1, and VPAC2 was 30, 34, and 34, respectively, suggesting that PAC1 mRNA is expressed at a higher level than is VPAC1 or VPAC2 mRNA in the primary CGC culture. In addition, PAC1 mRNA expression decreased after stimulation, which was attenuated by nicardipine (Fig. 3B[a]). On the other hand, expression of VPAC1 and VPAC2 mRNA did not change significantly for at least 12 hr after KCl stimulation (Fig. 3A[b] and [c]).
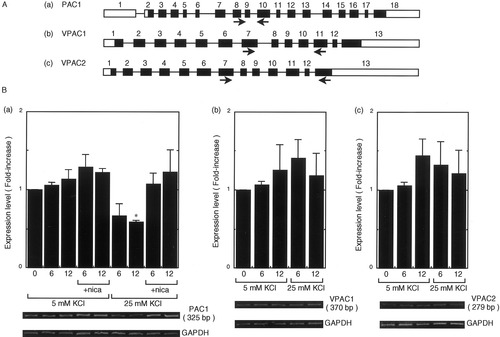
Changes in PACAP and VIP receptor mRNA expression induced by membrane depolarization. A: Structure of PAC1 (a), VPAC1 (b), and VPAC2 (c) genes. Boxes, exons; open boxes, noncoding regions; solid boxes, coding regions; arrows, primer positions for RT-PCR. B: Experimental procedure was the same as that in Figure 1. Data represent the mean ± SE from at least three independent experiments. *P < 0.05 vs. control (0 hr).
Attenuation of Apoptosis of CGCs Through PACAP/VIP-Specific Receptors
We demonstrated previously that endogenously synthesized PACAP has a neurotrophic effect on CGCs cultured at 5 mM KCl (Tabuchi et al., 2001). In the present study, we investigated the effect of VIP on CGCs. Using Hoechst staining, we measured the fraction of apoptotic cells among all CGCs 48 hr after treatment with VIP or PACAP. As shown in Figure 4, apoptosis of CGCs was induced by 5 mM KCl but attenuated when the KCl concentration was restored to 25 mM (Fig. 4A; compare 5 mM KCl to 25 mM KCl). Treatment of CGCs with VIP also attenuated apoptosis, the effect of which was saturated by the addition of VIP at 500 nM. A stronger attenuating effect of PACAP38 on CGC apoptosis was detected at 100 nM (Fig. 4A,B; compare 100 nM VIP to 100 nM PACAP38). Further addition of VIP (500 nM) to PACAP38 (100 nM) tended to increase the level of apoptosis prevention compared to the addition of PACAP38, the effect of which was not additive (data not shown).
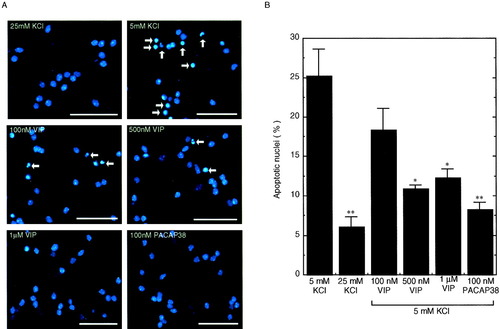
Effect of exogenous VIP on apoptosis of CGCs induced by deprivation of membrane depolarization. After CGCs had been cultured for 24 hr in 5 mM KCl, treatment with KCl elevation, 50 nM to 1 μM VIP, or 100 nM PACAP38 was carried out, and CGCs were stained with Hoechst 33258 48 hr after stimulation. A: Photomicrographs of CGCs stained with Hoechst 33258. Arrows, cleaved or condensed apoptotic nuclei. Scale bar = 25 μm. B: Percentages of apoptotic nuclei were quantified by the methods described in text. Data represent the mean ± SE from at least four independent experiments. *P < 0.05; **P < 0.01 vs. 5 mM KCl at the same time.
VIP-mediated prevention of the apoptosis induced at 5 mM KCl was abolished completely by the VPAC1/2 receptor antagonist, Ac-(Y1, D-F2)-GRF(1–29)NH2 (Liu et al., 2000), but not by the PAC1 and VPAC2 receptor antagonist, PACAP(6–38) (Beaudet et al., 2000; Liu et al., 2000) (Fig. 5), indicating that the exogenously added VIP affected the CGCs specifically through the VPAC1 receptor. The viability of CGCs at 25 mM KCl, however, decreased on the addition of Ac-(Y1, D-F2)-GRF(1–29)NH2, the effect of which was less than that of PACAP(6–38) (Fig. 5). The simultaneous addition of both antagonists tended to limit CGC survival at 25 mM KCl more effectively than did the addition of either antagonist alone, although the effect was not additive (Fig. 5).
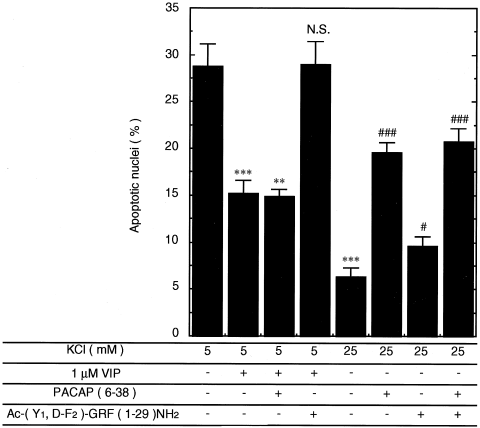
Effects of PACAP/VIP receptor antagonists on attenuation of apoptosis achieved by exogenously added VIP or membrane depolarization. CGCs cultured as described in the legend to Figure 4 were treated with 1 μM VIP or 25 mM KCl in the presence or absence of 10 μM Ac-(Y1, D-F2)-GRF(1–29)NH2 or 10 μM PACAP(6–38). Percentage of apoptotic nuclei was measured as described in text. Data represent the mean ± SE from at least five independent experiments. **P < 0.01; ***P < 0.001 vs. 5 mM KCl at the same time. #P < 0.05; ###P < 0.001 vs. 25 mM KCl at the same time. N.S., nonsignificant vs. 5 mM KCl.
DISCUSSION
We demonstrated that the mRNA expression of the VIP gene was increased by membrane depolarization in cultured CGCs (Fig. 1B[a]). This increase was inhibited completely by nicardipine (Fig. 2A[a]), supporting its dependency on Ca2+ influx through L-VDCCs. Changes in the immunoreactivity of CGCs to VIP corresponded with changes in VIP mRNA expression (Fig. 2A[b]), indicating that the activity-dependent increase in VIP mRNA expression couples with protein synthesis. In contrast, mRNA expression of secretin, a member of the same family as PACAP and VIP, was detected but not increased by membrane depolarization (Fig. 1B[b]). Because immunoreactivity to VIP and PACAP was observed in most cells under the depolarizing conditions (Fig. 2A[b]; see Tabuchi et al., 2001), it is plausible that induction of VIP and PACAP mRNA expression, at least in part, is caused in the same cells in culture. The cycle numbers required to detect amplified DNA bands by RT-PCR were approximately 32–34 with the PACAP, VIP, and secretin mRNAs, suggesting that in CGCs, these three kinds of mRNA are at similar levels of expression.
We and other groups have demonstrated previously that the transcription factor CREB (cAMP-responsive element binding protein) is also involved in Ca2+ signal-mediated activation of BDNF gene promoters I (Tabuchi et al., 2002) and III (Tao et al., 1998, Tabuchi et al., 2000). A series of studies thus strongly support the notion that CREB is a key regulator for Ca2+ signal-mediated gene expression in neurons. Because the VIP gene also accommodates the cAMP-responsive element (CRE) in its promoter, which is located 70 base pairs upstream of the transcription initiation site (Hahm and Eiden, 1998), it seems highly likely that VIP gene activation is mediated by Ca2+ signals through the binding of CREB to the CRE, which may account for the coordinated expression of PACAP and VIP in CGCs. In support of this, we have found that Ca2+ signal-mediated activation of the PACAP gene is controlled predominantly by CREB (unpublished data).
We found that three types of receptors, PAC1, VPAC1 and VPAC2, were expressed in CGCs (Fig. 3). PAC1 mRNA seems to be expressed more abundantly than is the VPAC1 or VPAC2 mRNA, as estimated from the number of cycles required for RT-PCR (Fig. 3). A similarly abundant expression of PAC1 mRNA is observed in other regions of the brain, including the hypothalamus, hippocampus, and neocortex (Basille et al., 2000). Expression of PACAP and VIP receptors, however, was not enhanced by membrane depolarization and, in fact, the expression of the PAC1 gene was reduced under the conditions (Fig. 3B[a]). This reduction was blocked by nicardipine (Fig. 3B[a]), implying that PAC1 gene expression can be downregulated by Ca2+ signals evoked via L-VDCCs. Neurotrophin-3 (NT-3) and c-jun genes have been identified already as genes whose expression is downregulated by Ca2+ signals (Ichikawa et al., 1998).
Although the PACAP gene does not require de novo protein synthesis for activation mediated by Ca2+ signals (Tabuchi et al., 2001), the VIP gene does, at least in part (Fig. 2B). It is established that c-fos is induced by Ca2+ influx into CGCs, resulting in an increase in the DNA-binding activity of the transcription factor activator protein-1 (AP-1) to the 12-o-tetradecanoylphorbol-13-acetate (TPA)-responsive element (TRE) (Sakurai et al., 1992). It is therefore possible that some transcription factor such as AP-1, whose expression is dependent on de novo protein synthesis, is involved at least partly in VIP gene activation. In support of this notion, several TREs are located in the promoter of the VIP gene (Hahm and Eiden, 1998). More studies regarding transcriptional regulation of the VIP gene promoter would reveal how transcription factors such as CREB and AP-1 are involved in Ca2+ signal-mediated activation of the gene.
We also demonstrated the protective effect of exogenously added VIP on the apoptosis of CGCs under nondepolarizing conditions (Fig. 4). Exogenously added VIP attenuated apoptosis of CGCs but the effect of PACAP38 was stronger than that of VIP at the same concentration (Fig. 4A,B). This VIP mediated-attenuation of apoptosis seemed to involve the specific interaction of VIP with VPAC1 receptor because Ac-(Y1, D-F2)-GRF(1–29)NH2, a VPAC1/2 receptor antagonist, completely abolished VIP-mediated attenuation of apoptosis but PACAP(6–38), a PAC1/VPAC2 receptor antagonist, did not (Fig. 5). As for the activity-dependent survival of CGCs, thought to be dependent largely upon endogenously synthesized and secreted neurotrophic factors, the administration of Ac-(Y1, D-F2)-GRF(1–29)NH2 partly reduced CGC survival under the depolarizing condition (Fig. 5), implying a role for activation of VPAC1/2 receptors in the activity-dependent survival of CGCs. Because the antagonizing effect of PACAP(6–38) was greater than that of Ac(Y1, D-F2)-GRF(1–29)NH2, however, and the simultaneous use of the antagonists did not result in an additive attenuation of CGC survival (Fig. 5), it seems likely that endogenously synthesized PACAP mostly contributes to activity-dependent CGC survival mainly through PAC1, whereas endogenously synthesized VIP contributes only slightly through the VPAC1 receptor, which may explain the observation that the simultaneous addition of exogenous PACAP38 and VIP did not result in an additive attenuation of the apoptosis (data not shown). This predominant involvement of PACAP in CGC survival was supported also by the observation that the neuroprotective activity of PACAP was mimicked by VIP at high concentrations (Cavallaro et al., 1996). This was due probably to abundant PAC1 expression in CGCs, the high affinity of PACAP for the PAC1 receptor, and possibly a differential activation of intracellular transduction pathways transmitted via PAC1 and VPAC1 receptors (Moody et al., 2003). Because BDNF has a strong neurotrophic effect on CGCs (Ichikawa et al., 1998), it seems highly possible that BDNF and PACAP are the major endogenous neurotrophic factors responsible for the activity-dependent survival of CGCs.
It has been reported recently that the neuroprotective action of VIP requires the presence of astroglia, which results in the release of several neurotrophic factors including cytokines and activity-dependent neurotrophic factor (ADNF) from astroglia (Blondel et al., 2000). PACAP has been identified already as an inducer for several kinds of glial and neuronal responses (Vaudry et al., 2000b). There thus seems to be several modes of action by endogenously synthesized PACAP and VIP on neuronal and glial cells through differential activation of PACAP/VIP receptors, the mRNA expression of which is coordinately regulated in an activity-dependent manner in neurons.




