NMDA receptor function in mouse models of Huntington disease
Abstract
Huntington disease (HD) is an autosomal dominant disorder in which degeneration of medium-sized spiny striatal neurons occurs. The HD gene and the protein it encodes, huntingtin, have been identified but their functions remain unknown. Transgenic mouse models for HD have been developed and we examined responses of medium-sized striatal neurons recorded in vitro to application of N-methyl-D-aspartate (NMDA) in two of these. The first model (R6/2) expresses exon 1 of the human HD gene with approximately 150 CAG repeats. In the R6/2 an enhancement of currents induced by selective activation of NMDA receptors as well as an enhancement of intracellular Ca2+ flux occurred in both presymptomatic and symptomatic mice. These alterations appeared specific for the NMDA receptor because α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor-mediated currents were reduced in symptomatic R6/2s. In R6/2 animals there were parallel increases in NMDA-R1 and decreases in NMDA-R2A/B subunit proteins as established by immunohistochemistry. The second model (YAC72) contains human genomic DNA spanning the full-length gene and all its regulatory elements with 72 CAG repeats. The phenotypical expression of the disorder develops more gradually than in the R6/2. In YAC72 mice we found similar but less marked increases in responses of medium-sized striatal neurons to NMDA. These findings indicate that alterations in NMDA receptor function may predispose striatal neurons to excitotoxic damage, leading to subsequent neuronal degeneration and underscore the functional importance of NMDA receptors in HD. J. Neurosci. Res. 66:525–539, 2001. © 2001 Wiley-Liss, Inc.
The genetic abnormality in Huntington disease (HD) has been shown to be due to an unstable expansion of trinucleotide (CAG) repeats within the coding region of the gene (The Huntington Disease Collaborative Research Group, 1993). The function of the encoded protein (huntingtin) and the mechanisms by which it causes neuropathology are unknown.
A major hypothesis to explain the neuronal damage in HD involves excitotoxicity (DiFiglia, 1990; Freese et al., 1990; Beal et al., 1993; Ferrante et al., 2000). This theory postulates that subpopulations of striatal neurons are hypersensitive to glutamate released by cortical and thalamic afferents due primarily to changes in their postsynaptic receptors [N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), kainate (KA)]. This theory is based on studies demonstrating that excitotoxic lesions of the striatum, typically generated by more selective activation of NMDA receptors in animals, produce effects that resemble the neuropathological and neurochemical changes of HD (Coyle, 1979; Schwarcz et al., 1984; Beal et al., 1986; Choi, 1988; DiFiglia, 1990; Bordelon and Chesselet, 1999).
Until the generation of transgenic mouse models of HD (Mangiarini et al., 1996; Reddy et al., 1998; Hodgson et al., 1999), direct tests of altered glutamate receptor function have not been possible. Here we examined changes in glutamate receptors and the potential physiological mechanisms that may increase striatal neuronal vulnerability in two different mouse models of HD. We focused on the R6/2 transgenic, which contains exon 1 and promoter sequences of the human HD gene inserted into the mouse genome, and carries 141–157 CAG repeats (Mangiarini et al., 1996). The transgenic demonstrates an aggressive form of the disease, with a progressive neurological syndrome that includes alterations in transmitter and receptor expression and signaling mechanisms (Cha et al., 1998; Bibb et al., 2000; Luthi-Carter et al., 2000; Menalled et al., 2000), motor deficits (Carter et al., 1999), and learning disabilities (Lione et al., 1999; Murphy et al., 2000). There are similarities between the neuropathology of the R6/2 and human findings (Davies et al., 1997; Turmaine et al., 2000), especially cases of juvenile HD. We performed the most extensive studies on the R6/2 model because its symptoms develop rapidly (within the first 60 days). We examined electrophysiological changes in two different age groups of R6/2 transgenics and their littermate controls (WT).
The YAC72 transgenic was used for comparative purposes to corroborate alterations in NMDA receptor function observed in the R6/2 (Hodgson et al., 1999). The YAC72 uses yeast artificial chromosomes containing human genomic DNA spanning the full-length HD gene, and all its regulatory elements with a 72 CAG expansion. By 7 months of age YAC72 transgenics exhibit hyperactivity, physiological changes in hippocampal neurons, and by 12 months show degeneration of striatal medium-sized spiny neurons.
Previously, we demonstrated increased NMDA receptor sensitivity using a cell swelling assay in R6/2 transgenics and a CAG knock-in model (Levine et al., 1999). The present experiments provide a direct examination of the electrophysiological responsiveness of NMDA receptors at the cellular level in multiple murine HD models.
MATERIALS AND METHODS
Experiments were performed on two age groups of R6/2 transgenics and their age-matched WT littermate controls. We use the terms presymptomatic (before development of overt motor signs) and symptomatic (after motor abnormalities were visible) R6/2 mice to differentiate the two groups. Although we use the term presymptomatic to define the younger group, studies have shown that R6/2 animals before and at this age display subtle behavioral alterations (Lione et al., 1999). Fifty-seven R6/2 transgenic mice were used [11 presymptomatic and four WTs, 40 ± 5 (mean ± SE) days of age; 25 symptomatic and 17 WTs, 80 ± 5 days]. R6/2 mice and WTs were obtained from our colony at UCLA. All transgenic YAC72 mice were symptomatic. Nine YAC72 were used (four transgenic and five WT controls, 415 ± 8 and 419 ± 8 days of age, respectively). The YAC72 mice were obtained from the colony at the Center for Molecular Medicine and Therapeutics, (Vancouver, BC, Canada) and were transported to UCLA. All experimental procedures were carried out in accordance with the USPHS, Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at UCLA.
Slice Preparation
All mice were anesthetized with halothane, decapitated and brains were placed in ice-cold oxygenated artificial cerebrospinal fluid (ACSF) [in mM: NaCl 130, NaHCO3 26, KCl 3, MgCl2 5, NaH2PO4 1.25, CaCl2 1, glucose 10 (pH 7.2–7.4)]. Coronal sections containing the striatum were cut (∼350 μm) and placed in oxygenated ACSF (same as above except CaCl2 2 mM and MgCl2 2 mM). After at least 1 hr, the slice was transferred to a custom-made recording chamber. Neurons were visualized in the slice using a fixed-stage upright microscope (Olympus, Model BX50WI) and submerged in continuously flowing oxygenated ACSF (25°C, 4 ml/min). The slice was illuminated with near infrared (IR) light (790 nm, Ealing Optics, Hollston, MA), and cells were visualized with a 40× water-immersion objective using differential interference contrast (DIC) optics. The image was detected with an IR-sensitive CCD camera (Hamamatsu C2400, Tokyo, Japan).
Whole-Cell Voltage Clamp
Patch electrodes (3–6 MΩ) were filled with the following internal solution (in mM): Cs-methanesulfonate 130, CsCl 10, NaCl 4, MgCl2 1, MgATP 5, EGTA 5, HEPES 10, GTP 0.5, phosphocreatine 10, leupeptin 0.1 (pH 7.25–7.3, osmolality 280–290 mOsm/L). An Axopatch 200B (Axon Instruments, Foster City, CA) was used for recording. A 3M KCl agar bridge was inserted between the extracellular solution and the Ag-AgCl indifferent electrode. Tight seals (2–5 GΩ) were obtained by applying negative pressure. The membrane was disrupted with additional suction to obtain the whole-cell configuration. Access resistances ranged from 10–25 MΩ and were compensated 60–85%. There were no significant differences between mean access resistances for neurons obtained from transgenic and WT groups. Recordings were not corrected for junction potentials, which ranged from 2–3 mV. Leak currents, when present, were subtracted on-line using the Axopatch 200B amplifier. Membrane capacitances and input resistances were measured by applying a 10 mV depolarizing voltage command and using the membrane test function integrated in the pClamp8 software (Axon Instruments, Foster City, CA). Cells were held at −70 mV to minimize contributions of voltage-gated currents. These currents also were blocked using: tetrodotoxin (TTX, 1 μM), Cd2+ (100 μM), and tetraethylammonium (TEA, 20 mM). Non-NMDA receptor activation was blocked with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 5 μM).
After the basic membrane properties were determined a series of depolarizing step voltage commands were applied with slices bathed in standard ACSF. Because Cs+ in the internal solution blocked most of the K+ conductances, this protocol evoked mainly inward currents (Na+ and Ca2+). Although it is impossible to clamp the large Na+ currents, the amplitude of this current permitted an estimate of the quality of the recording and the health of the neuron. Only cells that produced Na+ currents >4 nA (range 4–10 nA; average 6 nA) were included in the data set. Na+ currents were then blocked with TTX and the remaining whole cell Ca2+ currents were examined using the same voltage protocol or a slow ramp (from −100 to 10 mV in 4.4 sec). Ca2+ influx was measured using Fura-2 during application of the slow ramp voltage command (see below). After these measurements, Cd2+, TEA and CNQX were added to block voltage- and ligand-gated currents.
The effective concentration of NMDA that produced reliable currents at −70 mV holding potential was determined to be 50 μM. Other concentrations tested were 10 and 100 μM; the lower concentration did not produce consistent NMDA currents at the −70 mV holding potential and the higher concentration proved to be lethal in many cells from transgenic animals. Therefore, in these slice experiments we examined only one concentration of NMDA. NMDA was applied for 3 min followed by a 6–8 min wash. The peak current was measured and normalized by dividing by cell capacitance (Alzheimer et al., 1993). In selected experiments NMDA receptor voltage-dependence was evaluated using a slow upward ramp voltage command (−70 to +30 mV) followed by a fast downward ramp (+30 to −90 mV). The NMDA current was determined by digitally subtracting the current before and after bath application of NMDA. Only the downward ramp was used to obtain data (Burgard and Hablitz, 1994). In some experiments, the standard ACSF was replaced with a Mg2+-free solution to assess the Mg2+-dependence of the NMDA receptor. After at least 60 min in this solution the response to NMDA was tested using the same ramp protocol. To verify specificity and examine non-NMDA receptor-mediated currents AMPA (5–25 μM, 3 min duration) was applied. During these experiments CNQX was replaced with 2-amino-5-phosphonovalerate (AP5; 50 μM) to block NMDA receptors.
Ca2+ Imaging
Fura-2 pentapotassium salt (200 μM, Molecular Probes, Eugene, OR) was included in the intracellular solution of the micropipette (EGTA was eliminated in these experiments). Once patched and filled with dye, neurons were imaged using a cooled CCD camera (AstroCam TE3/A/S from Perkin-Elmer Life Sciences, Cambridge, UK) mounted on the Olympus microscope. A monochromator (SpectraMASTER, Perkin-Elmer) excited the sample at alternating wavelengths of 340 nm and 380 nm using a 200–400 ms exposure time, at an image recording frequency of 0.2–0.3 Hz (for NMDA-induced Ca2+ influx) and 0.8 Hz (for voltage-gated Ca2+ influx). Regions of interest (ROI) approximately the size of the cell soma were designated within each patched neuron. Average emitted light intensity of this ROI was measured for each excitation wavelength in each frame. Software (Merlin™, Perkin-Elmer) calculated the ratio of light emitted at 340 nm excitation divided by light emitted at 380 nm excitation. Ca2+ concentrations are presented in Tables I and II as both ratio values and converted into nanomolar units using the Grynkiewicz equation (Grynkiewicz et al., 1985). Two measures were obtained, the influx of Ca2+ induced by a slow depolarizing ramp voltage command and, after blockade of voltage-gated Ca2+ currents with Cd2+, the influx of Ca2+ through the NMDA receptors induced by bath application of the agonist.
| WT | R6/2-Presymptomatic | WT | R6/2-Symptomatic | |
|---|---|---|---|---|
| Basic membrane properties | ||||
| Capacitance (pF) | 81 ± 13 (13) | 89 ± 5 (29) | 83 ± 5 (16) | 68 ± 4 (16) P < 0.025 |
| Input resistance (MΩ) | 89 ± 16 (13) | 126 ± 21 (29) | 106 ± 28 (16) | 209 ± 31 (16) P < 0.025 |
| Time constant (msec) | 1.7 ± 0.3 (13) | 1.7 ± 0.1 (29) | 1.7 ± 0.1 (16) | 1.4 ± 0.1 (16) P < 0.05 |
| Voltage-gated Ca2+ peak currents | ||||
| Step Ca2+ current (pA) | 703 ± 76 (11) | 795 ± 74 (28) | 951 ± 124 (11) | 540 ± 81 (13) P < 0.01 |
| Current density (pA/pF) | 8.9 ± 0.9 (11) | 9.3 ± 0.9 (28) | 12.2 ± 1.7 (11) | 8.0 ± 1.7 (13) P < 0.05 |
| Ramp Ca2+ current (pA) | 636 ± 104 (13) | 799 ± 102 (29) | 900 ± 133 (14) | 389 ± 71 (15) P < 0.025 |
| Current density (pA/pF) | 8.2 ± 1.5 (13) | 9.0 ± 1.1 (29) | 11.4 ± 1.8 (14) | 5.9 ± 1.1 (15) P < 0.01 |
| Peak Ca2+ concentration in response to voltage ramps | ||||
| Basal Ca2+ (nM) | 72.3 ± 7.2 (9) | 72.8 ± 5 (21) | 59.3 ± 5.5 (15) | 55.1 ± 3.8 (15) |
| Basal Ca2+ (ΔF/F) | 0.20 ± 0.017 (9) | 0.20 ± 0.012 (21) | 0.17 ± 0.013 (15) | 0.16 ± 0.009 (15) |
| % Change (nM) | 279 ± 44 (9) | 285 ± 34 (21) | 215 ± 38 (15) | 143 ± 20 (15) P < 0.05 |
| % Change (ΔF/F) | 185 ± 24 (9) | 188 ± 20 (21) | 151 ± 21 (15) | 109 ± 14 (15) P < 0.05 |
| Time to peak (sec) | 5.1 ± 0.3 (9) | 4.5 ± 0.1 (21) P < 0.05 | 5.2 ± 0.2 (15) | 5.8 ± 0.3 (15) P < 0.05 |
- * Values are mean ± S.E. (n).
| WT | R6/2-Presymptomatic | WT | R6/2-Symptomatic | |
|---|---|---|---|---|
| NMDA current and current density | ||||
| NMDA current (pA) | 166 ± 44 (10) | 368 ± 106 (22) P < 0.05 | 237 ± 38 (15) | 356 ± 96 (14) |
| Current density (pA/pF) | 1.9 ± 0.3 (10) | 4.3 ± 1.2 (22) P < 0.05 | 2.9 ± 0.4 (15) | 5.7 ± 1.6 (14) P < 0.05 |
| Time to peak NMDA current (sec) | 291 ± 24 (10) | 312 ± 24 (20) | 226 ± 9 (15) | 227 ± 11 (14) |
| Peak Ca2+ concentration in response to NMDA | ||||
| % Ca2+ change (ΔF/F) | 19 ± 3 (6) | 36 ± 7 (12) P < 0.05 | 31 ± 4 (15) | 91 ± 32 (13) P < 0.05 |
| % Ca2+ change (nM) | 23 ± 3 (6) | 44 ± 9 (12) P < 0.05 | 37 ± 6 (15) | 131 ± 55 (13) P < 0.05 |
| Time to peak Ca2+ influx (sec) | 294 ± 31 (6) | 304 ± 34 (12) | 244 ± 11 (15) | 249 ± 12 (13) |
- * Values are mean ± S.E. (n).
Acute Neuron Dissociation
Some slices from R6/2 animals also were used for acute dissociation of neurons. Slices were incubated for 1–6 hr at room temperature in NaHCO3 buffered saline bubbled with 95% O2, 5% CO2 (in mM): 126 NaCl, 2.5 KCl, 2 MgCl2, 2 CaCl2, 26 NaHCO3, 1 Na2HPO4, 1 pyruvic acid, 5 μM glutathione, 0.1 NG-nitro-L-arginine, 1 kynurenic acid, 10 glucose, 15 HEPES, pH 7.4 with NaOH, 300–305 mOsm/L. After 1 hr incubation, a slice was placed in low Ca2+ isethionate solution (in mM): 140 Na isethionate, 2 KCl, 4 MgCl2, 0.1 CaCl2, 23 glucose, 15 HEPES, pH 7.4, 300–305 mOsm/L, and the dorsal striatum was dissected and placed in an oxygenated cell-stir chamber (Wheaton Inc., Millville, NJ) containing papain (Calbiochem, La Jolla, CA; papain, 1–2 mg/ml) in HEPES-buffered Hank's balanced salt solution (HBSS, Sigma Chemical Company, St. Louis, MO) at 35°C. After 20–40 min of enzyme digestion, tissue was rinsed three times with the low Ca2+-isethionate solution and mechanically dissociated with a graded series of fire-polished Pasteur pipettes. The cell suspension was plated into a 35 mm NUNCLON dish containing HEPES-buffered HBSS saline on the microscope stage.
Whole-cell recordings from dissociated neurons used standard techniques (Hamill et al., 1981; Bargas et al., 1994). The internal solution consisted of (in mM): 180 N-methyl-D-glucamine (NMDG), 40 HEPES, 2 MgCl2, 10 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 12 phosphocreatine, 2 Na2ATP, 0.2 Na3GTP, 0.1 leupeptin, pH 7.2–7.3 with H2SO4, 265–270 mOsm/L. The external solution consisted of (in mM): 135 NaCl, 20 CsCl, 5 BaCl2, 10 glucose, 10 HEPES, 0.001 TTX, 0.02 glycine, pH 7.3 with NaOH, 300–305 mOsm/L.
Recordings from acutely dissociated neurons were obtained with an Axopatch 200A amplifier. Electrode resistance was 2–4 MΩ in the bath. After seal rupture, series resistance (4–10 MΩ) was compensated (70–90%) and periodically monitored. Recordings were made only from medium-sized cells that had short (<75 μm) proximal dendrites. NMDA (100 μM, 3 sec duration every 20 sec) was applied with a gravity-fed ‘two-pipe’ system. The array of application capillaries (ca. 600 μm i.d. per capillary) was positioned a few hundred μm from the cell under study. Solution changes were effected by changing the position of the array with a DC drive system, controlled by a SF-77B perfusion system (Warner Instruments Co., Hamden, CT) synchronized by pClamp. Solutions could be delivered within 2–3 msec with this system.
Immunohistochemistry
Data for immunohistochemical studies were obtained from an additional group of 16 age-matched pre- and symptomatic R6/2 and WT animals. Tissues from a R6/2 and an age-matched WT pair were mounted in the coronal plane and sectioned on a cryostat (10 μm). Sections from R6/2 and WT were processed simultaneously to minimize histological variation between samples and permit comparisons between R6/2 and WT tissue sections. Each antisera reaction was performed at least twice in each of four different pairs of R6/2 transgenic and WT brains for each antibody. Affinity-purified rabbit-derived primary antisera (NMDA-R1, NMDA-R2A/B, GluR 2/3, and GluR 1; obtained from Chemicon, Temecula, CA) were applied to slide mounted sections, diluted in phosphate-buffered saline (pH 7.2) and incubated overnight at 4°C in a moist environment. Antisera dilutions used were NMDA-R1, 1:50; NMDA-R2A/B, 1:25; GluR 2/3, 1:100; and GluR 1, 1:50. Unbound primary antisera were rinsed off and fluorescently coupled secondary antisera (goat anti-rabbit rhodamine-X, Molecular Probes) were applied at 1:200 dilution for 1–2 hr at 4°C in a moist environment. Unbound secondary antisera were rinsed off after the incubation and the sections were examined with a confocal microscope (BioRad, Richmond, CA, MRC 600). Image acquisition parameters were established using the cortex of WT tissue. Settings were optimized to use the full range of the laser (0–255 gray scale) at the smallest possible pinhole setting and beam intensity for the laser. Ten accumulated scans of the laser were used to produce the images, and data were stored without any further enhancement or analysis. Experimental images were obtained from the WT striatum first, followed by acquisition of data from the R6/2 using these same settings. A specific order of image acquisition also was followed such that regions of interest were acquired from the lateral, central, and then the medial aspect of the striatum for each side, in each of the three sections mounted on the glass microscope slides. This experimental standard allowed comparisons to be made between age-matched pairs of R6/2 and WT striata in each pre- and symptomatic group for each antibody evaluated. Data were obtained from the dorsal half of the striatum to correlate with the areas used for electrophysiological recordings. Additional quantitative assessments of the change in fluorescent staining intensity were made by computing average gray scale values for the same regions of the striatum in the matched pairs of R6/2 and WT mice. Quantitative data from the images are presented as percent change from WTs.
Statistics
Values in the tables, figures and text are presented as means ± SE. Differences among group means were assessed with appropriate t-tests or ANOVAs. Welch's approximation to the t-test for unequal variances was used when group variances were not homogeneous (Welch, 1947). For post-hoc evaluations using ANOVAs, the Bonferroni t-test was used because this test is one of the more conservative approaches using multiple comparisons. Differences among distributions were evaluated with distributional tests for detecting changes in variance between two populations that have similar central tendencies (Gibbons, 1985). Multiple Pearson Product-Moment correlations were performed to examine associations among different measures obtained from the same neurons. In the text, only P-values and the type of test used are reported. Details of the statistical analyses are available from the authors upon request. Differences for all statistical tests were considered statistically significant when P < 0.05.
RESULTS
R6/2 Transgenics
Presymptomatic R6/2 mice did not display overt behavioral abnormalities like limb clasping, gross changes in motor coordination and appeared similar to their WT littermate controls. Symptomatic mice experienced considerable weight loss compared to WT animals (20 ± 1 g for R6/2 transgenics and 31 ± 2 g for WTs; P < 0.01). All symptomatic animals had obvious abnormal motor behaviors including limb clasping, poor motor coordination, tremors and some displayed seizures.
Medium-sized striatal cells were identified easily by infrared videomicroscopy in both pre- and symptomatic R6/2 mice and WT controls (Fig. 1A,B). Biocytin injections in some of these neurons verified their identity as medium-sized spiny neurons (Fig. 1D–G). In general, somata of medium-sized neurons from symptomatic R6/2 mice were smaller than those from their WT counterparts, as we demonstrated previously (Levine et al., 1999). In addition, in slices from symptomatic R6/2 mice small spheres (∼1–2 μm diameter) could be seen throughout the neuropil, particularly in the ventral striatum (Fig. 1C). These particles were not observed in presymptomatic R6/2 mice or in the YAC72 transgenic model. The composition or possible pathological significance of the particles was not explored further.
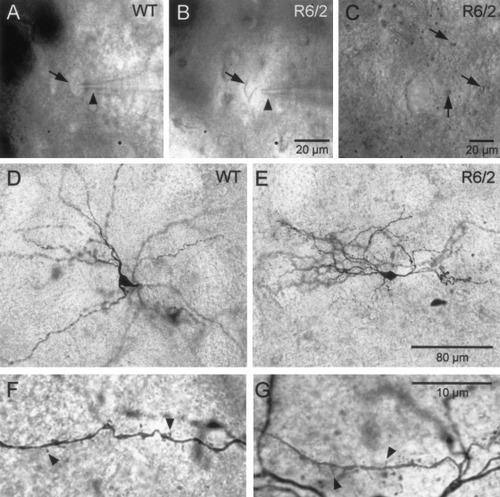
Images of recorded neurons. A,B: DIC-IR images of medium-sized neurons (arrows) with patch pipettes attached (arrowheads) recorded from a WT (A) and symptomatic R6/2 (B) slice. Calibration in B refers to A. C: shows small dark particles (arrows) observed in symptomatic R6/2 transgenics. D,E: Biocytin-filled medium-sized spiny neurons from a WT (D) and an R6/2 transgenic (E). Calibration in E refers to D. F,G: Higher magnifications of dendritic processes in D and E, respectively to illustrate spines (arrowheads). Calibration in G refers to F.
Basic Membrane Properties
There were no statistically significant differences between basic membrane properties of neurons obtained from presymptomatic R6/2 and WT mice although there was a tendency for neurons from transgenics to have a higher mean input resistance (Table I). Neurons from symptomatic R6/2 mice had significantly lower mean capacitances, significantly higher mean input resistances, and significantly lower mean time constants (Table I).
Voltage-Gated Ca2+ Currents and Concentration
A series of depolarizing step voltage commands, from a holding potential of −70 mV, induced inward Na+ and Ca2+ currents in neurons from all animals. After blockade of Na+ channels by TTX, the peak amplitudes of voltage-gated Ca2+ currents were measured using both step and slow ramp voltage commands (Fig. 2). Both protocols gave similar results. In neurons from presymptomatic R6/2 mice, peak Ca2+ currents and current densities were slightly higher than in neurons from WTs (Table I). These differences were not statistically significant (Table I). In marked contrast, in neurons from symptomatic R6/2 animals, peak Ca2+ current amplitudes and densities were significantly reduced compared to those from WT controls using either protocol (Fig. 2; Table I).
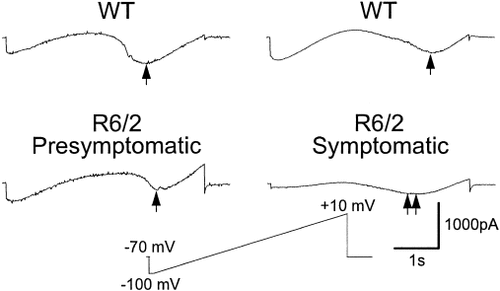
Examples of whole-cell Ca2+ currents in neurons from pre-symptomatic R6/2 transgenics and their age-matched WT (left two traces) and symptomatic R6/2 transgenics and age-matched WT (right two traces). Currents were evoked by a 4.4 second ramp command from −100 to +10 mV (bottom center). Peak inward Ca2+ currents are shown at arrows. Note the marked decrease in peak Ca2+ current in the neuron from the symptomatic R6/2 transgenic (double arrow).
Correlative measures of intracellular Ca2+ concentration using Fura-2 were made during ramp voltage command protocols. Two procedures were used to measure intracellular Ca2+ concentration. In the first, Ca2+ concentrations were converted into nanomolar units using the Grynkiewicz equation (Grynkiewicz et al., 1985). The second method presents values as ratio units (Table I, ΔF/F). In general, changes measured using the Grynkiewicz equation tended to be larger than those obtained using ratiometric comparisons. There were no differences in mean basal Ca2+ concentration for neurons obtained from transgenic and WT mice in either pre- or symptomatic groups (Table I). There was, however, a statistically significant decrease in basal Ca2+ concentration between the pre- and symptomatic age groups for both R6/2 transgenics and WTs (Table I; ANOVA, P < 0.05). This decrease may represent a maturational effect. In neurons from presymptomatic mice, there was no difference in mean percent change in Ca2+ concentration between transgenic and WT mice (Table I). Neurons from R6/2 transgenics reached peak values, however, significantly faster than neurons from WT controls (Table I). In marked contrast, neurons from symptomatic mice had mean peak changes in Ca2+ concentrations that were significantly lower, and significantly more time was required to reach the peak concentration (Table I). The marked decreases in intracellular Ca2+ influx in response to voltage ramps correlate with the decreases in Ca2+ conductances in the neurons from symptomatic mice.
NMDA and AMPA Currents
NMDA (50 μM, 3 min duration) was bath applied to striatal slices in the two age groups while holding the cell membrane potential at −70 mV and after blockade of voltage-gated Na+, K+, Ca2+ conductances and non-NMDA receptors (Fig. 3). The current evoked by NMDA could be blocked by AP5 (50 μM, Fig. 5C). Mean peak membrane currents and current densities were significantly larger in neurons from presymptomatic R6/2 transgenics than WT controls (Table II). Mean peak NMDA-induced currents and densities also were larger in neurons from symptomatic R6/2 transgenics than in WTs (Table II). This effect was only statistically significant for current densities in the symptomatic mice (Table II). There were no differences between WT and R6/2 transgenics in the time to reach the maximum current (Table II).
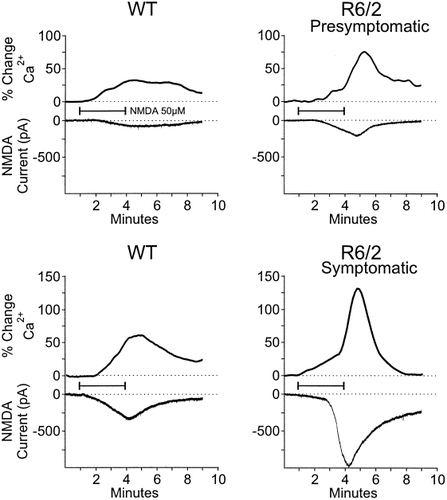
Changes in NMDA current and percent change in intracellular Ca2+ in striatal cells from pre-symptomatic R6/2 transgenics and their age-matched WTs (top two panels) and symptomatic R6/2 transgenics and their age-matched WT (bottom two panels). In each panel top traces show bath application of NMDA (50 μM, 3 min) produces an increase in intracellular Ca2+ and bottom traces show the induced NMDA current. Greater increases in intracellular Ca2+ and NMDA current occurred in both the pre- and symptomatic R6/2 transgenics compared to WTs. Horizontal line indicates application of NMDA. Ca2+ concentration is expressed as percent change of nM units.
Inspection of the data revealed a marked increase in variation in peak current amplitudes and current densities of neurons from R6/2 transgenics compared to WTs (Table II). In both pre- and symptomatic R6/2 transgenics two populations of cells emerged; one population with peak NMDA currents and densities similar to WT animals, and a second population with much higher peak currents and current densities. In both WT groups only two neurons had peak currents greater than 400 pA [WTs age-matched to presymptomatic R6/2s = 1/10 (10%); WTs age-matched to symptomatic R6/2s = 1/15 (7%)]. In contrast, in R6/2 transgenics approximately 30% of the cells had peak currents larger than 400 pA [presymptomatic = 6/22 (27%); symptomatic = 5/14 (38%)]. Differences between distributions were statistically significant when evaluated with distributional measures for detecting changes in variance between two populations that have similar central tendencies (P < 0.02) (Gibbons, 1985). Because there appeared to be two populations of neurons in both pre- and symptomatic R6/2 mice, we divided neurons from the R6/2 transgenics into two groups (“most-responsive” and “least-responsive” neurons) with a between group cutoff of 400 pA (equivalent to about 4 pA/pF for current density) in subsequent analyses. We used this cutoff because it represented less than 10% of the population of neurons obtained from WT mice.
In both pre- and symptomatic R6/2 transgenics, the population of most-responsive neurons displayed markedly increased amplitude and duration of current density responses to application of NMDA compared to least-responsive neurons and neurons from WT mice (Fig. 4, top panels). Differences in current density of most-responsive neurons were significantly greater than those of least-responsive and WT neurons 4–6 min after NMDA application began (ANOVA, Tukey's post-hoc test, P < 0.05) and remained elevated for at least 9 min in presymptomatic mice. In symptomatic mice, differences in current density of most-responsive neurons were significantly greater than those of least-responsive and WT neurons 2–9 min after NMDA application began (ANOVA, Tukey's post-hoc test P < 0.05). In the most-responsive neurons from the presymptomatic R6/2 transgenics NMDA current density peaked at about 5 min whereas in the symptomatic neurons current density changes peaked at 3 min.
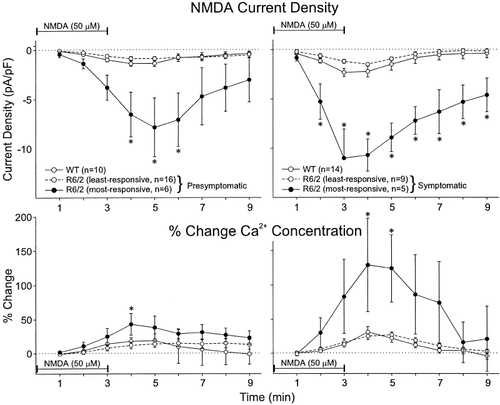
Time course of concurrent changes in mean NMDA-induced current densities (±SE) and mean percent changes (±SE) in Ca2+ concentration in pre-symptomatic R6/2 transgenics and their age-matched WT (left two graphs) and symptomatic R6/2 transgenics and their age-matched WTs (right two graphs). Transgenic groups were divided into least- and most-responsive neurons (see Results for details and justifications). Asterisks indicate that the most-responsive R6/2 transgenic groups were statistically significantly different from their respective WT and least-responsive transgenic group. Horizontal line indicates application of NMDA. Percent change in Ca2+ concentration determined as in Figure 3.
The increased mean peak current and density were specific to activation of NMDA receptors. Bath application of AMPA (5–25 μM; 3 min duration) produced significantly larger mean peak membrane currents and densities in neurons from WTs compared to those from R6/2 mice (1,502 ± 164 [n = 10] vs. 858 ± 153 [n = 7] pA for peak current [P < 0.025] and 19 ± 2 vs. 13 ± 2 pA/pF for peak current densities [P < 0.05] in neurons from WT and R6/2 mice respectively; data obtained from the symptomatic group only).
The inversion of group differences with exposure to AMPA provides evidence against differential space clamp limitations accounting for the enhanced responses to NMDA in slices from R6/2 transgenics and WT. The fact that voltage-gated Ca2+ currents also were reduced in transgenic animals argues that unclamped Ca2+ currents that were not blocked by Cd2+ are less likely to contribute significantly to the observed enhancement of NMDA currents in these mice.
Intracellular Ca2+ concentration changes induced by exposure to NMDA also were evaluated using Fura-2. Ca2+ concentration changes were expressed using two measures, and both procedures produced similar results (Table II). In the neurons from presymptomatic R6/2 mice there was a significant increase in peak intracellular Ca2+ concentration after exposure to NMDA compared to WT animals (Table II). In symptomatic mice the increment was even larger but with considerable cell–cell variation (Table II).
Similar to current density changes, we also examined the time course in the change in intracellular Ca2+ concentration (Fig. 4, bottom graphs). Neurons from pre- and symptomatic R6/2 transgenics were divided into the two populations (most-responsive and least-responsive neurons) based on a peak current response of 400 pA as indicated above. The most-responsive population of neurons from presymptomatic R6/2 mice displayed slightly higher increases in intracellular Ca2+ than neurons in the other two groups from the 3–6 min time points. Only the difference at 4 min was statistically significant (ANOVA, Tukey's post-hoc test, P < 0.05). In the symptomatic group, the most-responsive neurons displayed much larger increases in intracellular Ca2+ from 3–7 min, but there was considerable cell–cell variability. Differences were statistically significant at only 4–5 min (ANOVA, Tukey's post-hoc test, P < 0.05).
In contrast to NMDA, the Ca2+ increase due to activation of AMPA receptors was small but similar in R6/2 transgenics and WT [28 ± 5 (n = 10) vs. 25 ± 5% (n = 8) in WT and R6/2 respectively; data obtained only from the symptomatic group]. This result emphasizes the specificity of the increased Ca2+ response being due to NMDA receptor activation.
Correlations Between Ca2+ Conductances and Responses to NMDA
Pearson Product-Moment correlations were computed to determine relationships between altered Ca2+ conductances and responses to NMDA. In both pre- and symptomatic groups there were no significant correlations between Ca2+ conductances (either peak whole-cell current or current density and peak NMDA current or current density) for transgenics or WT neurons. Correlation coefficients ranged from +0.257 to −0.244. This finding indicates that the changes in responses to NMDA in R6/2 mice were independent of the changes in voltage-gated Ca2+ conductances and these outcomes represent different effects. In both groups of WT mice there was a series of low but consistent positive correlations between Ca2+ influx during the ramp voltage command and Ca2+ influx during NMDA receptor activation. Correlation coefficients ranged from +0.304 to +0.572 (2/8 were statistically significant). This suggests that the degree of intracellular Ca2+ increase might reflect some intrinsic property specific to striatal neurons. This relationship was absent in neurons from both pre- and symptomatic R6/2 transgenics (R's ranged from −0.443 to −0.079; none were statistically significant). The lower correlations were probably due to the marked changes in Ca2+ flux in the R6/2 transgenic animals, the increased responses to NMDA, and the decreased responses to ramp voltage commands.
Voltage-Dependence
One explanation for the increased NMDA current in the R6/2 transgenic is altered voltage-dependence of the NMDA receptor. In a separate group of neurons from symptomatic R6/2 mice and WT controls (three R6/2 mice, seven neurons; three WT mice, four neurons), changes in NMDA receptor voltage-dependence were evaluated by digital subtraction of the current evoked by a downward ramp voltage command (+30 to −90 mV) before and after bath application of NMDA (50 μM; 1–2 min) (Fig. 5A, inset). NMDA currents showed strong voltage-dependence in both control and transgenic mice. Peak NMDA currents occurred at about −30 mV in symptomatic R6/2 transgenics and WTs (Fig. 5A). The NMDA receptor current density was significantly greater in R6/2 transgenics from −70 mV to −20 mV membrane potentials compared to WTs (ANOVA, Tukey's post-hoc test, P < 0.05).
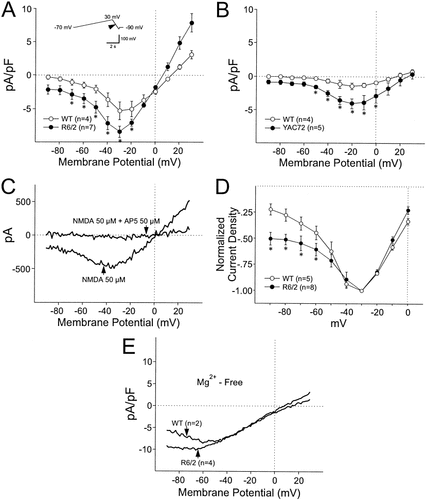
A,B: Current density-voltage plots in response to NMDA (50 μM) for neurons from symptomatic R6/2 (A) and YAC72 (B) transgenics and their respective age-matched WT controls. Plots (mean ± SE) obtained in response to a ramp voltage command from −70 mV to +30 mV and then to −90 mV (inset) 1 min after application of NMDA began. Data obtained from the downward ramp (arrow in inset). Data for each neuron was the result of subtraction of ramp-induced currents in the presence of NMDA from ramp-induced currents before NMDA was applied. Asterisks indicate transgenic groups differed significantly from their respective WT controls. There was an increase in NMDA current density in both HD transgenics. N's for each group in parentheses. C: Blockade of the NMDA response in the presence of AP5 in a neuron from a symptomatic R6/2 transgenic. When NMDA was applied alone, the ramp voltage command produced a typical current-voltage plot. In the presence of NMDA and AP5, the same ramp voltage command produced no current. Data obtained by subtraction as indicated in A and B. D: Current densities were normalized by setting the value for each neuron at −30 mV equal to −1. R6/2 transgenics displayed a statistically significant less steep negative slope conductance between −90 and −30 mV (asterisks). E: Effect of removal of Mg2+. In neurons from R6/2 transgenics greater current densities occurred only at the most hyperpolarized membrane potentials (−60–−90 mV) in the absence of Mg2+. At membrane potential from −50 mV–+30 mV, however, the current densities were similar. Data obtained by subtraction as indicated in A and B.
The negative slope conductance tended to be less steep in R6/2 animals. To quantify these conductances, current densities were normalized to −1 at the −30 mV holding potential where the peak for NMDA current density amplitude occurred (data obtained by subtraction from two WT mice, five neurons and three R6/2 mice, eight neurons from the symptomatic group). Normalized current densities between −90 and 0 mV in 10 mV steps based on the value at −30 mV were computed for each cell. The rate of change of normalized current density between −90 and −30 mV was significantly greater in cells from the symptomatic WTs than in the R6/2 transgenics (ANOVA, P < 0.025) (Fig. 5D).
After removal of Mg2+ (three symptomatic R6/2 mice, four neurons; two WT mice, two neurons), the current evoked by NMDA was greater in R6/2 transgenics at membrane potentials more negative than −50 mV but similar at the other less hyperpolarized membrane potentials (Fig. 5E). These findings suggest that the voltage-dependence or the NMDA receptor sensitivity to Mg2+ in R6/2 transgenics may be altered implying that subunit composition of the receptor may be changed.
Dissociated Neurons
We examined NMDA evoked currents in acutely dissociated neurons from symptomatic R6/2 transgenic and WT mice. These experiments were performed in Mg2+-free solution to determine if alterations in the Mg2+ sensitivity of the NMDA receptor could contribute to the increased NMDA responses that occurred in the most-responsive R6/2 neurons in slices. In addition, the membrane potential was clamped at −40 mV to inactivate Ca2+ conductances. We did not use Cd2+ in these experiments because the rapid peak NMDA response is decreased markedly in acutely dissociated neurons by application of Cd2+. Na+ and K+ conductances were blocked as described above. A rapid application system was used in these experiments in which NMDA was pressure ejected within several msec. With this application system two components of the response to NMDA become evident, a rapid desensitizing peak response followed by a steady state current (Fig. 6).
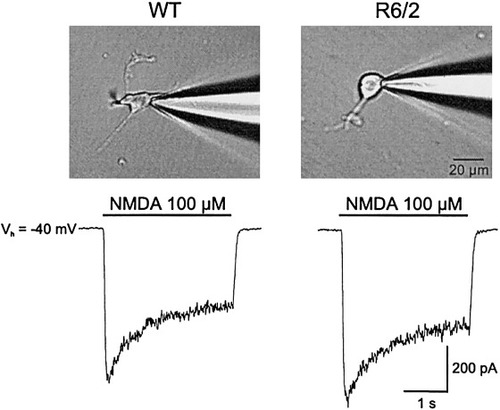
Examples of NMDA-evoked currents in isolated medium-sized neurons from age-matched WT and symptomatic and R6/2 mice. Top panels show patch pipette attached to isolated neurons. Bottom traces show current responses to application of NMDA (100 μM, 3 sec). Note two components to the current response, a rapid peak that desensitizes followed by a steady-state current.
Acutely dissociated medium-sized striatal neurons were obtained from four pairs of symptomatic R6/2 transgenics and age-matched WTs. Basic membrane properties were not significantly different between groups (n = 12 and 13 cells in WT and R6/2 transgenics, respectively). Cell capacitances were 6.7 ± 0.8 pF in WTs and 7.3 ± 1.1 pF in R6/2 transgenics, input resistances were 2.9 ± 1 GΩ in WTs and 2.4 ± 1 GΩ in R6/2 transgenics, and time constants were 108 ± 9 μsec in WTs and 143 ± 26 μsec in R6/2 transgenics. Similar to neurons in slices, voltage-gated Ca2+ conductances were reduced in cells from symptomatic R6/2 animals (242 ± 63 pA in WTs vs. 160 ± 61 pA in transgenics); however, the difference was not statistically significant.
Mean currents evoked by 100 μM NMDA were slightly larger in neurons obtained from the R6/2 transgenics compared to those from WTs (349 ± 83 vs. 321 ± 45 pA for the desensitizing peak; 198 ± 50 vs. 163 ± 25 for the steady-state current, in neurons from R6/2 and WT mice, respectively). No least responsive neurons were found in the population of R6/2 cells examined. Note also that there was an increased variation in both mean desensitizing and steady-state currents in the dissociated neurons from the R6/2, similar to the findings in slices. These results underscore the possibility that NMDA receptors in R6/2 transgenics may have a differential Mg2+ sensitivity than neurons from WT mice because Mg2+ removal reduced the differences in NMDA-induced current amplitudes.
Immunohistochemistry
NMDA receptor expression of NMDA-R1 and NMDA-R2A/B subunit proteins were examined using immunohistochemistry. These subunit proteins were chosen because the R1 subunit is a constituent of all functional NMDA receptors and is used as the prototype for morphological detection of the receptor. The R2 subunits (A–D) comprise the other principal NMDA receptor subunit types. The 2A/B subunits contain the Mg2+ binding pocket (Williams et al., 1998) of the NMDA receptor. In pre- and symptomatic groups of R6/2 and WT mice, NMDA-R1 subunit staining was detected within striatal somata and neuropil (Fig. 7A,B). Positively reacting neurons were medium-sized in diameter and showed a strong cytoplasmic expression pattern. Stained NMDA-R1 processes could be seen extending through the neuropil and some were contiguous with reactive cell bodies. There was also a distinct, punctate stain within some axons of passage coursing within the internal capsule. There was a small but consistent increase in NMDA-R1 protein staining intensity within somata and neuropil in the presymptomatic R6/2 vs. the WT striatum (Fig. 7A). To provide quantitative estimates of changes, mean intensities of all pixels were computed for 2–3 equal-sized regions of neuropil (regions that did not have fibers of passage) in matched pairs of R6/2 and WT mice. These were averaged for each animal and the percentage change with respect to the WT was calculated. The striata from presymptomatic R6/2 mice displayed a small increase (occurring in each of four pairs) in intensity (7 ± 6%). This difference was not statistically significant. In symptomatic mice, NMDA-R1 subunit staining was more pronounced especially within the neuropil and processes in the R6/2 tissue (Fig. 7B). Processes could be followed for long distances through the neuropil. There was a statistically significant increase in mean staining intensity in the R6/2 transgenics compared to the WTs (21 ± 9%, t-test, P < 0.05).
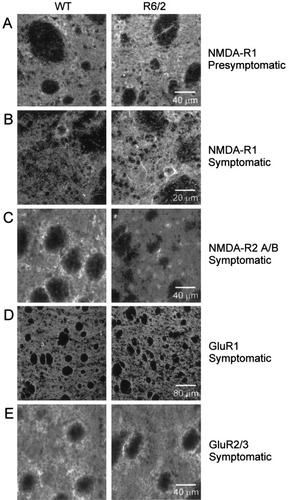
Immunohistochemistry of glutamate receptor subunit proteins in striata from pre- and symptomatic R6/2 and age-matched WT mice. A: The NMDA-R1 subunit showed a slight elevation in staining intensity in the striatum of a presymptomatic R6/2 transgenic compared to an age-matched WT control. Immunofluorescence showed a neuropil reaction surrounding medium-sized, positively stained neurons in both R6/2 and WT. Axons of passage within the internal capsule fiber bundles showed limited staining for the subunit. B: NMDA-R1 staining intensity was elevated markedly in the striatum of a symptomatic R6/2 transgenic compared to its paired WT control. Intensely stained processes were visible emanating from reactive neurons in the symptomatic R6/2 striatum. Reactive axons within the internal capsule also were evident compared to the WT. C: NMDA-R2A/B subunit expression was attenuated in the striatum of a symptomatic R6/2 transgenic compared to its paired WT control. Staining for these subunits was detected within a population of medium-sized neurons and the surrounding neuropil. No axonal reaction was present in the axons of passage in the internal capsule. D: GluR1 subunit expression was equivalent in a symptomatic R6/2 striatum and its paired WT control. An intense staining reaction was distributed within the neuropil and a small group of larger sized neurons in both R6/2 and WT striata. E: Striatal staining for the AMPA receptor subunits GluR2/3 was decreased slightly in the neuropil of the striatum of symptomatic R6/2 compared to its age-matched WT. Subpopulations of medium diameter striatal neurons also exhibited these receptor subunits, whereas the axons in the internal capsule were not reactive.
NMDA-R2A/B was examined in the symptomatic group. Expression of this subunit occurred in both somata and the neuropil (Fig 7C). No staining could be detected within the axons of the internal capsule. The intensity of the NMDA-R2A/B staining pattern was reduced significantly in the symptomatic R6/2 vs. the age-matched WT (23 ± 4%, t-test, P < 0.05). These results suggest that populations of NMDA receptors in the symptomatic R6/2 striatum may have reduced Mg2+ binding and thus could be less sensitive to voltage regulation.
In contrast to the NMDA receptor subunit changes, little difference was noted in the AMPA receptor subunit proteins, GluR1 and GluR2/3. The GluR1 subunit is expressed ubiquitously, is a constitutive element of AMPA receptors, and is employed to distinguish the receptor morphologically (Catania et al., 1995). Staining for the GluR1 receptor was distributed robustly within the striatal neuropil and within a small number of intensely reactive larger neurons (Fig. 7D). The majority of the medium-sized striatal neurons exhibited sparse staining for the GluR1 subunit, and were difficult to distinguish from neuropil expression (Ariano et al., 1998). There were no differences in either the quality or quantity of fluorescent staining for the GluR1 protein in matched sets of symptomatic R6/2 and WT striata. The GluR2/3 subunits also were evaluated as an indicator of striatal AMPA receptors. The morphological expression was seen in medium-sized neurons, within the neuropil, and a slight reaction was noted within the axons of passage in the internal capsule. Minimal alterations in the staining intensity were detected (1 ± 0.6% decrease in staining intensity in R6/2 compared to WT). These immunohistochemical findings add further support to the physiological assessment of AMPA currents showing small decreases in conductance changes in the symptomatic R6/2 neurons vs. those from the WT striata.
YAC72 Transgenics
The YAC72 transgenics expressing full-length mutant huntingtin with 72 glutamine repeats were examined when animals were between 1–2 years of age. These animals have selective neural loss predominantly involving the medium spiny neurons of the lateral striatum and develop behavioral abnormalities much slower than the R6/2 transgenics. YAC72 transgenics were less impaired behaviorally compared to the symptomatic R6/2 transgenics when evaluated in the present experiments. The direction of the changes in membrane capacitance and input resistance were similar between the YAC72 and symptomatic R6/2 transgenics but were smaller in magnitude in the YAC72 (88 ± 11 [n = 10] vs. 82 ± 7 [n = 10] pF for membrane capacitance and 105 ± 33 [n = 10] vs. 189 ± 44 [n = 10] MΩ for input resistance in neurons from WT and YAC72 mice, respectively). Time constants were unchanged in YAC72 transgenics (1.7 ± 0.2 [n = 9] vs. 1.7 ± 0.3 [n = 8] msec for neurons from WT and YAC72 mice, respectively).
YAC72 transgenics showed similar, although less dramatic, alterations in responses to NMDA application as the R6/2 transgenic, indicating that increased responsiveness was not unique to the R6/2 model. NMDA currents were examined using the ramp protocol before and after bath application of NMDA. NMDA current densities were significantly larger in YAC72 transgenics than in WT mice from −50 mV to 0 mV (Fig. 5B; ANOVA, Tukey's post-hoc test, P < 0.05). No obvious changes in voltage-dependence between neurons from YAC72 and WT mice were apparent and NMDA current peaked at about −20 mV in each group.
When the membrane was clamped at −70 mV, the direction of changes were similar to those observed in the R6/2 mice. Peak NMDA currents were higher in YAC72 transgenics (189 ± 43 pA in WTs vs. 244 ± 101 pA in YAC72 transgenics). Current densities were higher (2.3 ± 0.6 pA/pF in WT vs. 3.1 ± 1.4 pA/pF in transgenics). Basal Ca2+ levels were similar in neurons from YAC72 and WTs (52 ± 4.6 vs. 52 ± 3.3 in neurons from WT and YAC72 mice, respectively). The Ca2+ increase during NMDA application was higher in the transgenics (29 ± 5% in WTs vs. 46 ± 22% in YAC72 transgenics). The differences in peak currents and densities and percent Ca2+ were not statistically significant because of the high variability in the values from transgenic neurons, an effect also observed in the R6/2 transgenics. Because only a small cohort of YAC72 animals were available, we were not able to record from a sufficient group of these neurons to determine if two subpopulations were present as observed in the R6/2 transgenic.
DISCUSSION
The principal finding of these experiments was increased responses to NMDA receptor activation in a sub-population of medium-sized neurons in both pre- and symptomatic R6/2 transgenics. Increased responsiveness to NMDA also was observed in neurons from symptomatic YAC72 transgenic mice. The increased responses to NMDA were accompanied by increased Ca2+ concentration in the cell. Changes in Ca2+ concentration were not due to increased activation of voltage-gated Ca2+ conductances as these channels were blocked with Cd2+. Moreover, voltage-gated Ca2+ conductances were reduced in symptomatic R6/2 transgenics. In contrast to the increased response to NMDA, there was a significant decrease in the response to AMPA in symptomatic R6/2 mice, and AMPA did not cause a differential elevation in intracellular Ca2+ between R6/2 transgenics and WT mice. These electrophysiological findings were supported by immunohistochemistry. Striatal NMDA-R1 subunit protein staining was enhanced, especially in symptomatic R6/2 transgenics. There also were minimal changes in the AMPA receptor subunit proteins, GluR1 and GluR2/3. Therefore, the increase in NMDA current and the concurrent elevation in internal Ca2+ were due to selective changes in NMDA receptors in the R6/2 transgenics.
Similar enhancements in NMDA currents and Ca2+ concentrations in a subpopulation of striatal neurons occur in a third transgenic model evaluated in our laboratory (Chase et al., 1998; Laforet et al., 1998, 2001). The transgene in this model contains 100 CAG repeats in a human huntingtin cDNA that encodes the first one third of the gene. These transgenic mice develop motor deficits at about 2–3 months after birth that progress in severity with age. Symptomatic animals with this HD construct, tested about 1 year of age, exhibited a population of medium-sized striatal neurons that displayed increased responses to NMDA and concurrent increased Ca2+ concentration compared to WT controls. Thus, increased NMDA receptor-mediated responses in populations of medium-sized striatal neurons are common to the R6/2, the YAC72 and the 100 CAG repeat transgenic. The severity of change in NMDA receptor function may correlate with the length of the CAG expansion. Changes in NMDA receptor sensitivity were greater in symptomatic R6/2 mice that have about 150 CAG repeats and transgenic mice with 100 CAG repeats than in the YAC72 transgenic with 72 CAG repeats. Our previous work supports this contention in that NMDA-induced cell swelling was greater in knock-in mice with 94 CAG repeats compared to mice having a 71 CAG expansion (Levine et al., 1999). Increased length of the CAG expansion may not be the only factor because in the transgenic mice shorter forms of the construct that produces the mutant huntingtin (as in the R6/2 and CAG 100 transgenic) appear to cause more severe effects (Tobin and Signer, 2000).
The NMDA responsiveness of medium spiny striatal neurons could be separated into two distinct groups in both the pre- and symptomatic ages of R6/2 mice, although the low number of YAC72 neurons recorded precluded making comparable distinctions in this model. Similar subpopulations have been detected in the CAG100 HD transgenic model (Laforet et al., 2001). There is a differential sensitivity to neurodegeneration in HD of striatal medium spiny neurons (Richfield et al., 1995). Enkephalin-containing spiny neurons degenerate first in human HD whereas other types of medium-sized neurons (substance P, somatostatin, calretinin) are less vulnerable to the disease (DiFiglia, 1990). Gene expression studies also have shown early alterations in enkephalin-containing striatal neurons in HD mouse models (Luthi-Carter et al., 2000; Menalled et al., 2000), and we have detected early losses in met-enkephalin immunohistochemistry in the R6/2 and CAG100 HD transgenic (Ariano, unpublished observations). It is possible that the striatal neuronal subpopulation that displays the most elevated response to NMDA application may produce enkephalin and be more susceptible to the disease in the HD models.
There are a number of mechanisms that could account for the enhanced NMDA responses in HD models. The most parsimonious explanation is that an increase in NMDA receptor numbers occurs in a subset of striatal neurons. There is little evidence, however, for this and quantitative in situ hybridization and receptor binding autoradiography do not detect increases in the R6/2 (Cha et al., 1998). An alternative hypothesis is that the functional properties of the NMDA receptor have been altered by the mutation. For example, the subunit composition of the receptor may have changed. Immunohistochemistry showed that the R1 subunit of the NMDA receptor is elevated significantly in the R6/2. This elevation also has been detected in the CAG100 transgenic (Ariano, unpublished observations). Several additional observations support the alternative of functional receptor changes and provide evidence for differences in Mg2+ sensitivity. There was an increased NMDA current in the R6/2 at more hyperpolarized membrane potentials. This outcome was associated with a reduction in the steepness of the negative slope conductance in the R6/2. When the Mg2+ concentration was reduced markedly, there was still some Mg2+ block in the WT whereas the current voltage relationship in the R6/2 transgenic was more linear, especially at hyperpolarized holding potentials. In the acutely dissociated neurons when the membrane was held at −40 mV in a low Mg2+ bath there was little difference in peak NMDA currents between neurons from R6/2 transgenics and WTs, again emphasizing that Mg2+ sensitivity may be altered. Finally, immunohistochemical experiments demonstrated a marked reduction in staining for the NMDA-R2A/B subunits. The NMDA-R2A/B subunits provide the Mg2+ binding region for the receptor (Williams et al., 1998). A decrease in the expression of the NMDA-R2A/B subunits would significantly alter the properties of NMDA receptor functioning. Another possibility is that changes in protein kinase C may alter the Mg2+ block of NMDA receptors (Chen and Huang, 1992).
Cell culture studies have shown a relationship between NMDA receptor function and mutated huntingtin. Using HEK 293 cells, receptors composed of NR1/NR2B subunits, produced larger currents in response to NMDA application when cells were co-transfected with mutated huntingtin (Chen et al., 1999). Preliminary studies from the same laboratory have used acutely dissociated striatal neurons derived from the YAC72 transgenics and showed increased NMDA-evoked peak currents (Chen et al., 2000). YAC72 striatal cultures have confirmed an increase in vulnerability of neurons expressing mutated huntingtin after stimulation of NMDA receptors (Zeron et al., 2001). Primary striatal cultures from the R6/2 model demonstrate that glutamate and NMDA produced larger increases in intracellular Ca2+ levels (Hoyt and Higgins, 2000), supporting our studies of neuronal susceptibility in the R6/2 (Levine et al., 1999).
R6/2 medium-spiny neurons exhibit a depolarized resting membrane potential (Levine et al., 1999). This outcome could contribute to decreased Mg2+ blockage in the R6/2, especially in dendrites outside the voltage clamp. The resting membrane potentials are not depolarized in the 100 CAG repeat model (Laforet et al., 2001), or in the YAC72 (Klapstein and Levine, unpublished observations). This indicates that multiple mechanisms may contribute to increased NMDA responses. We have demonstrated decreased K+ conductances in two HD models, the R6/2 and the 100 CAG (Ariano et al., 2000). Altered K+ conductances that affect the membrane potential could provide an environment in which striatal neurons become susceptible to prolonged depolarization-induced toxic events that would lead to subsequent cellular degeneration.
Changes in cortical projection neurons and the corticostriatal input have been observed in HD (DiFiglia et al., 1997). Cytoplasmic accumulation of mutant huntingtin occurs in pyramidal neurons and is associated with degeneration of the corticostriatal pathway (Sapp et al., 1999). Changes in cortical pyramidal neurons take place early in the disease in the CAG 100 transgenic model (Laforet et al., 2001). Preliminary experiments in the R6/2 show marked degenerative changes in cortical pyramidal neurons using the Golgi impregnation technique (Fisher, Zanjani, Chesselet and Levine, unpublished observations). Aggregation of N-terminal huntingtin occurs in axon terminals in HD mouse models, and would contribute to neuronal dysfunction (Li et al., 2000). Thus, the postsynaptic changes in striatal NMDA receptor function demonstrated in the present study may be reinforced by alterations in presynaptic inputs that may denervate spines of striatal neurons. In the R6/2 transgenic, the cytoarchitecture of striatal spiny dendrites is altered significantly in biocytin-filled neurons and in Golgi analysis (Klapstein et al., 2000). The lack of alterations in responsiveness to NMDA in acutely dissociated R6/2 neurons may be related to the removal of these peripheral dendritic processes by the dissociation procedure. The hyper-responsive NMDA receptors would have been removed in this preparation.
Reductions in striatal excitotoxicity have been reported in vivo in the R6/1 and R6/2 1–2 weeks after infusion of glutamate receptor agonists (Hansson et al., 1999; Morton and Leavens, 2000). In contrast, we find that striatal NMDA receptors are hyper responsive after acute application of NMDA in a subset of R6/2 striatal neurons. The resistance to excitotoxicity is not due to loss of striatal neuron responsiveness (MacGibbon et al., 2000). Underlying changes in corticostriatal afferents, and not the postsynaptic striatal environment may produce reduced excitotoxicity in the in vivo paradigm. Generation of excitotoxicity in vivo requires an intact glutamatergic input to the target structure that is subject to excitotoxic challenge. Kainic acid lesions of the hippocampus require an intact perforant pathway (Köhler et al., 1978) and kainate-induced degeneration of striatal neurons is dependent upon the integrity of the corticostriatal pathway (McGeer et al., 1978). We have observed reduced excitatory input to striatal neurons in two of the HD models. Stronger electrical stimulation of the corticostriatal pathway is required to induce excitatory postsynaptic potentials in cells from symptomatic R6/2 and in the transgenic with 100 CAG repeats (Klapstein et al., 2000; Laforet et al., 2001). In addition, we have preliminary evidence of reduced spontaneous excitatory postsynaptic currents in R6/2 mice (Cepeda et al., 2001). It is possible that reduced glutamate inputs, coupled with the decrease in AMPA receptor responsiveness, combine to diminish or prevent NMDA receptors from being continuously activated in the R6/2 transgenic animals after injection of excitotoxins in vivo.
In conclusion, we present evidence for increased responsiveness of NMDA receptors in multiple mouse models of HD. The alterations in NMDA receptor function may predispose subpopulations of striatal neurons to excitotoxic damage and can lead to subsequent degeneration. These findings provide a physiological rationale for development of pharmacological treatments for HD that target NMDA receptors.
Acknowledgements
We greatly appreciate the skillful assistance of Ahrin Koppel, Mary Kay Lobo, Eve Jokel, Ehud Gruen, Lindsey Christian and Timothy Lobo. We thank Donald Guthrie, PhD, Professor of Biomathematics and Psychiatry and Prabha Siddarth, PhD, for consultation regarding the statistical analyses. We thank Dr. Marie-Françoise Chesselet for her useful discussions and critique of a previous version of this manuscript. This work was supported by grants from the Hereditary Disease Foundation (MRH, MAA and MSL), MRC/CIHR of Canada and the Canadian Genetic Disease Network (MRH and BRL), and the Huntington Disease Society of America (MRH). Dr. Michael Hayden is an established investigator of the BC Children's Hospital.




