Water maze learning and forebrain mRNA expression of the neural cell adhesion molecule L1
Abstract
L1 and NCAM, two cell adhesion molecules of the immunoglobulin superfamily, have been implicated in the formation of neural circuits, synaptic plasticity, and cognitive function. In this study, we sought to investigate whether differences in the steady-state levels of L1 and NCAM expression in specific brain regions could account for individual differences in learning abilities. Using adult male Wistar rats, we evaluated mRNA levels of L1, NCAM, and the NCAM180 isoform in different brain regions (hippocampus, thalamus, striatum, prefrontal and frontal cortices) immediately after submitting rats to a massed training protocol in the water maze. The results showed that untrained and trained rats exhibited similar levels of mRNA for these molecules, which supports the view that training did not influence their immediate level of expression. However, in most of the brain regions we investigated (with the exception of prefrontal and frontal cortices), L1 mRNA levels were positively correlated with the latency to find the hidden platform in the water maze task and with posttraining plasma corticosterone levels. However, no correlations were observed for total NCAM or NCAM180 mRNA in the brain regions examined in this study. Given that animals with a slower spatial acquisition curve exhibited more anxiety-like responses, including thigmotactic behavior in the water maze and increased corticosterone levels, and that recent genetic studies indicate a role for L1 in anxiety, the current findings suggest a relationship among L1, anxiety, and cognitive processes. © 2003 Wiley-Liss, Inc.
Learning abilities can vary considerably among individuals of the same species (James, 1890; Schwegler and Crusio, 1995; Habib et al., 2000). Although rodent studies indicate that the variance in learning performance found within a specific strain is particularly large in aged populations (Rowe et al., 1998; Schulz et al., 2002), a certain variability is also present in young animals (Venero et al., 2002a), which might be predictive of cognitive impairments in the elderly (Dellu et al., 1994). Investigation of the neurobiological mechanisms involved in individual differences in learning abilities could, therefore, have an impact on the understanding of neural processes involved in many domains of memory research (such as normal learning, ageing-related cognitive decline, and intelligence).
The remodelling of neural circuits is widely accepted as representing a fundamental mechanism of information processing and storage (Malenka and Nicoll, 1999; Martin et al., 2000). Recent findings indicate that individual differences in the acquisition of a hippocampus-dependent spatial learning task, the Morris water maze (Morris et al., 1982), are followed by a learning-related differential expression of asymmetric synapses in the hippocampus, as evaluated at the level of the contacts between mossy fiber terminals and CA3 pyramidal cells at 24 hr posttraining (Sandi et al., 2003). Synaptic density was higher in animals that showed a poorer acquisition curve, which suggested that the neural circuits subserving learning in fast and slow learners might show a differential training-induced regulation and/or represent neural substrates with different properties for the acquisition of spatial responses. In support of the latter possibility, evidence has been presented indicating that differential performance in spatial learning tasks might be related to a priori differences in hippocampal morphometry (Crusio et al., 1993; Bernasconi-Guastalla et al., 1994; Schwegler and Crusio, 1995).
L1 and NCAM, two cell adhesion molecules of the immunoglobulin superfamily, have been implicated in the formation of neurocircuitry (Takei et al., 1999). Both are cell surface macromolecules, which, through Ca2+-independent homophilic and heterophilic cell–cell interactions, play key roles in morphogenesis, regeneration, and behavioral and synaptic plasticity (Jucker et al., 1996; Schachner, 1997; Rønn et al., 2000). Functional perturbation through the administration of antibodies or antisense oligonucleotides (Scholey et al., 1993, 1995; Lüthi et al., 1994; Rønn et al., 1995; Sandi et al., 1995; Arami et al., 1996) and genetic studies (Cremer et al., 1994; Lüthi et al., 1996; Wolfer et al., 1998) have implicated these adhesion molecules in learning-related synaptic plasticity. In addition, we have recently shown that exposure of rats to a chronic stress regime, which induces cognitive alterations in hippocampus-dependent tasks, resulted in increased L1 but decreased NCAM mRNA and protein levels in the hippocampus (Sandi et al., 2001; Venero et al., 2002b), suggesting that the degree of expression of these molecules in hippocampal circuits might be relevant for their functional implications in behavior.
Clinical studies further support a key role for these molecules in cognition. Mutations of L1 in humans produce a spectrum of neurologic abnormalities and mental retardation (for reviews see Fransen et al., 1997; Weller and Gärtner, 2001). NCAM, which is expressed in the brain as three main isoforms of 120, 140, and 180 kDa (NCAM-120; NCAM-140, and NCAM-180, respectively) molecular weight, has been found to be altered in an isoform-specific manner in the hippocampus and prefrontal cortex of schizophrenic patients (Barbeau et al., 1995; Vawter et al., 1998).
Given the critical importance of cell adhesion molecules in neural connectivity and cognition, we hypothesised that individual differences in learning abilities might be related to a differential expression of L1 and NCAM in specific brain regions. Therefore, we sought to assess their expression levels in relation to performance variability. Because protein levels of these adhesion molecules can be modulated by activity within a few minutes (Kamiguchi et al., 1998; Miñana et al., 2001), and training experience has indeed been shown to alter L1 and NCAM protein content in the hippocampus (Merino et al., 2000), we decided to evaluate the abundance of L1 and NCAM transcripts in the hippocampus (dorsal and ventral dentate gyrus, CA1, and CA3) and other forebrain regions (prefrontal and frontal cortices, striatum, and thalamus) immediately after subjecting rats to a massed training protocol in the water maze.
MATERIALS AND METHODS
Subjects
Male Wistar rats (Harlan Iberica), weighing 150–175 g on arrival, were housed in groups of three per cage, under temperature- and light-controlled conditions (22°C ± 2°C; 12:12 light-dark cycle; lights on at 7 AM). They had free access to food and water in a colony room. Approximately 5 weeks after arrival, they were handled daily for 4 days before being weighed. Rats were then matched in groups of two according to their body weight, and each of the two matched animals was assigned randomly to one of the two experimental groups. Behavioral experiments were conducted between 9:00 AM and 2:30 PM All efforts were made to minimize the number of animals used. Animal care procedures were in accordance with the NIH guidelines for the care and use of laboratory animals.
Water Maze Learning
The Morris water maze was a black circular pool (2 m diameter, 45 cm high) filled with water (30 cm depth) at 24°C ± 1°C. The pool was divided into four quadrants of equal size. An invisible escape platform (11 cm diameter) was placed in the middle of one of the quadrants (1.5 cm below the water surface) equidistant from the sidewall and middle of the pool. The behavior of the animal (latency, distance, and swim speed) was monitored by a video camera, mounted in the ceiling above the center of the pool, and a computerized tracking system (Ethovision 1.90, Noldus IT, The Netherlands).
Four different starting positions were equally spaced around the perimeter of the pool. The training session consisted of eight consecutive training trials started from one of the four start positions, used in a random sequence similarly for each rat. A trial began by placing the rat into the water facing the wall of the pool at one of the starting points. If the rat failed to escape within 120 sec, it was guided to the platform by the experimenter. Once the rat reached the platform, it was allowed to stay there for 30 sec and, then, placed in a holding cage for an intertrial interval of 30 sec. Because we wanted to evaluate whether CAM mRNAs are differentially expressed depending on the performance of rats in the water maze task, cognitive status of the animals was defined on the basis of the latency to find the platform throughout the training trials. Trial 1 was excluded because it was the first time the rats were challenged with the water maze requirements and, therefore, they did not have any information regarding platform location. Thigmotactic swimming, i.e., the behavior that an animal displays when swimming close to the walls of the water maze, was also quantified. For measuring thigmotactic swimming, the maze was divided into two circles. Time spent in the outer ring of the pool (18 cm wide) was designated as “thigmotactic swimming.”
Immediately after being submitted to the massed training procedure in the water maze (n = 11), rats were decapitated for the subsequent in situ hybridization analyses. To ensure that analyses were performed in all animals at the same delay from the start of the training procedure, decapitation took place in all animals 22 min after the beginning of the first training trial. The remaining rats, which were not subjected to behavioral testing (untrained, n = 9), were also decapitated at the same time to obtain their brains for the subsequent measurements of CAM mRNAs.
In Situ Hybridization
Immediately after decapitation, the brain was removed and immediately frozen in dry-ice-cooled isopentane. Subsequently, the brains were coded and stored at −80°C until required for further processing. In situ hybridization was performed essentially as described elsewhere (Süsens et al., 1997). In brief, antisense RNA probes labeled with α-[35S]UTP were generated with T3 or T7 RNA polymerase from linearized cDNA subclones of L1, total NCAM, and NCAM180 according to the manufacturer's instructions (Ambion). Probes were resuspended in hybridization mix containing 50% formamide, 1× Denhardt's solution, 4× SSC, 5% dextran sulfate, 500 μg/ml single-stranded DNA, 250 μg/ml yeast tRNA, and 10 mM dithiothreitol (DTT) to 4 × 106 cpm/ml. Sagittal sections (14 μm thickness) were cut on a cryostat at −18°C by using the rat brain atlas of Paxinos and Watson (1997) as an anatomical reference (∼0.70–0.90 mm lateral to the midline for prefrontal and frontal cortex evaluation and 2.10–2.40 mm lateral for hippocampus, striatum, and thalamus analysis). Care was taken to select identical anatomical levels of sections from trained and control animals by using eosin-stained reference slides. Sections were thaw mounted onto gelatin-coated slides and stored at −70°C until further processing.
Briefly, in situ hybridization was performed as follows: slides were thawed at room temperature for 10 min, fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS), acetylated, dehydrated, and subjected to in situ hybridization at 55°C for 18 hr. The slides were washed with 4× SSC and subsequently treated with RNase A (10 mM Tris-HCl, pH 8.0, 500 mM NaCl, 1 mM EDTA, 20 μg/ml RNase A) for 30 min at 37°C. After three washes in decreasing salt concentrations for 10 min at room temperature, a 30-min high-stringency wash was performed in 0.1× SSC at 55°C. After an additional wash in 0.1× SSC for 5 min, sections were dehydrated and exposed to high-resolution X-ray films (Kodak Biomax MR) for 72 hr. Specificity of the signals was verified by comparing antisense with sense controls. The probe for L1 comprised 3,277 nucleotides of the mouse cDNA (nucleotides 1–3,277; acc. No. X12875) cloned into the SmaI site of pBluescriptII SK (in both directions) and linearized with Xba I both for the antisense direction and the sense direction. The probe for total NCAM consisted of 250 nucleotides of the mouse cDNA (nucleotides 1,391–1,640; acc. No. X15049) cloned into pBluescriptII SK using ApaI and EcoRI and linearized with Asp718 for the antisense direction and with EcoRI for the sense direction. The probe for NCAM180 contained 294 nucleotides of the mouse cDNA (nucleotides 353–646; acc. No. X15052) cloned into pBluescriptII SK between the SpeI and the BamHI site and linearized with BamHI for the antisense direction and with XbaI for the sense direction.
Image Analysis
For the quantification of autoradiographic films, images were captured by high-resolution (600 × 600 dpi), eight-bit (256 gray levels) microdensitometry with a flat-bed scanner (AGFA Arcus II). The images of the autoradiographs were converted to gray values and analyzed for optical density measurements (corresponding to mRNA levels) with image-analysis software (LeicaQwin). Mean intensity of pixels was registered for circumscribed areas. The film background was subtracted. Experimental values given in Results are the mean optical densities of six sections for each animal, with the experimenter blind to the nature of the section being analyzed. We have previously validated that the results obtained with this technique and with a real-time, high-resolution microimager are equivalent (Venero et al., 2002).
Corticosterone Measurement
After decapitation, trunk blood was collected, and samples were centrifuged (3,000 rpm for 20 min at 4°C). Plasma was stored at −35°C. Corticosterone was measured using a radioimmunoassay kit (Coat-A-Count; Diagnostics Products Corporation). The intraassay variability of the RIA was 3.6%. Its sensitivity (minimal detectable concentration) was 5.7 ng/ml.
Statistical Analysis
All results are expressed as mean ± SEM. ANOVA with repeated measures (i.e., trial for results from the water maze, and brain region for the in situ hybridization data) was performed to assess overall effects of either water maze training or “maze performance” on the studied variables. Differences detected by ANOVAs were also further evaluated with Student's t-test post hoc comparisons. In addition, and regardless of the outcome of the repeated-measures ANOVAs, Student's t-tests were performed for in situ data from each brain region to assess possible differences between the groups at any specific region. Pearson correlational analyses were performed to evaluate possible associations among the behavioral, neurobiological, and endocrine parameters in the water maze-trained animals. Significance was accepted at P ≤ .05.
RESULTS
Water Maze Performance
The acquisition learning curve of the animals in the water maze indicates a gradual decrease in escape latency with progressive training. The ANOVA shows a significant effect of trial on escape latency (F7,80 = 5.04, P < .0001; Fig. 1a).
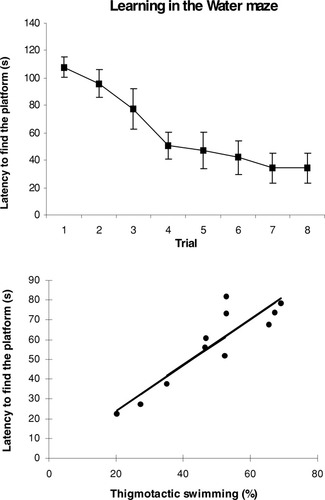
A: Acquisition learning curve of rats in a massed training protocol, in the Morris water maze. Results are the mean ± SEM from the 11 trained rats. B: Linear relationship between the latency to find the platform across the trials and thigmotactic behavior in the water maze.
Inspection of swimming paths during the course of training led us to distinguish important individual differences in the thigmotactic behavior of the animals. There was an interesting positive correlation between mean latency to finding the platform across trials 2–8 and thigmotactic swimming behavior in the pool (r = 0.90, P < .001; Fig. 1b).
L1 mRNA Expression
Figure 2 shows autoradiographic images illustrating the expression of L1, total NCAM, and NCAM180 mRNA from undisturbed and water maze-trained rats. The possible effect of water maze training on immediate expression of L1 mRNA was evaluated (Table I). ANOVAs indicated a lack of effect of training on L1 expression both throughout the different hippocampal subregions (DG-dorsal, DG-ventral, CA1, and CA3; F1,18 = 0.25, n.s.) and in a number of other brain regions analyzed [prefrontal cortex (PFC), frontal cortex (FC), thalamus, and striatum; F1,18 = 0.11, n.s.].
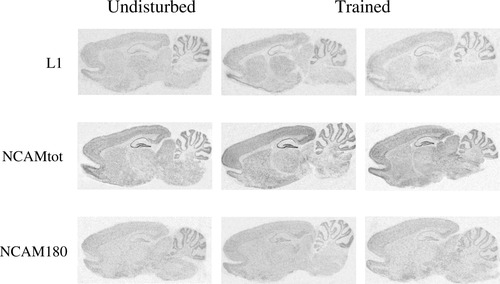
Digitized autoradiographic images showing the expression of L1, total NCAM, and NCAM180 mRNA levels in sagittal sections from untrained (left column) and water-maze-trained rats (a slow learner in the center column and a fast learner in the right column).
| Hippocampus | Other brain areas | |||||||
|---|---|---|---|---|---|---|---|---|
| DG-dorsal | DG-ventral | CA3 | CA1 | PFC | FC | Striatum | Thalamus | |
| L1 mRNA | ||||||||
| Untrained | 26.5 ± 2.3 | 26.2 ± 2.2 | 40.2 ± 3.7 | 33.8 ± 3.5 | 23.2 ± 2.4 | 28.5 ± 3.7 | 13.3 ± 1.4 | 17.7 ± 1.4 |
| Water maze | 29.7 ± 1.0 | 26.5 ± 1.3 | 41.5 ± 2.0 | 34.9 ± 1.4 | 22.3 ± 1.5 | 29.9 ± 2.6 | 13.3 ± 0.7 | 18.1 ± 0.8 |
| Total NCAM mRNA | ||||||||
| Untrained | 46.6 ± 1.3 | 46.4 ± 1.4 | 39.9 ± 1.5 | 37.5 ± 1.9 | 20.3 ± 1.4 | 24.8 ± 2.0 | 14.7 ± 0.6 | 13.0 ± 0.6 |
| Water maze | 46.3 ± 1.8 | 46.2 ± 2.0 | 39.1 ± 2.6 | 37.8 ± 2.1 | 19.5 ± 1.0 | 24.6 ± 1.1 | 14.3 ± 0.4 | 12.6 ± 0.5 |
| NCAM180 mRNA | ||||||||
| Untrained | 35.1 ± 1.3 | 31.6 ± 1.0 | 30.3 ± 1.1 | 27.8 ± 1.1 | 16.8 ± 1.3 | 19.5 ± 1.2 | 11.3 ± 0.5 | 12.1 ± 0.8 |
| Water maze | 34.8 ± 1.3 | 31.5 ± 1.4 | 30.7 ± 1.6 | 27.1 ± 1.0 | 16.7 ± 1.1 | 19.5 ± 1.5 | 11.0 ± 0.4 | 11.9 ± 0.7 |
- * Densitometric analysis was performed on autoradiograms following in situ hybridization. Values are expressed as the mean optical densities (a.u.) ± SEM for 9–11 rats per group. DG, dentate gyrus; PFC, prefrontal cortex; FC, frontal cortex.
Correlational analyses were performed to relate data from the behavioral performance in the water maze (mean latency to find the platform across trials 2–8) and L1 mRNA expression in the hippocampus. We found that the latency to find the platform positively correlated with L1 mRNA expression in each of the hippocampal subregions examined (DG-dorsal: r = 0.71, P < .02; DG-ventral: r = 0.57, P < .07; CA1: r = 0.62, P < .05; CA3: r = 0.60, P < .05), indicating that the animals that took longer to find the platform and to learn its location expressed higher levels of L1 mRNA (Fig. 3). Conversely, the better the performance, the lower the L1 mRNA expression.
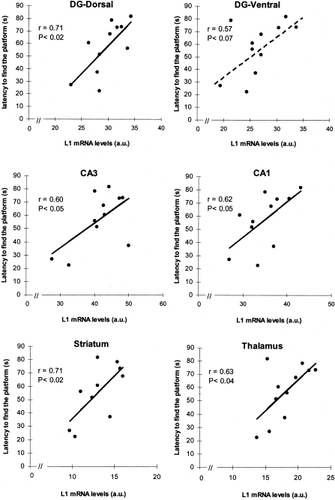
Correlations between latency to finding the platform over the massed training protocol and L1 mRNA levels in different brain areas.
Significant correlations were also found between the latency to find the platform across trials 2–8 and L1 mRNA expression in the striatum (r = 0.71, P < .02) and the thalamus (r = 0.63, P < .04), indicating again that, the better the performance, the lower the L1 mRNA expression (Fig. 3). However, no evidence for a correlation between these two parameters could be detected for the PFC (n.s.) or FC (n.s.).
Total NCAM mRNA Expression
As can be seen in Table I, the comparison of total NCAM mRNA expression between untrained and water maze-trained rats indicated that spatial learning did not influence the immediate expression of mRNA for NCAM, either at the level of the hippocampus (F1,18 = 0.01, n.s.) or in the other forebrain areas examined (F1,18 = 0.19, n.s.). Further correlational analyses between the latency to finding the platform (across trials 2–8) and total NCAM mRNA expression approached significance only for the PFC (r = 0.55, P < .08) and lacked significance for all other areas (all P > .05).
NCAM180 mRNA Expression
ANOVAs performed on NCAM180 mRNA levels also indicated a lack of effect of water maze training on the immediate expression of mRNA for this molecule both in the hippocampus (F1,18 = 0.03, n.s.) and in other forebrain regions (F1,18 = 0.06, n.s.; see Table I). In addition, correlational analyses did not indicate any significant correlation between acquisition curve in the water maze and the expression of NCAM 180 mRNA in any of the brain areas analyzed.
Corticosterone Levels
As expected, corticosterone levels were significantly higher in water maze-trained animals (457.1 ± 39.5 ng/ml) than in untrained controls (16.8 ± 3.1 ng/ml; F1,18 = 99.9, P < .00001). Correlational analyses between water maze performance and plasma corticosterone values indicated that posttraining levels of the steroid showed positive correlations with the latency to find the platform (trials 2–4: r = 0.79, P < .004; trials 2–5: r = 0.88, P < .0003; trials 2–6: r = 0.83, P < .002, see Fig. 4; trials 2–7: r = 0.70, P < .017; trials 2–8: r = 0.59, P = .057). In addition, corticosterone values showed positive correlations with tigmotactic swimming displayed by rats during the first six training trials (data not shown; trials 1–4: r = 0.68, P < .03; trials 1–5: r = 0.68, P < .03; trials 1–6: r = 0.63, P < .04), indicating that, the higher the thigmotactic swimming, the higher the steroid levels.
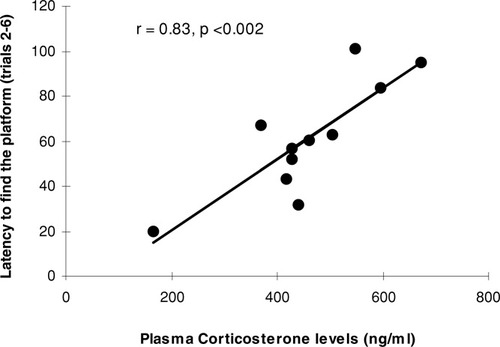
Linear relationship between plasma corticosterone levels exhibited by rats immediately after water maze training and their performance on water maze training (mean escape times averaged over trials 2–6).
In addition, we performed Pearson's correlational analyses to assess possible correlations between plasma corticosterone levels and mRNA expression for each CAM in each of the brain regions evaluated. For analyses involving L1 mRNA levels, although no correlations were found at any of the hippocampal subregions (all P > 0.2), significant positive correlations were seen in all other forebrain regions assessed (PFC: r = 0.84, P < .001; FC: r = 0.85, P < .001; striatum: r = 0.67, P < .03; thalamus: r = 0.81, P < .003), indicating that, the higher the L1 mRNA expression, the higher the corticosterone levels, and vice versa (Fig. 5). As for analyses involving total NCAM and NCAM180 mRNA levels, no correlations were found with the lone exception of total NCAM in the PFC, whose expression also showed a positive correlation with plasma corticosterone levels (r = 0.69, P < .02).
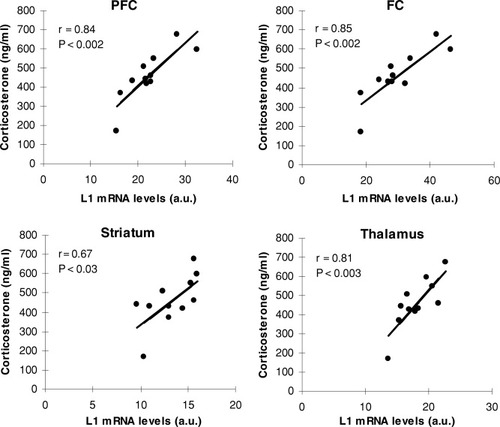
Correlations between plasma corticosterone levels exhibited by rats immediately after water maze training and L1 mRNA levels in different brain areas.
DISCUSSION
We investigated whether individual differences in spatial learning performance in rats might be related to the mRNA expression of the neural cell adhesion molecules L1 and NCAM (all isoforms) and the NCAM180 isoform. Our results showed that, as evaluated immediately after the end of the experiment, water maze training did not induce changes in the mRNA levels of L1, total NCAM, or NCAM180, which supports the view that they were not regulated by the preceding experience of spatial learning. It is interesting that, except for the FC and PFC, a correlation between learning performance and L1 mRNA levels was found in all other areas analyzed (hippocampus, thalamus, and striatum), indicating that, the higher the L1 expression, the slower the rats were to learn the task. Although the association between the hippocampus and spatial learning is well-established (Morris et al., 1982; Moser and Moser, 1998), the observed correlations at the level of the thalamus and the striatum, which are classically related to procedural learning (Nakahara et al., 2001), might seem unexpected. However, it should be noted that recent studies have also implicated both the thalamus (Sziklas and Petrides, 1999; Van Groen et al., 2002) and the striatum (Gallagher et al., 1990; Devan and White, 1999; De Leonibus et al., 2003) in the acquisition of spatial orientation learning. However, no significant correlations were found between spatial learning in the water maze and total NCAM or NCAM180 mRNA expression. Therefore, the current findings support the view that differential patterns of overall L1 gene expression might be related to individual differences in spatial learning abilities.
A similar association between increased L1 mRNA and protein levels in the hippocampus and impaired water maze performance was recently reported in chronically stressed rats (Sandi et al., 2001; Venero et al., 2002b). The fact that, in our study, L1 was the molecule that showed an overall correlation with learning abilities appears to be in line with the fact that L1 is, to date, the only adhesion molecule to be associated with a hereditary alteration in cognitive function, i.e., mental retardation (Kenwrick et al., 2000). However, it should be noted that neurological disorders related to L1 have been reported to be related to mutations in the L1 gene and that, so far, no link has been established between the extent of L1 gene expression and human diseases. In the present study, there were important individual differences in the spatial learning abilities of animals trained in a one-session massed spatial learning protocol. Under the same experimental conditions, some rats exhibited a slower acquisition curve than others, although all animals showed progressive learning throughout the training trials. This suggests that, although more slowly acquiring animals require a greater effort to learn the task, their learning rate is notably superior to that of rats with hippocampal lesions (Morris et al., 1982), cognitively impaired aged rats (Rowe et al., 1998; Schulz et al., 2002), or mice with gene manipulations that impair hippocampal synaptic plasticity (Chen and Tonegawa, 1997). Therefore, it is important to note that they should be considered not severely impaired in their performance but slower in their learning abilities.
L1 is found at regions of contact between neighboring axons and on the growth cones and has been implicated in axon growth during development, neuronal cell migration, synaptogenesis, myelination, and neuronal cell survival (Brümmendorf et al., 1998; Kenwrick et al., 2000; Hortsch, 2000). In addition, through its interactions with cytoskeletal components, L1 may also convert signals from the cell surface into structural changes (Hortsch et al., 1998). In addition to homophilic binding with other L1 molecules in the vicinity, a wide array of molecules (including other members of the Ig superfamily, integrins, extracellular matrix proteins, and a variety of proteoglycans) binds to the extracellular domains of L1 and influences their functions. In addition, L1 has been detected as one of the proteins involved in the N-methyl-D-aspartate receptor (NMDAR) multiprotein complexes (NRC), where it has been suggested to be involved in the structural organization of the NRC at the synapse and/or to participate in trans-synaptic signalling pathways (Husi et al., 2000).
Genetic studies in rodents have provided strong evidence for the relevance of this molecule in nervous system development and function. Thus, mutant mice with a constitutive deletion of the L1 gene exhibit extensive malformations in the development of the nervous system (Dahme et al., 1997), with consequent alterations in their behavioral phenotype (Fransen et al., 1998). In spite of this, LTP was not found to be altered in mice constitutively lacking L1 (Bliss et al., 2000).
However, given that changes in synaptic function and cognition in L1 knockout mice could result from abnormal development, such genetic studies are limited in that they do not allow us to infer functional roles of L1 in the adult brain. Two experimental approaches have been used to date to address specifically the functional roles of L1 in the adult brain: antibody intervention studies and conditionally L1-deficient mice. Antibodies against L1 were shown 1) to reduce long-term potentiation (LTP) in CA1 neurons when applied to rat hippocampal slices (Lüthi et al., 1994) and 2) to attenuate long-term retention of spatial information in the water maze when continuously intraventricularly infused in rats (Arami et al., 1996). Furthermore, recent data obtained in mutant mice in which the L1 gene was inactivated after cessation of the major developmental events indicated that, compared with controls, conditionally mutant mice showed 1) no defects in LTP in the CA1 region, 2) no differences in escape latencies to learn the water maze task after having been subjected to a previous pretraining phase (cued platform), and 3) decreased anxiety (Law et al., submitted). Immunohistochemical experiments demonstrated that the conditionally L1-deficient mice showed a strong reduction in the amounts of L1 immunoreactivity in the hippocampus, cerebral cortex, striatum, and to a lesser extent thalamus and hypothalamus, which gives a pattern of differential expression of L1 between mutant and control mice similar, in terms of regional distribution, to that described in our study for fast and slow learners.
There are three possible explanations for the observed individual differences in L1 mRNA levels. First, there might have been changes in L1 transcription induced by the training. Second, exposure of animals to the water maze procedure could have led to alterations of L1 mRNA stability. Third, the differences could be due to different L1 mRNA steady-state levels, already existing before the water maze training.
If individual changes in L1 transcription accounted for the different L1 mRNA amounts, these modifications of transcription rate would have to occur within a time window of 22 min, namely, the time between beginning of the water maze procedure and decapitation of the animals. For the immediate-early genes (IEGs) arc, zif268, and c-fos, a significant increase in mRNA was observed in rat hippocampi 30 min after water maze training (Guzowski et al., 2001). After the exploration of a novel environment, Guzowski et al. (1999) could even detect an induction of arc mRNA in CA1 and CA3 within 5 min. Although cell adhesion molecules are believed to be “late effector” rather than “immediate-early” genes (Sheng and Greenberg, 1990; Guzowski, 2002), we thus cannot completely rule out transcriptional changes induced by the water maze procedure.
The second mechanism proposed above, regulation of mRNA stability, is involved in controlling the expression of several genes in the central nervous system, e.g., amyloid precursor protein (Malter, 2001). Quattrone et al. (2001) demonstrated that control of GAP-43 mRNA stability by ELAV-like proteins is essential for spatial learning in mice. It is possible that the half-life of L1 mRNA is influenced by similar mechanisms, although the time schedule of the training protocol in the above-mentioned study differs significantly from the one that we applied.
The third explanation, different L1 mRNA steady-state levels, implies that performance in the water maze task correlates with the amount of L1 mRNA in the respective animal's brain at the beginning of the task. The fact that we did not observe significant differences in L1 mRNA expression between the untrained and the trained group of animals (see Table I) supports this interpretation. However, one cannot exclude that opposite changes of L1 mRNA levels in individual animals during the water maze training lead to the same result. Therefore, further experiments will be required to clarify the regulatory mechanisms underlying the correlation of L1 mRNA expression with the performance of rats in the water maze task.
In this regard, it is important to note that the high levels of thigmotactic swimming displayed by animals with a slower acquisition curve in our study have been previously validated as an index of anxiety (Simon et al., 1994). The idea that the longer latencies to find the hidden platform shown by slow learners are related to anxiety is further supported by ongoing experiments in our laboratory indicating that pretraining of rats in the water maze task eliminates the differences in the acquisition rate between fast and slow learners reported here (A.I. Herrero, C. Sandi, and C. Venero, unpublished observations). This observation also fits with the lack of differences reported in water maze learning between conditionally L1-deficient and control mice that had been pretrained with a cued platform (Law et al., submitted). Hence, given the proposed role of L1 in regulating exploratory and anxiety-related behaviors (Law et al., submitted), and given the performance characteristics of slow learners in our study, it might be hypothesized that their higher L1 mRNA expression could be related to a slower rate of learning through a primary influence on anxiety processes. This is further supported by the strong positive correlations found in the current study between plasma corticosterone levels and L1 mRNA expression in all brain regions examined, with the sole exception of the hippocampus [a counterintuitive finding, given the widely accepted regulatory role of the hippocampus on the functioning of the hypothalamus-pituitary-adrenocortical axis and therefore, eventually, on glucocorticoid production (de Kloet et al., 1998)], in that anxiety is well known to be associated with increased glucocorticoid activation (Landgraf et al., 1999). In addition, considerable evidence supports the existence of a close interaction between anxiety and cognitive processes (McNaughton, 1997; Hindmarch, 1998; Ohl et al., 2003). Therefore, our study constitutes another experimental approach to unraveling the role of L1 expression in cognitive processes. Unlike genetic or interventive studies, which aim to evaluate the impact of extremes of L1 expression (i.e., its absence, overexpression, or interference with its function), our approach is addressed to seeking for the role of this molecule when it is expressed within its normal range of variation in Wistar rats. Further studies will be addressed to investigating whether anxiety responses, as evaluated in an elevated plus maze, are related to L1 expression throughout the brain and could help to predict spatial learning abilities. In addition, it will be interesting to test whether early environmental experience, which has an impact on anxiety behavior in the adult (Gutman and Nemeroff, 2002), would also influence the predicted associations among L1 expression, anxiety, and cognitive function.
NCAM genetic studies have also implicated this molecule in the development of the nervous system (Rafuse et al., 2001) and in learning (Cremer et al., 1994), aggression (Stork et al., 1997), and anxiety (Stork et al., 1999) behaviors. However, in our study, total NCAM or NCAM180 mRNA expression was not significantly associated with water maze performance.
Finally, a correlation was also found between performance in the maze and posttraining corticosterone levels. Although the implications of this finding are limited by the fact that the higher steroid levels were observed in slow learners, which, hence, swam for a more prolonged period than fast learners, it should be noted that all animals were sacrificed at the same time with regard to the beginning of the training session. Because corticosterone release is a slowly triggered response that requires neuroendocrine activation of the hypothalamus-pituitary-adrenal system, it might be argued that plasma levels of the steroid, as measured 22 min after the beginning of training, were predominantly dependent not on swimming efforts during the few minutes before decapitation but on a previous activation of the neuroendocrine response initiated from the beginning of the training session. This would imply that the observed correlation might have been related not just to differential swimming length but to variability in the activation of the stress response in animals with different learning abilities. In this regard, higher corticosterone responses in slow learners might, again, be an index of heightened anxiety behavior in these animals. Although further studies are needed to test this possibility carefully, the extensive literature relating glucocorticoids to cognitive function (de Kloet et al., 1998; Sandi, 1998; Roozendaal, 2000) and anxiety (Korte et al., 1996; Gass et al., 2001) supports the value of including analysis of glucocorticoid function in subsequent studies aimed at gaining a deeper insight into the cognitive implications of cell adhesion molecules.
Acknowledgements
C.V. was the recipient of postdoctoral fellowship from Comunidad de Madrid and NATO and A.I.H. of a predoctoral fellowship from the Spanish Ministry of Science and Technology (MCYT). The authors thank M. Isabel Cordero and Nyika Kruyt for help with the training of the animals in the water maze, Birgit Hertlein and Birte Rossol for cloning the NCAM and NCAM180 plasmids, H. Chica Schaller for generously providing laboratory facilities for the in situ hybridization experiments, and Fabio Morellini and Samantha Gizerian for helpful comments on the manuscript.




