Astrocyte-targeted expression of interleukin-6 protects the central nervous system during neuroglial degeneration induced by 6-aminonicotinamide
Abstract
6-Aminonicotinamide (6-AN) is a niacin antagonist, which leads to degeneration of gray matter astrocytes mainly in the brainstem. We have examined the role of interleukin-6 (IL-6) in this degenerative process by using transgenic mice with astrocyte-targeted IL-6 expression (GFAP-IL6 mice). This study demonstrates that transgenic IL-6 expression significantly increases the 6-AN-induced inflammatory response of reactive astrocytes, microglia/macrophages, and lymphocytes in the brainstem. Also, IL-6 induced significant increases in proinflammatory cytokines IL-1, IL-12, and tumor necrosis factor-α as well as growth factors basic fibroblast growth factor (bFGF), transforming growth factor-β, neurotrophin-3, angiopoietin, vascular endothelial growth factor, and the receptor for bFGF. In accordance, angiogenesis was increased in GFAP-IL6 mice relative to controls after 6-AN. Moreover, oxidative stress and apoptotic cell death were significantly reduced by transgenic IL-6 expression. IL-6 is also a major inducer in the CNS of metallothionein I and II (MT-I+II), which were significantly increased in the GFAP-IL6 mice. MT-I+II are antioxidants and neuroregenerative factors in the CNS, so increased MT-I+II levels in GFAP-IL6 mice could contribute to the reduction of oxidative stress and cell death in these mice. © 2003 Wiley-Liss, Inc.
6-Aminonicotinamide (6-AN) is a niacin antagonist that shuts down the hexose monophosphate pathway, which is toxic to neuroglial cells (Krum, 1994, 1995;Haghighat et al., 1996; Penkowa et al., 1997, 2002; Hothersall et al., 1998; Krinke and Classen, 1998; Budihardjo et al., 2000; Penkowa and Hidalgo, 2000a). Injection of 6-AN leads to degeneration of gray matter macroglia of brainstem and spinal cord (Krum, 1994, 1995; Haghighat et al., 1996; Penkowa et al., 1997, 2002). The latter is followed by inflammation (Penkowa et al., 1997) and formation of reactive oxygen species (ROS), leading to oxidative stress (Penkowa and Hidalgo, 2000a; Penkowa et al., 2002), which can induce neurodegeneration and apoptosis (Sun and Chen, 1998; Cassarino and Bennett, 1999; Floyd, 1999).
Interleukin-6 (IL-6) is a multifunctional cytokine produced in activated cells during CNS inflammation (Hirano et al., 1990; Gadient and Otten, 1997; Gruol and Nelson, 1997; Benveniste, 1998; Muñoz-Fernandez and Fresno, 1998; Penkowa et al., 2000b; Lenzlinger et al., 2001). IL-6 is a main regulator of inflammatory and immune responses (Benveniste, 1998; Raivich et al., 1999;Van Wagoner and Benveniste, 1999; Brunello et al., 2000;Penkowa and Hidalgo, 2000a; Penkowa et al., 2000b;Morganti-Kossmann et al., 2002). Consequently, in IL-6 knock-out (IL-6KO) mice, the CNS inflammatory response is reduced (Klein et al., 1997; Eugster et al., 1998;Raivich et al., 1999; Penkowa et al., 2000b; Swartz et al., 2001). Moreover, transgenic IL-6 overexpression under the control of the glial fibrillary acdic protein (GFAP) gene promoter (GFAP-IL6 mice) induces neuroinflammation (Campbell et al., 1993; Brett et al., 1995; Fattori et al., 1995; Di Santo et al., 1996; Campbell, 1998a, b; Brunello et al., 2000; Giralt et al., 2002a), but, when a brain injury is superimposed, these increased IL-6 levels show significant antiinflammatory effects and lead to increased CNS repair (Fee et al., 2000; Swartz et al., 2001). Accordingly, posttraumatic tissue healing is significantly impaired, whereas neuronal cell death is increased, in IL-6KO mice during neuropathology (Murphy et al., 1999; Penkowa et al., 2000b; Swartz et al., 2001). Furthermore, local injection of IL-6 attenuates neurotoxic effects of N-methyl-D-aspartate (Toulmond et al., 1992) and is neuroprotective during cerebral ischemia (Loddick et al., 1998). Consistently with this, IL-6 is a member of the neuropoietin family of cytokines (Hopkins and Rothwell, 1995) and has neurotrophic and neuroprotective effects (Gruol and Nelson, 1997; Benveniste, 1998; Carlson et al., 1999; Gahring et al., 1999; Penkowa et al., 2000b; Swartz et al., 2001;Eskes et al., 2002; Pavelko et al., 2003). Furthermore, IL-6 is a major inducer of the antioxidant neuroprotective factors metallothionein I and II (MT-I+II; Hernandez et al., 1997; Carrasco et al., 1998; Penkowa et al., 2000b), which significantly inhibit brain damage and cell death during various neuropathological conditions and promote neuroregeneration and tissue repair (Penkowa et al., 1999a, b, 2000a, 2003; Carrasco et al., 2000; Hidalgo et al., 2001, 2002; Giralt et al., 2002a, b). Accordingly, MT-I+II can be used therapeutically against different CNS disorders (Penkowa and Hidalgo, 2000b; Giralt et al., 2002b; Penkowa et al., 2002).
Thus, IL-6 exerts both proinflammatory and neuroprotective roles in the injured CNS. To understand the mechanisms underlying such different roles, we have studied astrocyte-targeted overexpression of IL-6 in a model of CNS damage, subjection to an i.p. injection with the gliotoxic niacin antagonist 6-AN. We have used transgenic GFAP-IL6 mice, a well-known animal model of IL-6 overexpression (Campbell et al., 1993; Fattori et al., 1995; Brett et al., 1995; Di Santo et al., 1996; Campbell, 1998a, b; Brunello et al., 2000). The results clearly demonstrate that, although in the normal brain transgenic overexpression of IL-6 may be detrimental, during a toxic insult, such as after 6-AN, IL-6 is a neuroprotective factor.
MATERIALS AND METHODS
Animals
Construction and characterization of the GFAP-IL6 transgenic mice has been described previously (Campbell et al., 1993). Briefly, an expression vector derived from the murine GFAP gene was used to target expression of IL-6 to astrocytes. The GFAP-IL6 mice have a mixed C57B6 × SJL genetic background, and a colony is maintained in our laboratory by crossing heterozygous GFAP-IL6 males with C57B6 × SJL females. To study the role of metallothioneins (MTs) in the GFAP-IL6 animal model, we have recently undertaken the approach of crossing these mice with either MT1+2KO mice (Giralt et al., 2002) or TgMTI mice (Molinero et al., 2003). For this particular study, heterozygous GFAP-IL6 mice were crossed with MT-I+II-deficient mice, which have a 129/SvJ genetic background (Masters et al., 1994). Although all the offspring are heterozygous for MT-I+II, they were genotyped for identifying the GFAP-IL6 +/− and the GFAP-IL6 −/− animals as previously described (Penkowa et al., 2000a). Both groups share the same genetic background and so were used for this study; we refer to the GFAP-IL6 −/− animals as the “normal” or “wild-type” mice. Age and gender were matched for both groups.
All experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC). Also, all animals were acquired and cared for in accordance with the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals and with the principles presented in the Guidelines for the Use of Animals in Neuroscience Research by the Society for Neuroscience. Moreover, all animal protocols used were approved by our institutional animal experimentation committee, and all efforts were made to minimize animal distress and to reduce the number of animals used.
Experimental Procedures
To induce CNS injury, mice were injected intraperitoneally with 6-AN (Sigma Aldrich, St. Louis, MO; code A0630), an antimetabolite that shuts down the hexose monophosphate pathway, which is preferentially used by protoplasmic astroglia, which thereby suffer cytotoxic edema and cell death (Krum, 1995; Haghighat and McCandless, 1996; Penkowa et al., 1997). This injurious effect of 6-AN is confined to certain gray matter areas of hindbrain and spinal cord, whether or not these neurons project outside the CNS (Penkowa et al., 1997). As a result, only astroglia of the nuclei of brainstem and medulla spinalis are injured, whereas other CNS regions, such as the forebrain, appear to be resistant to 6-AN (Krum, 1995; Penkowa et al., 1997).
6-AN Administration
Eight-month-old female normal and GFAP-IL6 mice received an i.p. injection of 10 mg/kg body weight of 6-AN, which was dissolved in physiological saline. As a control for the 6-AN injection, other adult mice were injected with saline. The mice were killed 1 or 3 days after the injection. The brains were fixed for 24 hr in 4% paraformaldehyde. Brains were afterward dissected and cut as 3-μm-thick consecutive sections, which were processed for histochemistry and immunohistochemistry as previously described (Penkowa et al., 2000b). Four animals per group and type of procedure were used.
Histochemistry
Stainings for hematoxylin and eosin (H&E) and tomato lectin from Lycopersicon esculentum (Sigma; code L9389) were performed as previously described (Penkowa et al., 1999, 2000b).
Immunohistochemistry
Sections were incubated overnight at 4°C with one of the following primary antibodies: rabbit anti-cow GFAP 1:250 (Dakopatts, Glostrup, Denmark; code Z 334); rabbit anti-human S100 1:2,000 (Dakopatts; code Z311); rat anti-mouse MOMA (monocyte-macrophage marker-1) 1:20 (Serotec, Bicester, United Kingdom; code MCA947; a marker of lymphoid tissue macrophages); mouse anti-human CD-34 1:20 (Neomarkers, USA; code MS-363; a marker of myeloid and lymphoid progenitor cells as well as proliferating vessels); goat anti-mouse CD-20 1:200 (Santa Cruz Biotechnology, Santa Cruz, CA; code sc-7736; a marker of B lymphocytes); mouse anti-rat CD-3 (IgM) 1:50 (Serotec; code KD MCA 772; a marker of T lymphocytes); mouse anti-rat CD-4 1:50 [Serotec; code MCA 55R; a marker of T helper (Th) lymphocytes]; rabbit anti-rat MT-I+II (Gasull et al., 1993; Penkowa et al., 1997, 1999); mouse anti-human IL-1β 1:50 (Biogenesis, USA; code 5375-4329); rat anti-mouse IL-6 1:50 (Harlan Seralab, Indianapolis, IN; code MAS584); goat anti-mouse IL-12 1:50 (Chemicon Int., United Kingdom; code AB2130P); rabbit anti-mouse tumor necrosis factor-α (TNF-α) 1:100 (Biosource, USA; code AMC 3012); rabbit anti-human transforming growth factor-β1 (TGF-β1) 1:200 (Santa Cruz Biotechnology; code sc-146); rabbit anti-human basic fibroblast growth factor (bFGF) 1:100 (Santa Cruz Biotechnology; code sc-79); goat anti-human NT-3 1:20 (R&D Systems, Minneapolis, MN; code AF-267-NA); goat anti-human angiopoietin-1/4 1:100 (Santa Cruz Biotechnology; code sc-9360; marker of angiogenesis); rabbit anti-human vascular endothelial growth factor (VEGF) 1:50 (Neomarkers, USA; code RB-222P0; a marker of angiogenesis); rat anti-mouse perlecan (bFGF receptor, heparan sulfate proteoglycan) 1:30 (Neomarkers, USA; code RT-794-P0; a marker of angiogenesis); rabbit anti-mouse inducible nitric oxide synthase (iNOS; a marker for oxidative stress) 1:100 (Alexis Biochemicals, USA; code 210-503-R050); rabbit antimalondialdehyde (MDA; a marker for oxidative stress) 1:100 (Alpha Diagnostic Int., USA; code MDA 11-S); rabbit antinitrotyrosine (NITT; a marker for oxidative stress) 1:100 (Alpha Diagnostic Int., USA; code NITT 12-A); mouse anti-8-oxyguanine (a marker for oxidative stress) 1:100 (Chemicon Int.; code MAB-3560); and rabbit anti-human cleaved caspase-3 1:50 (Cell Signaling Technology Inc., USA; code 9661).
The primary antibodies were detected using biotinylated anti-mouse IgG 1:200 (Sigma; code B8774), or biotinylated anti-mouse IgM (μ-chain-specific) 1:50 (Jackson Immunoresearch, West Grove, PA; code 115-065-020), or biotinylated anti-rabbit IgG 1:400 (Sigma; code B3275), or biotinylated anti-rat IgG 1:1500 (Amersham, Buckinghamshire, United Kingdom; code RPN 1005), or biotinylated anti-goat/sheep IgG 1:20 (Amersham; code RPN 1025), followed by streptavidin-biotin-peroxidase complex (StreptABComplex/HRP; Dakopatts; code K377) prepared at the manufacturer's recommended dilution. These secondary and tertiary steps in the immunoreaction were performed for 30 min at room temperature. Afterward, sections were incubated with biotinylated tyramide and streptavidin-peroxidase complex (tyramide signal amplification, TSA indirect; NEN, Life Science Products, Boston, MA; code NEL700A) prepared following the manufacturer's recommendations. The immunoreaction was visualized using 0.015% H2O2 in diaminobenzidine (DAB)/Tris-buffered saline (TBS) for 10 min at room temperature.
To evaluate the extent of nonspecific binding in the immunohistochemical experiments, control sections were incubated in the absence of primary antibody. Results were considered only if these controls were negative.
In Situ Detection of DNA Fragmentation (TUNEL)
Terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP)-digoxigenin nick end labeling (TUNEL) was performed as previously described (Penkowa et al., 1999a).
Fluorescence
To detect which cell types suffer from oxidative stress or apoptotic cell death, we performed double- and triple-fluorescence stainings. To determine which cells suffer from oxidative stress, sections were pretreated as described above and incubated overnight at 4°C with mouse anti-human NF ready-to-use (Biogenex, San Ramon, CA; code AM073-10M) or mouse anti-porcine vimentin 1:50 (Dakopatts; code M0725; as a marker for both reactive astrocytes and macrophages) and simultaneously with rabbit anti-human iNOS, or rabbit anti-NITT, or rabbit anti-MDA (all as described above). The monoclonal antibodies were detected by using goat anti-mouse IgG linked with Texas red (TXRD) 1:50 (Southern Biotechnology, Birmingham, AL; code 1030-07), whereas the polyclonal antibodies were detected by using goat anti-rabbit IgG linked with fluorescein (FITC) 1:50 (Southern Biotechnology; code 4050-02).
To determine which cells suffer from apoptosis, triple stainings for TUNEL and cellular markers were performed. Sections were incubated with FITC-linked TUNEL (Oncor, Gaithersburg, MD; code S7111-KIT) according to the manufacturer's protocol and afterward incubated overnight at 4°C with rabbit anti-human neuron-specific enolase (NSE) 1:1,000 (Calbiochem, La Jolla, CA; code D05059) and mouse anti-porcine vimentin (as described above) simultaneously. The anti-NSE antibodies were detected by using TXRD-linked goat anti-rabbit IgG 1:40 (Jackson Immunoresearch; code 111-075-144), whereas antivimentin antibodies were detected by using goat anti-mouse IgG linked with aminomethylcoumarin (AMCA) 1:20 (Jackson Immunoresearch; code 115-155-146).
The sections were embedded in 20 μl fluorescent mounting medium (Dakopatts; code S3023) and kept in darkness at 4°C. To evaluate the extent of nonspecific binding of the antisera in the fluorescence stainings, control sections were incubated in the absence of primary antibody. Other standard control stainings were performed as previously described (Penkowa and Hidalgo, 2000a, b; Penkowa et al., 2000a, b). Results were considered only if these controls were negative. For the simultaneous examination and recording of the three stains, a Zeiss Axioplan2 light microscope equipped with a triple-band (FITC/TXRD/AMCA) filter was used.
Cell Counts
In addition to morphological analysis, cellular counts in the caudal border of the medial vestibular nuclei were carried out in a blinded manner in a 0.5-mm2 unilateral area of 3-μm brainstem sections. Cell counts were performed in three mice per group and in two sections from each mouse.
Statistical Analysis
The results of cellular counts were evaluated by two-way ANOVA, with genotype and 6-AN as the main factors.
RESULTS
General Results
The administration of 6-AN caused the animals to become hypoactive, and they developed weakness and slight motor impairments on the first day; by the day of sacrifice, the animals were clearly paralyzed in their extremities. Food was therefore put into the animal cages. We present the histochemistry and immunostaining results for the 3-day period.
H&E Stainings
Brainstem sections from control and GFAP-IL6 mice injected with saline did not reveal significant histological differences, as verified by H&E stainings (Fig. 1A,B). After 6-AN injection, both control and GFAP-IL6 mice showed tissue degeneration in specific gray matter areas of the brainstem; for instance, the medial vestibular nuclei, the nucleus of the solitary tract and the solitary nucleus, the cuneate nucleus, the pontine nuclei, the superior paraolivary nucleus, the medioventral periolivary nucleus, and the facial nucleus displayed signs of tissue degeneration as judged by H&E stainings both 3 days (Fig. 1) and 1 day after 6-AN injection (not shown). Despite the fact that no thorough quantitative analysis was performed, the damaged areas were similar in both genotypes as judged by this approach and the timings studied. Presumably, this decrease of H&E staining in specific brainstem nuclei reflects the well-known effect of 6-AN, which causes cytotoxic edema and acute cell death of astroglia and oligodendroglia (Hothersall et al., 1981; Krum and Rosenstein, 1993; Krum, 1994, 1995; Haghighat and McCandless, 1996). This was verified by GFAP and S100 stainings (see below).
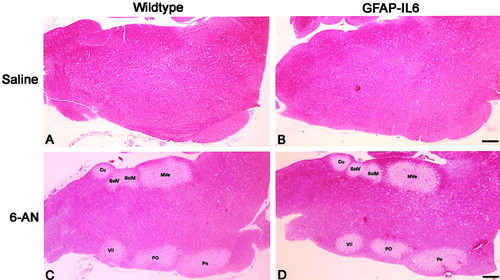
H&E-stained brainstem sections of saline- and 6-AN-injected wild-type and GFAP-IL6 mice. A and B show the brainstem of wild-type (A) and GFAP-IL6 (B) mice after saline injection. C and D show the brainstem of 6-AN injected wild-type (C) and GFAP-IL6 (D) mice. 6-AN induced tissue degeneration in certain gray matter areas of the brainstem such as in the medial vestibular nuclei (Mve), the nucleus of the solitary tract (SolM), the solitary nucleus (SolV), the cuneate nucleus (Cu), the pontine nuclei (Pn), the superior paraolivary nucleus and the medioventral periolivary nucleus (Po), and the facial nucleus (VII). Scale bars = 280 μm.
Astrocytes
Throughout brainstem sections from control mice injected with saline, GFAP expression was seen in some dispersed astrocytes situated in both gray and white matter (Fig. 2A,E). In saline-injected GFAP-IL6 mice, moderate reactive astrogliosis was observed in both anterior and posterior parts of the brainstem (Fig. 2B,F). After 6-AN injections, GFAP-positive astrocytes of specific degenerated gray matter areas of the brainstem (see Fig. 1) decreased in both wild-type and GFAP-IL6 mice (Fig. 2C,D). In contrast, the areas surrounding the affected areas showed a dramatic increase of GFAP immunoreactivity, which was further increased in the GFAP-IL6 mice (Fig. 2D,H) compared with wild-type mice (Fig. 2C,G, Table I).
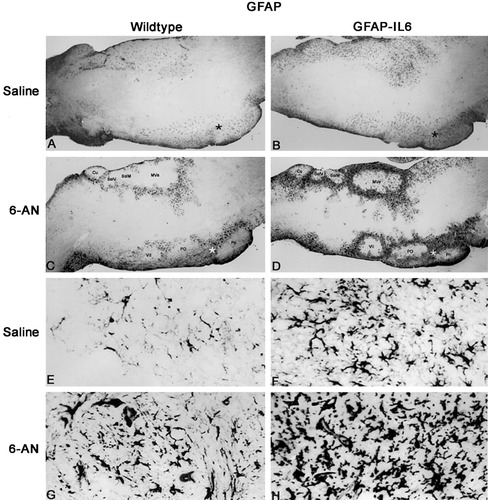
GFAP immunoreactivity in the brainstem of saline- and 6-AN-injected wild-type and GFAP-IL6 mice. A: After saline injection, some dispersed GFAP+ astrocytes are seen in the brainstem of wild-type mice. The star marks the area magnified in E. B: In GFAP-IL6 mice injected with saline, GFAP+ reactive astrogliosis is seen in the brainstem. Hence, several astrocytes are seen in posterior and anterior areas throughout the brainstem. The star marks the area magnified in F. C: After 6-AN treatment, the astroglia dissappear from certain brainstem gray matter areas of wild-type mice, and simultaneously GFAP+ reactive astrogliosis is observed around the degenerated gray matter areas. D: Also in 6-AN-injected GFAP-IL6 mice, astroglia disappeared from specific gray matter areas of the brainstem, such as the medial vestibular nuclei (Mve), the nucleus of the solitary tract (SolM), the solitary nucleus (SolV), the cuneate nucleus (Cu), the pontine nuclei (Pn), the superior paraolivary nucleus and the medioventral periolivary nucleus (Po), and the facial nucleus (VII). Reactive astrogliosis surrounding the damaged areas in GFAP-IL6 mice was significantly increased relative to wild-type mice. E: Higher magnification of the area marked by the star in A. F: Higher magnification of the area marked by the star in B, which shows the increased astrocytes of GFAP-IL6 mice relative to normal mice. G: Higher magnification of the area marked by a star in C, showing 6-AN-induced reactive astrogliosis of normal mice. H: Higher magnification of area marked by the star in D. As shown, GFAP-IL6 mice had significantly increased reactive astrogliosis compared with that in normal mice after 6-AN.
| Saline | 6-AN | |||
|---|---|---|---|---|
| Wild-type | GFAP-IL6 | Wild-type | GFAP-IL6 | |
| GFAP+ | 69 ± 1.7 | 130 ± 9.8 | 221 ± 8.3 | 446 ± 9.6 |
| S-100 | 83 ± 6.4 | 140 ± 12.6 | 187 ± 8.9 | 392 ± 7.0 |
| Lectin+ | 32 ± 4.1 | 51 ± 4.9 | 212 ± 5.9 | 355 ± 11.6 |
| MOMA-1+ | 2.3 ± 0.3 | 15.3 ± 3.4 | 196 ± 7.8 | 348 ± 10.7 |
| CD-34+ | 1.0 ± 0.6 | 12 ± 1.5 | 212 ± 7.8 | 370 ± 9.5 |
| CD-3+ | 1.7 ± 0.3 | 9.3 ± 1.2 | 20 ± 1.4 | 72 ± 6.1 |
| CD-20+ | 0.7 ± 0.33 | 5.7 ± 1.2 | 12 ± 2.3 | 33 ± 2.3 |
| MT-I+II+ | 6.3 ± 1.2 | 16 ± 2.5 | 169 ± 39 | 409 ± 11 |
| MDA+ | 2.0 ± 0.6 | 2.7 ± 0.9 | 172 ± 14.2 | 87 ± 7.6 |
| NITT+ | 2 ± 1.5 | 1.3 ± 0.3 | 184 ± 14.7 | 105 ± 23 |
| TUNEL+ | 1.3 ± 0.9 | 2.3 ± 0.7 | 195 ± 8.2 | 114 ± 7.1 |
| IL-1β | 1.3 ± 0.3 | 9.3 ± 1.2 | 63 ± 6.2 | 130 ± 12 |
| IL-6 | 1.0 ± 0.6 | 22 ± 5.3 | 66 ± 6.8 | 151 ± 6.0 |
| TNF-α | 2.0 ± 0.6 | 9 ± 0.6 | 61 ± 5.2 | 134 ± 3.8 |
| bFGF | 9.0 ± 1.5 | 26 ± 3.6 | 45 ± 5.4 | 103 ± 6.7 |
| TGFβ | 11 ± 1.7 | 24 ± 3.8 | 64 ± 6.7 | 124 ± 9.8 |
| NT-3 | 6.3 ± 1.2 | 13 ± 2.3 | 36 ± 4.1 | 84 ± 5.2 |
- * Cellular counts from a 0.5-mm2 area were carried out in 3-μm-thick sections of unilateral pons. The cell counts are from the caudal border of the medial vestibular nuclei, and they were carried out in a blinded manner. The main morphological features of these stainings are shown in Figures 1-8 in representative animals. Results were analyzed with two-way ANOVA, with strain and treatment as main factors. Both factors were significant for all variables (P at least <0.05).
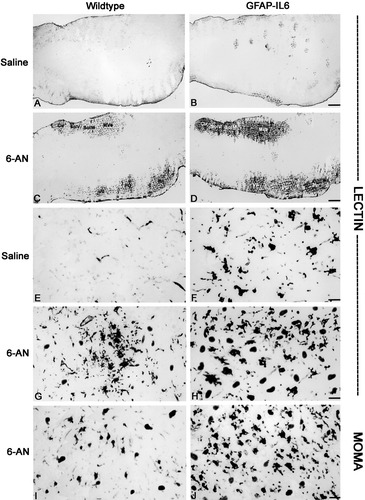
Lectin histochemistry and MOMA immunostainings in brainstem of saline- and 6-AN-injected wild-type and GFAP-IL6 mice. A: Lectin staining in control mice after saline injection. B: Lectin staining in GFAP-IL6 mice after saline injection. C: Lectin staining is increased in wild-type mice after 6-AN injection. Lectin+ macrophages/microglia are seen mainly in areas with astrocyte degeneration, such as the medial vestibular nuclei (Mve), the nucleus of the solitary tract (SolM), the solitary nucleus (SolV), the cuneate nucleus (Cu), the pontine nuclei (Pn), the superior paraolivary nucleus and the medioventral periolivary nucleus (Po), and the facial nucleus (VII). D: Lectin staining is significantly increased in 6-AN-injected GFAP-IL6 mice relative to controls. Again, the lectin+ macrophages/microglia infiltrated primarily the areas devoid of GFAP+ astrocytes (see this in Fig. 2). E: After saline, lectin staining was observed in some vessels (and very faintly in some ramified microglia) of control mice. F: Lectin histochemistry in GFAP-IL6 mice injected with saline showed round macrophages and stout, activated microglial cells throughout the brainstem. G: After 6-AN injection, control mice showed recruitment of activated lectin+ macrophages/microglial cells, which were seen primarily inside of the damaged gray matter areas. H: 6-AN treatment also induced macrophage/microglial activation in GFAP-IL6 mice. Again, macrophages/microglial cells were infiltrating the degenerated gray matter areas but were also numerous in other brainstem areas. As shown, the number of recruited lectin+ macrophages/microglial cells was significantly increased in GFAP-IL6 mice relative to controls. I,J: MOMA immunostaining showing lymphoid tissue macrophages in normal (I) and GFAP-IL6 (J) mice after 6-AN. The number of MOMA+ macrophages is significantly increased in GFAP-IL6 mice compared with that in normal mice. Scale bars = 240 μm in A–D; 42 μm in E–J.
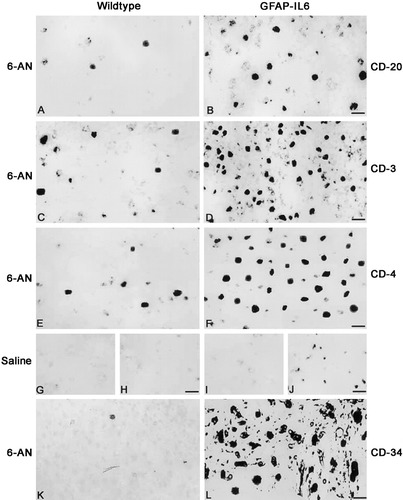
CD-20, CD-3, CD-4, and CD-34 immunostainings in brainstem of wild-type and GFAP-IL6 mice. A: CD-20 immunoreactivity of 6-AN-treated normal mice showing a few B lymphocytes in the brainstem. B: 6-AN-treated GFAP-IL6 mice show increased CD-20+ B cells in the brainstem. C: CD-3+ T cells in 6-AN-treated normal mice. D: CD-3+ T cells are significantly increased in GFAP-IL6 mice after 6-AN injection relative to normal mice. E: CD-4 immunoreactivity showing that most of the recruited T lymphocytes are Th cells in normal mice injected with 6-AN. F: Also in the 6-AN-treated GFAP-IL6 mice, most T cells are CD-4+ Th cells. G,H: Normal mice injected with saline show no significant CD-20 (G) or CD-3 (H) immunostaining. I,J: GFAP-IL6 mice treated with saline show no significant CD-20 staining (I), although some CD-3+ T cells are seen in the brainstem (J). K: 6-AN-treated normal mice show a few CD-34-stained cells. L: The numbers of CD-34+ myeloid and lymphoid progenitor cells of the bone marrow are significantly increased in 6-AN-treated GFAP-IL6 mice. Scale bars = 20 μm in A,B; 30 μm in C,D; 17 μm E,F; 33 μm in G–J; 16 μm in K,L.
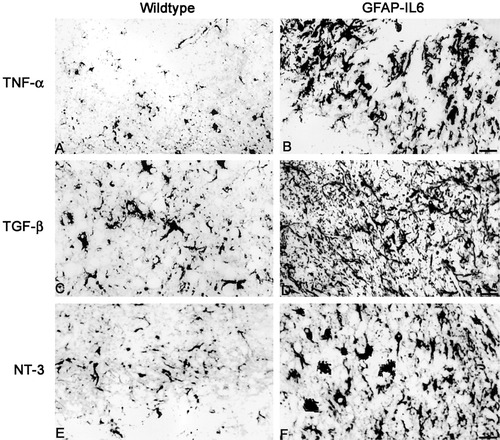
Immunoreactivity for TNF-α, TGF-β, and NT-3 in brainstem of 6-AN-injected wild-type and GFAP-IL6 mice. A: Normal mice show TNF-α staining in some macrophages and astrocytes after 6-AN. B: In GFAP-IL6 mice, the levels of TNF-α are significantly increased in macrophages and astrocytes. C,D: TGF-β immunostainings in normal (C) and GFAP-IL6 (D) mice showing increased levels in the latter. Mainly astroglia expressed TGF-β. E,F: Expression of NT-3 in normal (E) and GFAP-IL6 (F) mice. NT-3 was clearly increased by IL-6 overexpression, mainly in astrocytes, neurons, and microglia/macrophages. Scale bars = 44 μm in A,B,E,F; 50 μm in C,D.
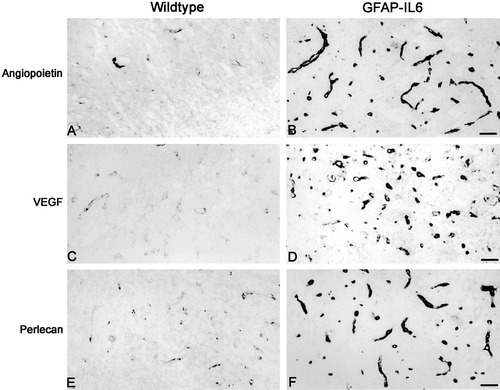
Immunostaining for angiogenesis and endothelial proliferation in brainstem of 6-AN-injected control and GFAP-IL6 mice. A,B: Angiopoietin expression in the vessels was low in normal mice (A) after 6-AN, whereas the GFAP-IL6 mice (B) show significantly increased angiopoietin immunostaining. C,D: VEGF expression in normal (C) and GFAP-IL6 (D) mice after 6-AN. The GFAP-IL6 mice showed increased VEGF in several small vessels relative to normal mice. E,F: Perlecan immunoreactivity in 6-AN-injected normal (E) and GFAP-IL6 (F) mice showing significantly increased levels in the latter. Scale bars = 46 μm.
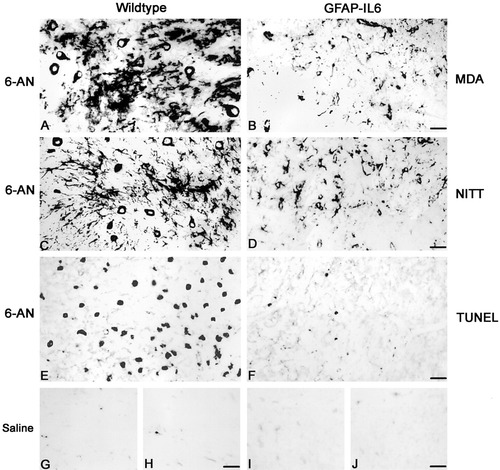
NITT and MDA immunoreactivity and TUNEL in the brainstem of 6-AN-treated wild-type and GFAP-IL6 mice. A,B: MDA immunostaining in 6-AN-treated normal (A) and GFAP-IL6 (B) mice. As shown, normal mice display increased MDA immunoreactivity after 6-AN. C,D: NITT immunoreactivity in 6-AN-treated normal (C) and GFAP-IL6 (D) mice. As shown, the control mice display increased NITT immunostaining after 6-AN. E,F: TUNEL stainings showing that, after 6-AN, normal mice (E) increase the number of TUNEL+ apoptotic cells relative to GFAP-IL6 mice (F). G–J: Saline-injected normal (G,H) and GFAP-IL6 (I,J) mice show no significant staining for oxidative stress marker NITT (G,I) or for apoptosis marker TUNEL (H,J). Scale bars = 43 μm in A,B; 49 μm in C,D; 40 μm in E–J.
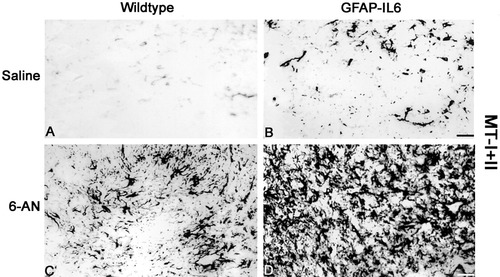
MT-I+II immunostainings in the brainstem of saline- and 6-AN-injected control and GFAP-IL6 mice. A: MT-I+II expression in saline-injected control mice showing MT-I+II in a few scattered glial cells. B: Saline-injected GFAP-IL6 mice show significantly increased MT-I+II expression relative to wild-type mice. C: After 6-AN, the wild-type mice showed increased MT-I+II levels in reactive atsrocytes and some macrophages/microglia. D: In 6-AN-injected GFAP-IL6 mice, a further and significant increase in MT-I+II expression was seen relative to wild-type mice. Hence, numerous reactive atsrocytes and macrophages/microglia showed increased MT-I+II. Scale bars = 44 μm.
By using S100 immunoreactivity, we confirmed that saline-injected GFAP-IL6 mice show a moderately increased number of astrocytes in both anterior and posterior parts of the brainstem relative to control mice. After 6-AN injections, the GFAP-IL6 mice showed significantly increased S100+ reactive astrogliosis compared with that of normal mice in the areas surrounding the damaged tissue (Table I).
Microglia/Macrophages and Their Progenitors
In brainstem sections of saline-injected normal mice, lectin staining was seen in some vessels, whereas no significant staining was seen in microglia (Fig. 3A,E). As expected, the saline-injected GFAP-IL6 mice showed activation of microglia/macrophages; many round macrophages and stout microglial cells were obvious throughout the brainstem (Fig. 3B,F). After 6-AN, the number of lectin-positive microglia/macrophages was significantly increased in all mice, but significantly moreso in GFAP-IL6 mice than in wild-type mice (Figs. 3C,D,G,H, Table I). Interestingly, in both genotypes microglia/macrophages infiltrated heavily the damaged gray matter areas, which were devoid of astrocytes. MOMA staining suggests that recruitment of macrophages from the lymphoid tissues is increased in the GFAP-IL6 mice compared with wild-type mice (Fig. 3I,JF). Moreover, CD-34+ bone marrow progenitor cells were also significantly increased in GFAP-IL6 mice relative to wild-type after 6-AN (Fig. 4K,L, Table I).
Lymphocytes
B and T lymphocytes were detected by using CD-20, CD-3, and CD-4 immunoreactivity. In saline-injected normal mice, no significant immunostainings for CD-20 (Fig. 4G), CD-3 (Fig. 4H), or CD-4 (not shown) were observed, which indicates that lymphocytes are not recruited to the normal brain. In saline-treated GFAP-IL6 mice, B cells were mostly absent (Fig. 4I), although some T cells were seen (Fig. 4J). Thus, IL-6 overexpression recruited T lymphocytes to the brainstem. After 6-AN, all mice showed increased B and T lymphocytes in the brainstem (Fig. 4, Table I); primarily, the lymphocytes accumulated in the degenerated gray matter areas, which were also infiltrated by macrophages (as shown in Fig. 3). Moreover, most of the recruited T cells were both CD-3 and CD-4 immunoreactive (Fig. 4), which indicates that these T lymphocytes are of the Th type. However, both B and T lymphocytes were significantly increased in the GFAP-IL6 mice relative to normal mice after 6-AN (Fig. 4, Table I). Furthermore, as shown by using CD-34 immunostainings (Fig. 4K,L), myeloid and lymphoid progenitor cells of the bone marrow were also increased in GFAP-IL6 mice relative to normal mice after 6-AN.
Cytokines and Growth Factors
Brainstem sections from wild-type mice injected with saline displayed low levels of the proinflammatory cytokines IL-1, IL-6, IL-12, and TNF-α, as well as the growth factors TGF-β, bFGF, and neurotrophin-3 (NT-3; Table I). As expected, the GFAP-IL6 mice showed moderately increased IL-6 expression. Moreover, the levels of IL-1, IL-12, and TNF-α were also mildly increased in saline-injected GFAP-IL6 mice, which also showed up-regulated levels of the growth factors TGF-β, bFGF, and NT-3 (Table I).
After 6-AN injection, wild-type mice increased the expression of IL-1β, IL-6, IL-12, TNF-α, TGF-β, bFGF, and NT-3 in inflammatory cells situated in the brainstem; interestingly, the GFAP-IL6 mice showed significantly further increased levels of IL-1β, IL-12, TNF-α, TGF-β, bFGF, and NT-3 relative to control mice (some of these are shown in Fig. 5, Table I). The proinflammatory cytokines IL-1β, IL-6, IL-12, and TNF-α were detected mainly in macrophages, reactive astrocytes, and lymphocytes. The growth factors TGF-β, bFGF, and NT-3 were detected primarily in reactive astroglia, and a few neurons and microglial cells also were positive.
Angiogenesis
Angiogenesis, as judged by using angiopoietin, VEGF, and perlecan immunoreactivity, was not pronounced in the brainstem of saline-injected normal mice (not shown), whereaas, as shown by Brett et al. (1995), the GFAP-IL6 mice showed an increased angiogenic potential with increased number of vessels (not shown). After 6-AN injection, expression of angiogenic factors increased around the degenerated areas. However, in the brainstem of the normal mice, angiogenic factors were only very mildly expressed, whereas the angiogenic response of GFAP-IL6 mice was very pronounced and angiogenesis was increased relative to the normal mice (Fig. 6).
Oxidative Stress and Apoptosis
To study oxidative stress, we have used immunoreactivity for iNOS (Sasaki et al., 2001), MDA (Lung et al., 1990), NITT (Ye et al., 1996), and 8-oxoguanine (Soultanakis et al., 2000). In brainstem sections of saline-injected mice, the iNOS, MDA, NITT, and 8-oxoguanine immunostainings were comparable in wild-type and GFAP-IL6 mice and showed that the normal CNS hardly suffers from oxidative stress (some of these are shown in Fig. 7, see also Table I). After 6-AN, oxidative stress as determined by using immunoreactivity for iNOS, MDA, NITT, and 8-oxoguanine was significantly increased in wild-type mice relative to GFAP-IL6 mice (see Fig. 7, Table I). The cells suffering from oxidative stress after 6-AN were mainly neurons and astrocytes, as verified by using triple immunofluorescence for MDA or NITT and cell markers. Apoptotic cell death was determined by using TUNEL, which showed that the normal CNS contains very few apoptotic cells (Fig. 7, Table I). After 6-AN injections, all mice increased the number of TUNEL+ cells, but GFAP-IL6 mice showed significantly fewer TUNEL+ cells than wild-type mice after 6-AN (Fig. 7, Table I). Apoptotic cells were mainly neurons and astrocytes, as verified by using fluorescence TUNEL and immunofluorescence (data not shown). In contrast to the 3-day time point, basically no TUNEL+ cells were observed 1 day after 6-AN (not shown).
MT-I+II Expression
Figure 8 and Table I show MT-I+II immunohistochemistry results. In accordance with previous data (Carrasco et al., 1998; Penkowa et al., 2000b; Molinero et al., 2003), MT-I+II immunoreactivity increased significantly in the CNS in GFAP-IL6 mice relative to wild-type mice (Fig. 8A,B). After 6-AN administration, wild-type and GFAP-IL6 mice showed clearly increased MT-I+II expression, which was most pronounced in the areas of reactive astrogliosis around the degenerated gray matter areas. As might be expected, the GFAP-IL6 mice showed a significantly greater MT-I+II up-regulation following 6-AN than wild-type mice (Fig. 8C,D, Table I).
DISCUSSION
In the present study, we have examined transgenic mice with overexpression of IL-6 (GFAP-IL6 mice) and proper controls following an i.p. injection with 6-AN. 6-AN induces astroglial toxicity and cell death in certain astrocyte populations, mainly those of the brainstem gray matter areas, followed by a CNS inflammatory response (Krum, 1994, 1995; Haghighat and McCandless, 1996;Penkowa et al., 1997, 1999b, 2002; Penkowa and Hidalgo, 2000a; Tyson et al., 2000).
In 6-AN-injected GFAP-IL6 mice, the degree of 6-AN-induced degeneration of brainstem gray matter areas was similar to that of wild-type mice. This was established by H&E as well as GFAP and S100 stainings, which showed that almost all the astrocytes disappeared from the degenerated gray matter areas of the brainstem. This indicates that the initial effects elicited by 6-AN are unaffected by IL-6 transgenic expression regarding astrocyte damage and death. However, reactive astrogliosis was observed surrounding these injured areas, and this was greater in the GFAP-IL6 mice than in the wild-type mice, suggesting that the transgenic expression of IL-6 potentiated the astrocyte response to the tissue injury elicited by 6-AN, which could have significant effects on the delayed responses of the CNS to 6-AN-induced gliotoxicity given the importance of astrocytes (Krum and Rosenstein, 1993;Xiao and Link, 1998; Benveniste, 1998; Raivich et al., 1999). GFAP and S100 stainings were also higher in saline-injected GFAP-IL6 mice, which is in agreement with previous studies showing that these mice display reactive astrogliosis in the hindbrain (Campbell et al., 1993; Brett et al., 1995; Campbell, 1998a, b; Giralt et al., 2002a; Molinero et al., 2003). Also, this is consistent with results observed in IL-6-deficient mice showing significantly reduced astrogliosis (Klain et al., 1997; Penkowa and Hidalgo, 2000a; Penkowa et al., 2000b).
In addition, the inflammatory responses of macrophages/microglia and lymphocytes, including their precursors, CD-34+ myeloid and lymphoid progenitor cells of the bone marrow, were clearly increased in CNS of the GFAP-IL6 mice relative to wild-type mice after 6-AN injection. The recruitment to the CNS of CD-34+ myeloid and lymphoid progenitor cells induced by IL-6 overexpression is shown for the first time. However, this effect fits with the fact that IL-6 is a multifunctional cytokine, which has profound effects on the three major systems, the immune system, the hematopoietic system, and the nervous system (Heinrich et al., 1990; Hirano et al., 1990; Kopf et al., 1994; Hauser et al., 1997). Hence, IL-6 functions synergistically with IL-3 to stimulate multilineage blast-colony formation in the bone marrow, and IL-6 also affects the further growth and differentiation of hematopoietic cells (for review see Hirano et al., 1990;Kopf et al., 1994; Peters et al., 1998). Accordingly, CD-34+ cells differentiate and grow in the presence of stem cell factor (SCF) and IL-6, causing increases in cell size and numbers relative to cells treated with SCF alone (Conti et al., 2002).
Moreover, IL-6, as expected, increased in GFAP-IL6 mice, but also other proinflammatory cytokines, such as IL-1β, IL-12, and TNF-α were increased in these mice (Campbell et al., 1993; Campbell 1998a, b). Along with proinflammatory cytokines, the antiinflammatory and/or trophic factors TGF-β, bFGF, VEGF, and NT-3 were also increased by 6-AN administration, and, again, this response was increased more in the IL-6-overexpressing mice than in wild-type mice. Furthermore, expression of the antiinflammatory and antioxidant proteins MT-I+II was significantly increased by IL-6 overexpression. This is in agreement with previous studies showing that IL-6 is the major inducer in the CNS of MT-I+II (Hernandez et al., 1997; Carrasco et al., 1998; Penkowa and Hidalgo, 2000a; Penkowa et al., 2000b). Because MT-I+II are inducers of antiinflammatory growth factors (Penkowa et al., 2000a, 2003), both increased IL-6 and increased MT-I+II may explain the increases in TGF-β, bFGF, VEGF, and NT-3 expression in GFAP-IL6 mice.
Additionally, the angiogenic response seen after 6-AN was significantly increased in GFAP-IL6 mice, which showed significantly increased immunoreactivity for angiopoietin, VEGF, and perlecan in the brainstem. Thus, vessel sprouting and vasculogenesis are induced by IL-6 overproduction, which is in agreement with other studies (Brett et al., 1995; Swartz et al., 2001) showing how IL-6 stimulates vascular sprouting and the number of vessels in the CNS. This could be an indirect effect, which is mediated by MT-I+II rather than by IL-6 per se. Thus, GFAP-IL6 mice with MT-I+II deficiency show significantly reduced angiogenesis relative to GFAP-IL6 mice with normal MT-I+II levels (Penkowa et al., 2000a). Also, MT-I overexpression increases angiogenesis significantly in GFAP-IL6 mice (Molinero et al., 2003). As well, MT-I overexpression and MT-II treatment induce CNS angiogenesis following brain damage (Giralt et al., 2002b;Penkowa et al., 2002). Thus, the IL-6-induced increases in MT-I+II levels along with growth factors may be responsible for the increased angiogenesis in the parenchyma. In addition, the IL-6-induced increases in growth factor expression could also contribute to increased angiogenesis, in that bFGF, TGF-β, NT-3, and VEGF are neuroprotective factors involved in angiogenesis and tissue regeneration (Pepper et al., 1992, 1993; Gómez-Pinilla et al., 1992;Logan et al., 1994; Hopkins and Rothwell, 1995; Mocchetti and Wrathall, 1995; Ebadi et al., 1997; Yoshida et al., 1997; Xiao and Link, 1998; Morisaki et al., 1999; Neufeld et al., 1999; Suda et al., 2000).
In addition to the increase in these inflammatory reactions, 6-AN-injected GFAP-IL6 mice also showed significantly reduced levels of oxidative stress as determined by immunoreactivity for iNOS, NITT, MDA, and 8-oxoguanine; moreover, 6-AN-induced apoptotic cell death as judged by TUNEL and cleaved caspase-3 immunostaining was considerably reduced relative to that in wild-type mice. It is likely that oxidative stress and apoptosis are linked (Sun and Chen, 1998; Floyd, 1999; Cassarino and Bennett, 1999). Hence, in general, the GFAP-IL6 mice appeared to suffer less from 6-AN-induced CNS tissue damage than wild-type mice at more advanced stages (3 days after 6-AN injection), in contrast to the initial stages. It is likely that such neuroprotection would be apparent also in H&E stainings carried out for longer times, which deserves further attention.
It is very likely that the IL-6-induced increase in MT-I+II contributes to the observed decreases in oxidative stress and apoptosis in GFAP-IL6 mice after 6-AN despite the increased ongoing inflammatory response. Thus, accumulating data show how MT-I+II are extremely competent antioxidants and scavengers of free radicals, thereby having important roles during oxidative stress (Thornalley and Vasak, 1985; Aschner, 1998; Simpkins, 2000; Van Lookeren Campagne et al., 2000; Viarengo et al., 2000; Hidalgo et al., 2001, 2002; Giralt et al., 2002). Thus, in vitro, MT-I+II inhibit DNA degradation and tissue damage from oxidative stress (Cai et al., 2000; Kling and Olsson, 2000; Rana and Kumar, 2000), and MT-I+II functionally substitute for Cu/Zn-superoxide dismutase (Cu/Zn-SOD) in vivo to protect cells from oxygen toxicity (Tamai et al., 1993). Moreover, cells from MT-I+II-deficient mice, in spite of their normal levels of reduced glutathione and other antioxidant enzymes, showed enhanced ROS production (Lazo et al., 1995). In addition, genetic MT-I+II deficiency increases significantly the levels of oxidative stress and apoptosis in vivo during CNS pathological conditions, such as during seizures (Carrasco et al., 2000), traumatic injury (Penkowa et al., 1999, 2000a), IL-6-induced cerebellar neuroinflammation (Giralt et al., 2002a), and experimental autoimmune encephalomyelitis (EAE; Penkowa et al., 2003). In this manner, reduced levels of oxidative stress and apoptosis were induced by MT-I overexpression and MT-II treatment in mice after 6-AN (Penkowa et al., 2002), traumatic brain injury (Giralt et al., 2002b), IL-6-induced cerebellar neuroinflammation (Molinero et al., 2003), and EAE (Penkowa and Hidalgo, 2000b). Thus, by inducing MT-I+II in the CNS, IL-6 can prevent oxidative stress, which leads to cell death (Cassarino and Bennett, 1999), and thereby IL-6 provides significant neuroprotection. This study was carried out in mice heterozygous for MT-I&II, so it will be interesting to determine in further studies whether more prominent effects are observed in animals with normal MT expression and/or MT overexpression; these studies are currently underway in our laboratories. We nevertheless anticipate that this will actually happen, judging by previous results obtained in MT-I-overexpressing mice (Penkowa et al., 2002).
On the other hand, we cannot rule out that IL-6 may also have direct antioxidant effects in the brain. However, it was demonstrated that IL-6 per se has no effects on nitric oxide synthesis (De Laurentiis et al., 2000). Therefore, we suggest that the decrease in oxidative stress and apoptosis observed in 6-AN-injected GFAP-IL6 mice is mediated by the increased MT-I+II in these mice.
In addition, IL-6 may have other indirect actions, such as stimulating other neurotrophic factors. This possibility has been shown in vivo when intrathecal IL-6 infusion in lumbar dorsal root ganglia induced brain-derived neurotrophic factor (BDNF; Murphy et al. 2000). In vitro, IL-6 alone or together with IL-1 induced production of nerve growth factor (Juric and Carman-Krzan 2000). Indeed, in this study, IL-6 overexpression induced the growth factors bFGF, TGF-β, NT-3, angiopoietin, and VEGF as well as the receptor for bFGF. Therefore, it is likely that, in addition to its own, innate neurotrophic activity, IL-6 induces additional trophic factors in the CNS. The proposed mechanisms through which endogenous IL-6 may protect the brain during pathological conditions should be explored further to determine the role of IL-6 in neuroprotection.
Acknowledgements
We thank Hanne Hadberg, Ha Nguyen, and Drs. Joaquín Hernández and Javier Carrasco for excellent technical assistance and Keld Stub for photographic assistance. These studies were supported by The Lundbeck Foundation, Scleroseforeningen, Lily Benthine Lunds Fond, Dir. Ejnar Jonasson's Fond, The Danish Medical Research Council, Novo Nordisk Fonden, Direktør Ib Henriksens Fond, Warwara Larsens Fond, The Danish Medical Association Research Fund, Schrøders Fond, Kathrine og Vigo Skovgaards Fond, Dir. Jacob Madsen's Fond, Fonden af 17.12.1981, Fonden til Laegevidenskabens Fremme, Gerda og Aage Haensch's Fond, Eva og Henry Frænkels Mindefond, Karen A. Tolstrups Fond, Dansk Parkinsonforening, Th. Maigaard's Eftf. Fru Lily Benthine Lunds Fond af 1/6 1978, Hans og Nora Buchards Fond, Holger Rabitz Mindelegat, Ragnhild Ibsens Legat for Medicinsk Forskning, and Hestehandler Ole Jacobsens Mindelegat (all to M.P.) and by Ministerio de Ciencia y Tecnología and Feder grant SAF2002-01268 and Direcció General de Recerca grant 2001SGR 00203 (to J.H.).




