FGF-2 modulates expression and distribution of GAP-43 in frog retinal ganglion cells after optic nerve injury
Abstract
Basic fibroblast growth factor (bFGF or FGF-2) has been implicated as a trophic factor that promotes survival and neurite outgrowth of neurons. We found previously that application of FGF-2 to the proximal stump of the injured axon increases retinal ganglion cell (RGC) survival. We determine here the effect of FGF-2 on expression of the axonal growth-associated phosphoprotein (GAP)-43 in retinal ganglion cells and tectum of Rana pipiens during regeneration of the optic nerve. In control retinas, GAP-43 protein was found in the optic fiber layer and in optic nerve; mRNA levels were low. After axotomy, mRNA levels increased sevenfold and GAP-43 protein was significantly increased. GAP-43 was localized in retinal axons and in a subset of RGC cell bodies and dendrites. This upregulation of GAP-43 was sustained through the period in which retinal axons reconnect with their target in the tectum. FGF-2 application to the injured nerve, but not to the eyeball, increased GAP-43 mRNA in the retina but decreased GAP-43 protein levels and decreased the number of immunopositive cell bodies. In the tectum, no treatment affected GAP-43 mRNA but FGF-2 application to the axotomized optic nerve increased GAP-43 protein in regenerating retinal projections. We conclude that FGF-2 upregulates the synthesis and alters the distribution of the axonal growth-promoting protein GAP-43, suggesting that it may enhance axonal regrowth. © 2003 Wiley-Liss, Inc.
Neurons of the adult central nervous system (CNS) of mammals fail to regenerate axons after nerve injury. In contrast, neurons of the peripheral nervous system (PNS) and the CNS of lower vertebrates such as fish and amphibians are able to regenerate and recover function after nerve injury. Several studies in those models have focused on the activation of intracellular signaling pathways of the injured neuron that promote axonal growth (Bernhardt, 1999; Goldberg and Barres, 2000). One neuronal protein closely related to axonal growth during development and regeneration is the membrane growth-associated phosphoprotein (GAP)-43 (Skene and Willard, 1981; Benowitz and Routtenberg, 1997). Constitutive basal levels of GAP-43 are found in a subpopulation of peripheral neurons and in axons of frog optic nerve (Hoffman, 1989; Chong et al., 1992; Stewart et al., 1992; Golding and Tonge, 1993; Andersen and Schreyer, 1999). Events such as neuronal differentiation, axonal growth, reactive synaptogenesis, and injury cause changes in GAP-43 expression (Skene, 1989; Benowitz et al., 1990; Oestreicher et al., 1997).
GAP-43 is localized in the soma, dendrites, and axons of rat retinal ganglion cells (RGCs) early during CNS development (Reh and Kljavin, 1989). The expression of this protein declines after target innervation, myelination of axons, and the formation of stable synapses. In embryonic cell cultures, GAP-43 is present in high concentrations in the growth cones and their filopodia (Burry et al., 1992), suggesting a role of GAP-43 in growth cone motility and in the interaction of growth cone with its environment (Meiri and Burdick, 1991). This expression declines during the differentiation of the growth cones into presynaptic terminals (Burry et al., 1992).
After optic nerve injury and peripheral nerve graft in rat and hamster, regenerating retinal ganglion cells are immunopositive to GAP-43 (Schaden et al., 1994; Ng et al., 1995) indicating that this molecule is upregulated in those cells. This evidence suggests an association of GAP-43 with axonal growth not only during development but also in axonal regeneration.
Growth factors alter the expression of intrinsic neuronal genes involved in cell survival and regeneration (Bregman, 1998; Horner and Gage, 2000). Basic fibroblast growth factor (FGF-2) is a member of the FGF family that has been shown to protect neurons against injury and degeneration (Viollet and Doherty, 1997). It has been demonstrated that the application of this factor stimulates neurite and axonal growth during development of the Xenopus visual system (McFarlane et al., 1995) and in RGCs in vitro (Bahr et al., 1989; Viollet and Doherty, 1997). FGF-2 also increases long-term cell survival in frog retinal ganglion cells after optic nerve axotomy (Blanco et al., 2000). Because FGF-2 is found in some ganglion and Müller cells of the frog retina and in the glial cells of the optic nerve (Blanco et al., 2000), we decided to investigate the possible involvement of this molecule in regeneration of optic nerve after axotomy. Although the effects of FGF-2 on axonal growth have been established in development and in vitro, the role of FGF-2 in axonal regeneration and the molecular mechanisms of these effects have not yet been elucidated.
The objective of this study was to characterize changes in GAP-43 expression during frog optic nerve regeneration after axotomy, and to determine if the application of FGF-2 after axotomy altered expression of this protein in retina and optic tectum.
MATERIALS AND METHODS
Surgical Technique and FGF-2 Application
Under 0.3% tricaine anesthesia, the right eyeball of a series of frogs (Rana pipiens) was approached from the palate and an incision made; the extraocular muscles were teased aside and the intraorbital section of the optic nerve was exposed. While avoiding large blood vessels, the nerve, with its meningeal sheath, was severed. The incision was sutured and the animal was allowed to recover in an individual cage. All protocols conformed to NIH guidelines and were approved by the institutional Animal Care and Use Committee.
For FGF-2 treatment (Chemicon, Temecula, CA), the growth factor was dissolved at a concentration of 25 μg/ml in phosphate-buffered saline (0.1 M PBS), pH 7.4. At the time of cutting, the optic nerve stump was lifted and placed on a strip of Parafilm and 5 μl of FGF-2 solution was applied with a Hamilton syringe directly to the cut end and allowed to soak for 5 min. This was equivalent to a total dose of 125 ng of FGF-2. The Parafilm was then removed and the incision sutured. Control applications consisted of 5 μl PBS applied for the same length of time.
Immunohistochemistry
Several eye-cups and tecta (n = 3–5) from control and experimental frogs (1, 2, 4, 8, and 12 weeks after operation) were removed and fixed in buffered 2% paraformaldehyde for 45 min. The tissues were placed in 30% sucrose for cryoprotection at 4°C overnight, then they were frozen and cryostat sections of 10–12 μm were cut. The sections were washed twice (5 min each) in PBS containing 0.3% Triton X-100 and 0.5% BSA and incubated 30 min in the same buffer containing 10% normal goat serum. They were then incubated with the monoclonal antibody against GAP-43 (1:125; Chemicon) diluted in 0.1 M PBS + 0.3% Triton X-100 + 0.5% BSA, for 2 hr at room temperature for the retinas, and overnight at 4°C for the optic tecta. A different monoclonal antibody (1:100; Novocastra Laboratories/Vector, Burlingame, CA) and a polyclonal antibody against GAP-43 (1:100; Sigma, St. Louis, MO) were also tested and similar results were obtained. After several washes with 0.1 M PBS + 0.3% Triton X-100 + 0.5% BSA, the sections were incubated with goat anti-mouse secondary antibody for 1 hr at room temperature. The sections were rinsed in PBS six times for 10 min each, and mounted in Polymount (Polysciences Inc., Warrington, PA). Preparations were viewed with a Zeiss Axioskop microscope equipped with a MicroMAX CCD camera (Princeton Instruments, Inc., Trenton, NJ).
For whole-mount preparations, retinas of operated frogs with and without FGF-2 application were dissected out and the vitreous body removed with the aid of 1 mg/ml collagenase/dispase/DNase cocktail diluted in frog Ringer solution for 15 min. After several washes in Ringer solution, the whole retinas were fixed 45 min in 4% paraformaldehyde and rinsed three times (15 min each) in PBS. The retinas were incubated 4 days in the primary anti-GAP-43 antibody (diluted in 0.1 M PBS + 10% normal goat serum + 3% Triton X-100 + 1% BSA) and washed three times in 0.1 M PBS + 3% Triton X-100 + 1% BSA for 15 min each. After 2 hr of secondary antibody incubation, the retinas were washed five times in PBS (15 min each) and flat mounted in Polymount.
GAP-43-Labeled Ganglion Cell Counts
GAP-43 expressing RGCs were counted in flat whole-mount retinas at 2, 4, 8, and 12 weeks after axotomy and after FGF-2 treatment. Cell counts in the ganglion cell layer were made taking two 0.6-mm2 sample areas of the superior, inferior, temporal, and nasal quadrants, 1 mm from the optic nerve, and two-thirds the distance to the periphery. Three retinas were averaged for each treatment.
Tetramethylrhodamine Dextran Amine Retrograde Labeling of RGCs
The retrograde labeling of RGCs with tetramethylrhodamine dextran amine (TDA; 3,000 MW; Molecular Probes, Eugene, OR) was carried out as described previously (Duprey-Diaz et al., 2002). Briefly, the optic nerve of four animals at 6 weeks after axotomy was cut close to the chiasm (at this time regenerated axons had reached the chiasm), a piece of cellulose acetate membrane filter saturated with TDA was inserted between the cut stumps, and the palate was sutured. After 48–72 hr, the labeled retinas were dissected and fixed as described above, and processed for immunocytochemistry.
Characterization of a R. pipiens GAP-43 fragment by RT-PCR
A small, conserved fragment of R. pipiens GAP-43 cDNA was cloned and sequenced. Degenerate primers (forward 5′ [CA]GIACIAARCARGTNGARAARAA 3′, reverse 5′ TTIC[GT]IRT[AGT]ATRTGICCNC[GT]RAA 3′) based on a highly conserved amino acid sequence of GAP-43 were used in a RT-PCR to isolate a 129-bp fragment of the R. pipiens GAP-43 gene. Reverse transcription of 0.7 μg of total RNA from the retina was carried out with Superscript II reverse transcriptase (100 U; Gibco BRL, Life Technologies AG, Switzerland) using random hexamers, according to the directions supplied by the manufacturer. The PCR conditions were: 95°C, 1 min × 1; 94°C, 1 min; 42°C, 1 min; 75°C, 1 min × 35 and 75°C, 5 min. The gel-isolated fragment was cloned into Bluescript as described previously (Baro et al., 1994) and sequenced by the Cornell Biotechnology Facility. Alignment of the Rana sequence with homologues from other species indicated that this region is 91% identical to the GAP-43 protein of Xenopus, rat, and human.
Semiquantitative RT-PCR
Semiquantitative RT-PCR was used to measure levels of GAP-43 gene expression in control and experimental animals. At various time points after sectioning the optic nerve (1, 2, 4, 8, and 12 weeks), control and experimental retinas and tecta were dissected and frozen on dry ice. Tissues from three animals were pooled and RNA was extracted using the Nucleospin Nucleic Acid RNA II Purification Kit (BD Bioscience, Palo Alto, CA). Two control and two experimental RNA extractions (6 animals) were carried out per time point. RNA quality was assessed with denaturing gel electrophoresis and concentrations were determined with a biophotometer. An aliquot of each RNA sample (0.7 μg) was then used to synthesize first strand cDNA. No controls were carried out to assess the efficiency of the reverse transcription.
Forward and reverse primers were designed based on the sequence information described above. An external standard was constructed by using these primers to amplify DNA from a Phi-X ladder (Promega, Madison, WI) and cloning the product into pBluescript (Stratagene, La Jolla, CA). The resultant 318-bp standard contained the forward and reverse primers flanking an unrelated segment of Phi-X DNA. Preliminary experiments were carried out with this standard to determine optimal cycle number and starting template concentration as described previously (Baro et al., 1997), using the GAP-43 primers. Then 0.02 μg/μl of cDNA from control and experimental animals were subjected to increasing numbers of amplification cycles, using the optimal concentration of 2 pg/μl external standard. The resulting amounts of DNA were quantified as described below and plotted against PCR cycle number. Amplification of both the external standard and GAP-43 began to saturate at 35 cycles in all preparations; all subsequent PCRs were therefore carried out at 30 cycles, within the linear range of amplification.
PCRs using the forward and reverse primers for GAP-43 were carried out with first strand retinal cDNA and a known amount of the standard. A PCR volume of 50 μl contained 10 μl cDNA, 2 pg/μl of external standard, specific primers against R. pipiens GAP-43 (1 pmol/μl, 1 μM of each), forward 5′ GAGGATGCTGACCAAA AAATTGAA 3′ and reverse 5′ GCTGGCCTGGATTTTGGTAG 3′, 1× 1.5 mM MgCl2 Taq buffer (Roche Diagnostic, Indianapolis, IN), 0.2 mM of dNTPs mix PCR grade (Roche Diagnostics) and 0.025 U/μl of Taq polymerase enzyme (Roche Diagnostics). Each PCR product was run in a 10% polyacrylamide gel and images were recorded and analyzed with the Gel Expert Software (Nucleo Tech Corp., San Mateo, CA). GAP-43/standard band intensity ratios were recorded and analyzed with the Gel Expert software (Nucleo Tech). Optical densities (OD) of the GAP-43 band and the standard band were determined. The GAP-43 OD was normalized by the standard OD. Three PCRs were carried out on each cDNA, giving a total of 6 PCRs for each condition.
In Situ Hybridization
In situ hybridization was carried out essentially as described by the Genedetect protocol for DIG-labeled oligonucleotide probes. The Genedetect Company (New Zealand) designed a 48mer digoxigenin-labeled oligonucleotide probe to the R. pipiens GAP-43 sequence. The eyes and tecta from control and experimental animals (4 weeks after axotomy and FGF-2 application) were dissected and fixed in 4% paraformaldehyde for 45 min, and then 16-μm cryostat sections were cut, air dried, and stored at −80°C. Just before use, tissue sections were washed in PBS, dehydrated, and air-dried. Sections were hybridized overnight at 37°C with 200 ng/ml of GAP-43 digoxigenin-labeled oligonucleotide probes in the hybridization solution (10% dextran sulfate, 4× SSC [1× = 150 mM NaCl, 15 mM sodium citrate, pH 7.2], 50% formamide, 10 mM dithiothreitol [DTT], 0.25 mg/ml Poly A, 0.25 mg/ml of denatured and sheared salmon sperm DNA, and 1× Denhardts buffer). After hybridization, sections were quickly washed with 1× SSC (10 mM DTT) at RT, two successive washes of 15 min were made with 1× SSC (10 mM DTT) at 55°C, with a further two washes of 15 min with 0.5× SSC (10 mM DTT) also at 55°C. The slides were then washed three times with Tris-buffered saline (TBS) and incubated 30 min in a blocking solution (TBS + 0.3% Triton X-100 + 2% normal sheep serum). The oligonucleotide probes were detected using a monoclonal anti-digoxigenin antibody (Roche Diagnostics) in a 1:100 dilution in the blocking solution. After 4 hr of incubation, the slides were washed with TBS six times (10 min each), incubated in the secondary antibody, and mounted in an anti-fading agent (Vectashield, Vector Labs).
Western Blots
Three retinas or tecta from control, axotomy plus PBS, and axotomy plus FGF-2 were dissected quickly and stored at −70°C until use. The samples were homogenized in 2 volumes of lysis buffer (10 mM HEPES, pH 7.6, 10 mM EDTA, 10 mM EGTA, 150 mM NaCl, 2.5% CHAPS, 0.2 mM phenylmethylsulfonyl fluoride (PMFS), 0.1 μg/ml leupeptin, 0.05 μg/ml pepstatin, 0.1 μg/ml aprotinin) and protein quantification was determined using Bio-Rad Protein Assay Dye Concentrate (Bio-Rad, Hercules, CA). Next, 50 μg of protein from each sample was separated on 8% SDS-polyacrylamide gel and transferred to a polyvinylidene fluoride (PVDF) membrane at 100V for 1 hr. The membranes were blocked for 4 hr at room temperature in a blocking solution containing 5% nonfat dry milk, 0.1% Tween-20 and TBS (50 mM Tris HCl, pH 7.5, and 150 mM NaCl). After blocking, the membranes were incubated overnight at 4°C with the primary antibody GAP-43 (1:1,000; Chemicon) diluted in blocking solution. The membranes were washed three times with 0.1% Tween-20 in TBS (15 min each) and incubated with an alkaline phosphatase-conjugated goat anti-mouse IgG antibody diluted in blocking solution (1:3,000; Bio-Rad). The membranes were washed with TBS as described before and then developed for analysis using a chemiluminescence method (Bio-Rad). Images were recorded and analyzed with the Gel Expert Software (Nucleo Tech). Optical densities (OD) of the GAP-43 band of four different Western blot experiments were determined and normalized relative to the averaged control data. The statistical significance was determined using Student's t-test.
RESULTS
GAP-43 Immunoreactivity After Optic Nerve Injury
In retinal sections from control animals, GAP-43 immunoreactivity was present only in the axons of the nerve fiber layer and optic nerve (Fig. 1A). In contrast to normal retinas, after optic nerve injury a subpopulation of large, α-like, RGCs were observed with GAP-43 immunoreactivity in their soma and dendrites (Fig. 1B). These labeled cells were observed throughout the period of regeneration, which is complete by approximately 12 weeks after axotomy. To determine if the GAP-43-containing RGCs are regenerating cells, retrograde labeling of these cells was carried out with TDA at 6 weeks after axotomy (when the axons are actively growing and have reached the optic tract). The majority of the GAP-43-immunostaining RGCs (Fig. 1C) were labeled retrogradely with TDA (Fig. 1D) indicating that these cells had regenerated axons to the chiasm. Many of the TDA-labeled RGCs were not labeled with GAP-43 in their cell bodies; however, all their axons were immunopositive for GAP-43 (Fig. 1C). RGCs with GAP-43 in the cell body comprised approximately 4% of the total TDA-labeled RGC population at this stage.
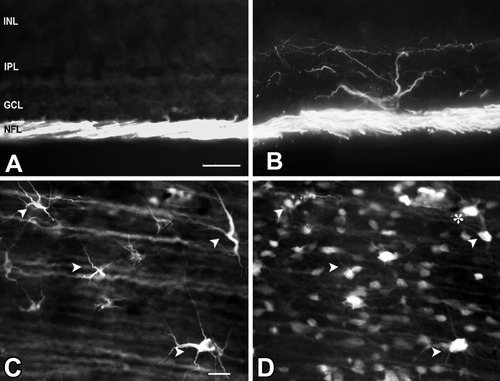
GAP-43 immunohistochemical staining of retinal sections. A: In control retinas, GAP-43 immunoreactivity is present in axons of ganglion cells that form the nerve fiber layer (NFL). B: Four weeks after axotomy, GAP-43 immunoreactivity is present in the cell body and dendrites of a subpopulation of large α-like RGCs. C: Whole-mount retina immunostained for GAP-43 6 weeks after axotomy. D: The same retina labeled retrogradely with TDA showing that GAP-43-immunoreactive RGCs are regenerating cells. Arrowheads, double-labeled cells; asterisk, back-filled RGC that does not contain GAP-43. Scale bar = 50 μm (A,B); 25 μm (C,D).
Counts of GAP-43-immunopositive RGCs in flat whole-mount retinas were carried out to determine the expression pattern of this protein in RGCs during regeneration (Fig. 2A). Two weeks after axotomy, the average number of GAP-43-positive RGCs per mm2 was 443 ± 49; an additional 30% more labeled cells were found at 4 weeks after axotomy (Student's t-test, P < 0.05). Eight weeks after axotomy, the number of RGCs staining with GAP-43 decreased significantly to almost the same values as those 2 weeks after axotomy (P < 0.05) and remained without a significant change at 12 weeks (436 ± 25).
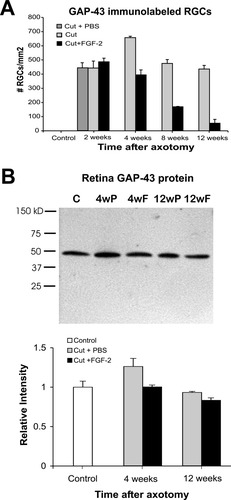
A: Histogram indicating the number of GAP-43 immunoreactive RGC cell bodies in 1 mm2 of whole-mount retinas during optic nerve regeneration and after FGF-2 application. Numbers of GAP-43 positive cells increase after axotomy and reach a maximum at 4 weeks. FGF-2 application significantly decreases the numbers of GAP-43-stained cells at 4, 8, and 12 weeks after axotomy (Student's t-test, P < 0.05). PBS treatment at axotomy is indistinguishable from axotomy alone. B: Representative Western blot and densitometric analysis of GAP-43 protein from control (C) and experimental retinas at 4 and 12 weeks after axotomy and PBS (P) or FGF-2 (F) treatment. Three different animals per experimental group were pooled and Western blot experiments were repeated four times.
GAP-43 Immunoreactivity Changes After FGF-2 Application
We next analyzed GAP-43 immunoreactivity in whole-mount flat retinas of FGF-2-treated optic nerves after axotomy. The number of GAP-43-labeled RGC cell bodies was significantly lower in FGF-2-treated retinas from 4, 8, and 12 weeks after axotomy than in the axotomized retinas without the treatment (Fig. 2A). Four weeks after axotomy and FGF-2 application, the number of RGCs labeled with GAP-43 decreased by 40% when compared to untreated axotomized retinas (Fig. 2A, Student's t-test, P < 0.05). At 4 weeks, there were no differences in the pattern of staining of GAP-43 between retinas with FGF-2 treatment and retinas without it (compare Fig. 3A,B). At 8 weeks after axotomy and FGF-2 treatment, only one-third of the cells labeled with GAP-43 remained as compared to untreated axotomized retinas (Fig. 2, P < 0.05). After 12 weeks of axotomy and FGF-2 treatment, the number of GAP-43-positive RGCs was markedly lower in comparison to all the other stages. A decrease of 85% in the labeled cells was found in the FGF-2-treated retinas versus untreated retina at this time (Fig. 2, P < 0.05). We also found in those FGF-2-treated retinas differences in the pattern of labeling of GAP-43, where mainly a network of nerve processes and dendrites was strongly labeled (compare Fig. 3C,D).
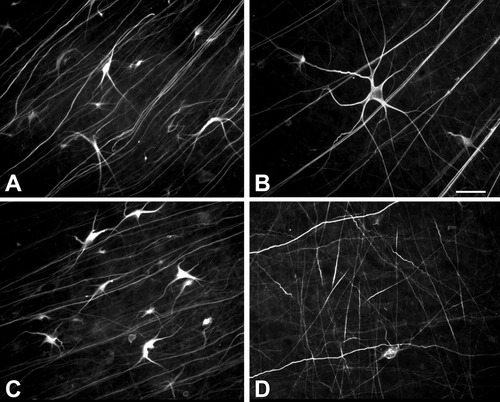
Whole-mount retinas immunostained for GAP-43 after axotomy and FGF-2 application to the optic nerve. A: At 4 weeks after axotomy, GAP-43 immunoreactivity is present in a subpopulation of large α-like RGCs. B: After FGF-2 application, no morphological changes in the GAP-43-stained RGCs are observed at 4 weeks. C: At 12 weeks after axotomy, GAP-43-immunoreactive cells remain present in untreated retina. D: FGF-2 treatment at 12 weeks results in a decrease of GAP-43-labeled RGCs, although many dendrites and axons remain stained. Scale bar = 50 μm.
GAP-43 Protein Levels Increase During Regeneration and FGF-2 Application
Western blot analysis showed that GAP-43 protein levels increased by 20% in the frog retina 4 weeks after optic nerve axotomy and PBS application when compared to normal retina (Fig. 2B). The levels of GAP-43 protein in retinas at 4 weeks after optic nerve axotomy and FGF-2 application were reduced back to control levels (Fig. 2B, P < 0.05). It should be noted that by this stage, about 25% of RGCs have been lost (Blanco et al., 2000). At 12 weeks after axotomy, GAP-43 protein levels were 10% lower in the retina with the FGF-2-treated cut nerve when compared to the PBS-treated axotomized retina, but the levels under both conditions were not different from control retinas. Again, it must be pointed out that by this time 50% of the RGCs had died, and FGF-2 treatment saved an additional 30% (Blanco et al., 2000), so the amounts of GAP-43 per cell were probably higher than in controls.
GAP-43 mRNA Levels Increase During Regeneration and FGF-2 Application
To determine whether GAP-43 expression was modulated after optic nerve injury and FGF-2 application, in situ hybridization and semiquantitative RT-PCR studies were conducted. Low levels of GAP-43 mRNA were detected by in situ hybridization in most cells in the RGC layer of the normal retina (Fig. 4A). These levels were noticeably increased 4 weeks after axotomy in retinas from both the PBS-treated and the FGF-2-treated optic nerves (Fig. 4C,D). No hybridization signal was detected in retinas with a sense probe (Fig. 4B). Because most cells in the GCL are ganglion cells with, in Rana pipiens, only 16% of the cells being displaced amacrines (Scalia et al., 1985), and because the GAP-43 signal was not detected in the inner nuclear layer where most amacrine cells are situated, it is reasonable to conclude that all cells with the GAP-43 hybridized signal were RGCs.
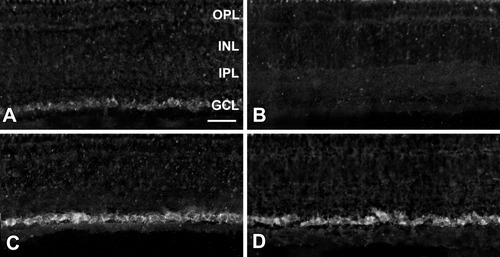
GAP-43 in situ hybridization of retinal sections. (A) In control retinas, low GAP-43 mRNA levels are present in cells of the ganglion cell layer (GCL). (B) Retina hybridized with a sense probe. (C) Four weeks after axotomy and PBS treatment and (D) FGF-2-treated retinas show an increase in the GAP-43 mRNA present in the RGCs. Scale bar = 50 μm.
Because retinal GAP-43 mRNA is restricted mainly to RGCs, we were able to use semiquantitative RT-PCR (Fig. 5) to quantify changes in retinal GAP-43 mRNA during axonal regrowth and after FGF-2 application, rather than using the technically challenging approach of quantitative, non-radioactive, in situ hybridization. In control retinas, GAP-43 mRNA was low; 1 week after axotomy, an increase of approximately sevenfold in GAP-43 mRNA was observed (Fig. 5B, P < 0.05). This increase continued until it peaked at 2 weeks, after which point these levels were maintained for 4, 8, and 12 weeks after axotomy when compared to control levels (Fig. 5B, P < 0.05).
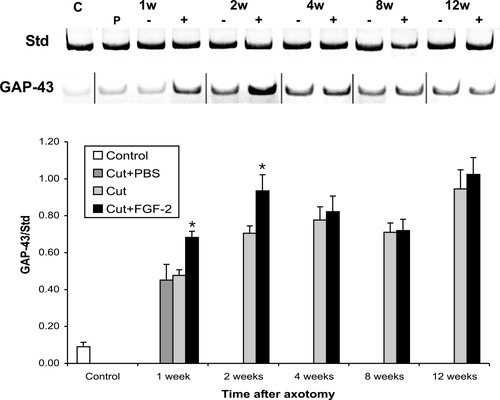
Semiquantitative RT-PCR measurement of GAP-43 mRNA in the retina. The ratio of GAP-43 mRNA to a standard was measured in control retinas, and at 1, 2, 4, 8, and 12 weeks after axotomy with and without FGF-2 application. A: A typical gel showing changes in GAP-43 mRNA expression after axotomy and FGF-2 application. B: Histogram of the data, showing a significant increase of GAP-43 mRNA after axotomy and an additional significant increase after FGF-2 application at 1 and 2 weeks. (Student's t-test, P < 0.05).
When FGF-2 was applied to the axotomized optic nerve stump, an additional increase of GAP-43 mRNA was detected. At 1 week after axotomy with the application of FGF-2, 30% more GAP-43 mRNA was measured compared to retinas with untreated or PBS-treated axotomized optic nerves (P < 0.05). After 2 weeks, the GAP-43 mRNA levels peaked with almost 20% more mRNA than in retinas from untreated axotomized nerves, and almost eight times the basal expression in control retinas (Fig. 5, P < 0.05). Between 4 and 12 weeks after axotomy, FGF-2 treatment did not produce significant changes in GAP-43 mRNA levels when compared to retinas from untreated nerves (Fig. 5B). Application of FGF-2 to the intact nerve or intraocularly to the normal retina did not lead to any statistically significant changes in GAP-43 mRNA levels in the retina when compared to control and PBS-treated retinas (data not shown).
GAP-43 Protein in Frog Optic Tectum Increases After FGF-2 Application
To determine the effects of axotomy and FGF-2 treatment on the expression of GAP-43 protein and mRNA in the tectum, immunohistochemistry, Western blots, and semiquantitative RT-PCR experiments were carried out, using control and deafferented tectal lobes. The optic tectum consists of nine layers. Layer 9 comprises seven laminae that contain retinal projections of myelinated and unmyelinated axons, and collateral dendrites of type b tectal neurons (Scalia et al., 1968; Potter, 1969). To visualize the GAP-43 staining pattern in the tectum after regeneration, tecta of control animals were compared to tecta of animals 4 and 12 weeks after axotomy and PBS treatment, and with the tecta of animals at 12 weeks after axotomy with FGF-2 treatment. In control tecta, GAP-43 immunoreactivity was observed in laminae B, D, F, and G of layer 9 (Fig. 6A). These are plexiform laminae that contain axons of RGC projections (Scalia et al., 1968; Potter, 1969). At 4 weeks after axotomy in deafferented tectal lobes, a loss of GAP-43-positive fibers was observed in laminae B, D, and F (Fig. 6B). At 12 weeks after injury, when the regenerating axons of the retina reach their target in the tectum, staining of GAP-43 was found mainly in laminae F and G (Fig. 6C). In tecta from FGF-2-treated nerves 12 weeks after surgery, an apparent increase in GAP-43 immunoreactivity was observed in laminae B, D, F, and G when compared to tecta from axotomized animals. (Fig. 6D).
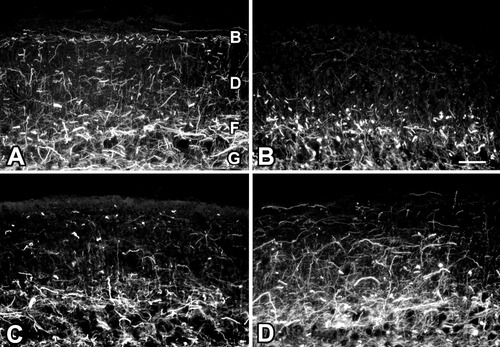
GAP-43 immunoreactivity in the frog optic tectum. A: GAP-43 immunoreactivity in the control optic tectum is observed in laminae of layer 9 that contain axonal projections of RGCs. B: Layer 9 shows a decrease in retinal processes stained with GAP-43 at 4 weeks after axotomy. C: At 12 weeks after axotomy, a slight increase in retinal processes labeled with GAP-43 is observed. D: Twelve weeks after axotomy and FGF-2 application, an additional increase in GAP-43-labeled retinal processes can be observed. Scale bar = 50 μm.
To quantify how GAP-43 protein is increased in the tectum, Western blots were carried out using extracts of deafferented tectal lobes (Fig. 7). GAP-43 protein showed a small but significant (P < 0.05), increase in the tectum 12 weeks after axotomy compared to controls. In the tecta where optic nerve was treated with FGF-2, however, an additional 30% increase was detected (Fig. 7A, P < 0.05).
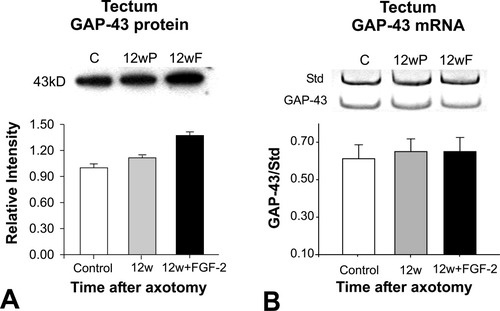
A: Representative Western blot and densitometric analysis of GAP-43 protein from control and experimental tecta 12 weeks after axotomy and PBS application (12wP) or FGF-2 (12wF) application. A small, significant increase in GAP-43 is observed at 12 weeks after axotomy when compared to control tecta; a larger increase is observed after FGF-2 application (Student's t-test, P < 0.05). B: RT-PCR for GAP-43 from total tectal tissues of control, 12 weeks after axotomy and PBS and 12 weeks after axotomy and FGF-2 application. GAP-43 mRNA levels in the tectum remain unchanged.
To test whether the increase of GAP-43 protein in the tectum was due to an increase in GAP-43 synthesis by tectal cells, semiquantitative RT-PCR was carried out using the isolated contralateral tecta of control and experimental animals 12 weeks after axotomy plus PBS, and axotomy plus FGF-2 application. The GAP-43 mRNA levels were unchanged in experimental tecta when compared to controls (Fig. 7B).
DISCUSSION
GAP-43 Is Present in Adult Frog RGCs
In this study, low basal levels of GAP-43 mRNA were found in normal adult frog retinas, and in situ hybridization indicated that it was restricted to RGCs. The protein itself was present only in axons of most RGCs. Strong GAP-43 immunoreactivity has been observed previously in the adult frog optic nerve (Golding and Tonge, 1993). In adult mammals, the protein is not normally expressed in RGC axons, only in sublaminae of the IPL (Moya et al., 1989; Doster et al., 1991; Kapfhammer et al., 1994). In the mammalian nervous system, GAP-43 expression and myelination of axons are inversely related (Kapfhammer and Schwab, 1994). We know that 97% of frog optic nerve axons are unmyelinated (Blanco and Orkand, 1996); whether there is a causal link with GAP-43 expression remains to be determined.
GAP-43 Is Upregulated by Axotomy
GAP-43 mRNA levels in RGCs are greatly increased after axotomy and this increase is sustained throughout the period of regeneration and reconnection to the target. Upregulation of GAP-43 synthesis is also observed in mammalian RGCs after axotomy (Fournier et al., 1997; Klocker et al., 2001). In zebrafish, however, GAP-43 expression is downregulated after target contact (Bormann et al., 1998). A similar discrepancy between fish and frog exists in the recovery from RGC hypertrophy, which may be due to slower refinement of retinotectal connections in the frog (Humphrey, 1988). In lizards, which do not recover a retinotectal projection at all, there is permanent upregulation of GAP-43 (Rodger et al., 2001). In contrast to the fish, in the frog a significant number of RGCs die after axotomy (Blanco et al., 2000). This could also slow the synaptic refinement process but may also produce changes in retinal circuitry that could prolong GAP-43 expression. There is evidence that sustained high levels of GAP-43 mRNA are found in adult neurons that undergo extensive periods of axonal arborization and synaptic plasticity or remodeling (Benowitz et al., 1990; Clayton et al., 1994).
Upregulation of GAP-43 could also be stimulated by target-derived growth factors. There is strong expression of brain-derived neurotrophic factor (BDNF) in the tectum 12 weeks after axotomy (Duprey-Diaz et al., 2002). BDNF treatment upregulates the expression of GAP-43 mRNA in RGCs after optic nerve injury (Fournier et al., 1997). It is possible therefore that high GAP-43 mRNA expression is maintained in the surviving RGCs in response to target-derived BDNF.
GAP-43 in Cell Bodies of RGCs After Axotomy
Concomitant with the upregulation of GAP-43 mRNA, there was a smaller increase in the amounts of the protein present in retina. Although intense immunostaining persisted in most RGC axons, after axotomy a small subpopulation of RGCs also contained GAP-43 in their soma and dendrites. A similar reappearance of GAP-43 is observed in mammalian RGCs in vitro (Meyer et al., 1994) and after optic nerve injury (Doster et al., 1991; Schaden et al., 1994). It is not clear why, in the frog retina, only a small subset of axotomized RGCs exhibit this accumulation of GAP-43 in the cell body and dendrites, even though most RGCs seem to upregulate the mRNA. The TDA experiments showed that these RGCs have extended their axons at least as far as the chiasm, so accumulation is not the result of a failure to regenerate. In the rat, there is evidence that the only RGCs capable of regeneration are those that accumulate GAP-43 (Schaden et al., 1994); however, this is clearly not the case in the frog. One possibility is that these RGCs in particular undergo more changes in synaptic inputs and exhibit more dendritic plasticity than do others. In cultured hippocampal neurons, GAP-43 immunoreactivity is found only in cell bodies and dendrites during the formation of synapses with neighboring neurons (van Lookeren Campagne et al., 1992). In the frog, we have observed dendritic remodeling in α-like ganglion cells after axotomy (Blanco et al., 1998), and this type of cell in particular accumulates GAP-43 in the cell body.
GAP-43 Expression and Distribution Is Modulated by FGF-2
Although axotomy alone produced a large increase in GAP-43 mRNA levels in RGCs, addition of FGF-2 to the cut nerve accelerated this increase in the first 2 weeks after axotomy. Paradoxically, FGF-2 treatment had the opposite effect on levels of the protein in the retina, and on the numbers of RGCs accumulating GAP-43. In the tectum, neither axotomy nor FGF-2 affected GAP-43 synthesis; however, treatment with the factor did increase the amounts of protein. Taken together, these results suggest firstly that FGF-2 enhances GAP-43 expression and secondly, that it affects the distribution of the protein, causing some of it to move from the retina to the tectum.
It has been shown that cultured motor neurons treated with FGF-2 upregulate GAP-43 mRNA (Piehl et al., 1998), so it is possible that it has a similar effect on frog RGCs. We saw no such effect, however, when FGF-2 was applied to the intact optic nerve, or intraocularly without axotomy. Frog RGCs have FGF receptors on both their axons and cell bodies (Blanco et al., 2000), so either site of application would be expected to activate GAP-43 synthesis. Rather, our results suggest that axotomy is a prerequisite for the effect of FGF-2; possibly, it must be transported retrogradely down the cut axons before it can affect GAP-43 gene transcription. Alternatively, axotomy may provide the retrograde signal to upregulate GAP-43 synthesis, and FGF-2 accelerates or strengthens this signal. BDNF also requires axotomy before it can enhance GAP-43 synthesis in rat RGCs (Fournier et al., 1997).
Our experimental evidence suggests that FGF-2 causes the redistribution of GAP-43 from the retina to the tectum. One possibility is that FGF-2 increases the numbers or accelerates the regrowth of GAP-43-containing RGC axons into the tectum. FGF-2 has been shown to accelerate growth of embryonic Xenopus RGC axons (McFarlane et al., 1995; Lom et al., 1998). The FGFR mediates the stimulation of neurite outgrowth by cell adhesion molecules (Williams et al., 1994), via the phosphorylation of GAP-43 (Meiri et al., 1998). In vitro, application of FGF-2 to hippocampal neurons induces the phosphorylation and translocation of GAP-43 from soluble to cytoskeleton-enriched fractions (Tejero-Diez et al., 2000).
Previous work in our laboratory demonstrated the stimulatory effect of FGF-2 on survival of axotomized RGCs (Blanco et al., 2000). In the present study, we have shown that FGF-2 upregulates the synthesis and alters the distribution of the axonal growth-related protein GAP-43, suggesting that FGF-2 may enhance axonal regrowth. One possibility is that this could lead to faster reconnection with the target and thereby allow survival of more axotomized neurons. We are currently investigating the molecular pathways by which FGF-2 promotes neuronal survival and axonal regrowth.
Acknowledgements
We thank C. del Cueto, W. Medina, and L. Quiñones for expert technical assistance. We also thank M. Duprey for TDA retrograde labeling and Dr. J. Blagburn for critical reading of the article. This work was supported in part by NIH-SCORE (SO6 GM08224 to R.E.B.), by a Research Centers in Minority Institutions award from the National Center for Research Resources, NIH (G12RR-03051 to R.E.B.), and by the APA Minority Fellowship Program (I.S.).




