Opposite effects of α1- and β-adrenoceptor stimulation on both glutamate- and γ-aminobutyric acid-mediated spontaneous transmission in cultured rat hippocampal neurons
Abstract
The effects of adrenergic receptor stimulation on spontaneous synaptic transmission were investigated in cultured rat hippocampal neurons by recording spontaneous excitatory and inhibitory postsynaptic currents (sEPSC and sIPSC). Noradrenaline (NA) inhibited sEPSC in a concentration-dependent manner, with maximal effect at 10 μM. The α1- and α2-adrenoceptor-selective agonists cirazoline and clonidine induced an inhibition of sEPSC appearance, whereas the β-adrenoceptor agonist isoproterenol elicited an increase. The inhibitory effect of NA was reversed by α1-adrenoceptor blockade. The participation of γ-aminobutyric acid (GABA)B-receptor stimulation in the inhibitory effect of NA was further examined. GABAB-receptor stimulation with baclofen induced a strong inhibition of bursting activity, which was fully reversed by the GABAB antagonist CGP 55845. By itself, CGP 55845 exerted a stimulatory effect on sEPSC frequency. In the presence of CGP 55845, the inhibitory effects of cirazoline and clonidine were maintained. NA (1, 10, and 100 μM) and α-adrenoceptor agonists decreased miniature EPSC and IPSC occurrence, whereas β-adrenergic stimulation increased it. In 50% of the cells examined, NA (1, 10 μM) had a stimulatory effect on sIPSC, whereas, in the remaining 50% of cells, NA (1, 10 μM) had an inhibitory effect. In all the cells, 100 μM NA induced an inhibition of sIPSC. The inhibitory effect of NA was due to α1-receptor stimulation, whereas the excitatory effect was due to β-receptor stimulation. In cultured hippocampal neurons, spontaneous excitatory and inhibitory synaptic transmissions are both similarly altered by adrenoceptor stimulation. However, in a subset of cells, low concentrations of NA mediate an increase of sIPSC via β-adrenoceptor activation. © 2002 Wiley-Liss, Inc.
Noradrenaline (NA) modulates both excitatory and inhibitory synaptic transmission within the hippocampal formation. The hippocampus is a target structure of noradrenergic inputs originating from the locus coeruleus (Loy et al., 1980). The actions of NA involve all adrenergic receptor subtypes, α1, α2, and β (for review see Vizi and Kiss, 1998). β-Adrenergic receptor stimulation generally mediates an increase in excitatory transmission at hippocampal synapses (Gereau and Conn, 1994), which can be involved in synaptic plasticity (Huang and Kandel, 1996; Katsuki et al., 1997; Huang et al., 1998). By contrast, α1-adrenergic receptors mediate an inhibition of excitatory synaptic transmission, as reported for area CA3 of the hippocampus (Scanziani et al., 1993). This inhibition may result from an increase in γ-aminobutyric acid (GABA) release as demonstrated in CA1 GABAergic interneurons (Bergles et al., 1996).
Surprisingly, adrenergic regulation of synaptic transmission is retained by hippocampal cultures in which neurons grow without the influence of adrenergic afferents. Indeed, as demonstrated in autaptic hippocampal neurons, NA may modulate negatively glutamate release via α2-adrenoceptors (Boehm, 1999) but also alter synaptic N-methyl-D-aspartate (NMDA) receptor phosphorylation status (Raman et al., 1996) via β-adrenoceptors. In this regard, we have reported that NA triggers phosphoinositide (PI) breakdown via α1-adrenoceptors in cultured hippocampal neurons of embryonic origin (Blanc et al., 1995; Aubert et al., 2001). However, although the stimulatory action of NA on PI hydrolysis decreases with time, mRNAs encoding all adrenoceptor subtypes are expressed throughout the development of these neurons. In fact, in mature cultures in which a network-driven activity can be recorded, i.e., after 15 days in vitro (DIV), α1-receptors are tonically activated, likely by an endogenous adrenergic ligand synthesized by a low number of neurons (Aubert et al., 2001). This tonic activation of α1-receptors results in a chronic increase of spontaneous excitatory postsynaptic currents (sEPSC). Other adrenoceptor subtypes, i.e., α2 and β, are also involved in this tonic enhancement of sEPSCs as demonstrated by the per se inhibitory effect of subtype-selective adrenoceptor antagonists (Aubert et al., 2001). In fact, tonically activated adrenoceptors provide a stimulatory effect only on action potential-dependent excitatory network-driven activity, in that miniature EPSCs (mEPSCs) isolated in the presence of tetrodotoxin (TTX) are insensitive to adrenoceptor antagonists. This does not preclude a more direct regulatory effect of adrenergic receptors on spontaneous excitatory synaptic transmission. To examine this possibility, we have investigated the impact of the stimulation of adrenoceptors by NA on both TTX-independent and TTX-dependent glutamate-mediated spontaneous synaptic transmission. In addition, in vitro maturation of hippocampal neurons leads to the formation of a network in which glutamate and GABA-receptor-mediated spontaneous synaptic transmission are intermingled and regulate each other. Indeed, glutamate-releasing cells chronically excite GABA-releasing cells, whereas GABA-releasing cells provide a tonic inhibition of glutamate-releasing cells (Vignes, 2001). Therefore, because any manipulation of glutamatergic transmission could also influence GABAergic transmission, adrenergic regulation on GABA-mediated synaptic transmission was investigated. The pharmacology of the effects of NA was defined with selective agonists and antagonists.
MATERIALS AND METHODS
Drugs
HEAT (BE 2254 or 2-[[b-(4-hydroxyphenyl)ethyl]aminomethyl]-1-tetralone), baclofen, CGP55845 ((2S)-3-[[(15)- 1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid), cirazoline hydrochloride (2- (2-cyclopropylphenoxymethyl)imidazoline), and clonidine hydrochloride (2-[(2,6-dichlorophenyl)amino]-2-imidazoline) were obtained from Tocris Cookson (United Kingdom). D,L-propranolol (1-[isopropylamino]-3-[1-naphthyloxy]-2-propanol), NA ((–)arterenol;3,4-dihydroxyphenylethanolamine), isoproterenol, and picrotoxin were purchased from Sigma-Aldrich (France). Tetrodotoxin was from Latoxan (France).
Hippocampal Cultures
For the preparation of hippocampal cultures, hippocampi were carefully dissected from E18 rat embryo brains taken from Sprague-Dawley pregnant female rats (“Centre d'Elevage Depré,” France) killed by decapitation. Hippocampi were then incubated for 12 min in Versene (Life Technologies, Bethesda, MD). After two washes in phosphate-buffered saline (PBS), cells were mechanically dissociated in culture medium using restricted, fire-polished pipettes. Culture medium contained DMEM/HAM-F12 (Life Technologies) supplemented with glucose (33 mM), glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), sodium bicarbonate (13 mM), HEPES (5 mM), insulin (87 μM), apo-transferrin (50 μg/ml), progesterone (20 nM), β-estradiol (1 pM), 3,5,3′-triiodotyronine (3 nM), putrescine (100 μM), and sodium selenite (46 nM). After centrifugation (4 min, 400g), the pellet of dissociated cells was dispersed in culture medium, and viable cells (trypan blue extruding cells) were counted. Cells were then plated in 0.5 ml of culture medium, at a density of 2 × 106, on rectangular culture dishes (Nunc, Naperville, IL) containing rectangular (10 × 11 mm) Thermanox (Nunc) coverslips previously coated with poly-L-lysine (7.5 μg/ml) and then with DMEM/F12 containing 10% fetal calf serum (Life Technologies). Cells were then maintained for 2–4 weeks at 37°C in a 5% CO2 atmosphere, in the same culture medium, without any changes.
Electrophysiological Measurements
On the day of the experiment, a coverslip was transferred to the recording chamber of an inverted microscope (IMT2; Olympus, Tokyo, Japan). Cells were superfused with the extracellular solution containing: 124 mM NaCl, 3 mM KCl, 26 mM NaHCO3, 1.25 mM NaH2PO4, 1.5 mM CaCl2, 1 mM MgSO4, 10 mM D-glucose (bubbled with O2/CO2: 95/5), at room temperature. Spontaneous postsynaptic currents (sPSCs) were measured using whole-cell recording with glass microelectrodes (4–5 MΩ resistance) filled with a solution comprising: 120 mM CsMeSO3, 1 mM NaCl, 1 mM MgCl2, 10 mM BAPTA, 5 mM N-(2,6-dimethyl-phenylcarbamoylmethyl)-triethylammonium bromide (QX-314), 5 mM HEPES (pH 7.3), 4 mM Mg-ATP, and 0.5 mM NaGTP. sEPSC were recorded at a holding potential of −60 mV and spontaneous inhibitory postsynaptic currents (sIPSCs) at 0 mV in cultured neuronal cells, irrespective of their size and shape. To isolate sEPSC, picrotoxin (50 μM) was included in the perfusate to inhibit GABAA-receptor-mediated sIPSC. Miniature PSCs (mEPSC and mIPSC) were obtained in the presence of tetrodotoxin (TTX; 500 nM). PSCs were measured with a patch-clamp amplifier (Axopatch 200 B; Axon Instruments, Burlingame, CA) and digitized (Digidata 1200 Interface; Axon Instruments). Signals were filtered at 1 kHz and sampled at 10 kHz. Continuous recording and analysis of PSCs were performed with John Dempster's software (WinCDR). Drugs were applied via the perfusion (flow rate ∼5 ml min–1). To calculate PSC amplitude and frequency, events were analyzed for 200 sec before and 200 sec during the application of drugs. Data are presented as mean ± SEM, and the statistical significance of the difference between experimental and control data was assessed using Student's t-test (P < 0.05 considered significant and indicated by an asterisk).
RESULTS
Adrenergic Regulation of Spontaneous Excitatory Synaptic Transmission
Effect of increasing concentrations of NA and reversal by α1-receptor blockade.
sEPSCs were recorded in cultured hippocampal cells from a developmental stage of 15 DIV and in the presence of the GABAA antagonist picrotoxin (50 μM). Under these conditions, sEPSCs occurred as large bursts, with a frequency ranging from 0.05 to 0.3 Hz. Perfusion of NA at different concentrations (from 1 to 100 μM) induced a significant concentration-dependent reduction of the burst frequency (Fig. 1a). Burst frequencies in the presence of NA at 1 μM, 10 μM, and 100 μM were, respectively, 86.3% ± 5.4% (n = 6), 32% ± 6.6% (n = 5), and 29.5% ± 8.1% (n = 4) when expressed as a percentage of control frequency (Fig. 1b). Maximal inhibitory effect of NA was obtained at 10 μM, and the EC50 of the NA effect was ∼3 μM (Fig. 1b). On average, NA significantly affected burst amplitude at a concentration of 100 μM. At this concentration, burst amplitude was 78% ± 2.5% (n = 4) of control amplitude (Fig. 1b).
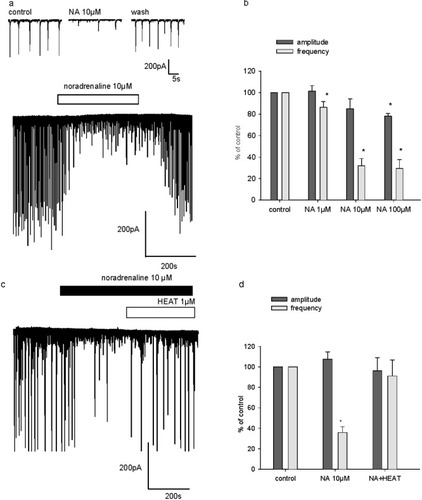
Effect of NA on spontaneous excitatory postsynaptic currents (sEPSC) and reversal by α1-adrenoceptor blockade. a: Single example illustrating the inhibitory action of 10 μM NA on sEPSC in a 21 DIV cultured hippocampal neuron. Sample traces have been extracted and shown on a smaller time scale. b: Pooled data on the effect of NA (1 μM, 10 μM, and 100 μM) on sEPSC frequency and amplitude. Data are expressed as a percentage of control frequency and amplitude, which were 0.9 ± 0.4 Hz and 219.3 ± 33.7 pA for NA at 1 μM (n = 6), 0.7 ± 0.3 Hz and 233.2 ± 37.6 pA for NA at 10 μM (n = 5), and 0.8 ± 0.4 Hz and 259 ± 35.4 pA for NA at 100 μM (n = 4), respectively. c: A recording illustrating the effect of the α1 antagonist HEAT (1 μM) on NA-induced (10 μM) depressant action on sEPSC in a 21 DIV neuron. d: Pooled data obtained in four distinct cells. On the graph, sEPSC frequency and amplitude are expressed as a percentage of control frequency and amplitude, which were 0.13 ± 0.02 Hz and 297 ± 60 pA, respectively. Data are means ± SEM. *P < 0.05.
NA induces an enhancement of phosphoinositide hydrolysis via α1-receptors (Aubert et al., 2001). Therefore, we next verified whether the inhibitory effect of NA on spontaneous excitatory activity was due to α1-adrenoceptor stimulation. For this purpose, we applied HEAT, a selective α1-adrenoceptor antagonist. HEAT was used at 1 μM, because, at this concentration, this compound fully antagonizes the increase of phosphoinositide breakdown mediated by 100 μM NA in cultured hippocampal neurons (Aubert et al., 2001). At 10 μM, NA reduced burst frequency to 35.9% ± 5.7% of control. HEAT mediated a recovery of burst frequency to 91% ± 15.7% of control (n = 4; Fig. 1c,d). The α2-adrenoceptor antagonist yohimbine (1 μM, n = 3) was also tested but did not reverse the NA-mediated inhibitory effect on sEPSC (data not shown). Therefore, NA-mediated inhibition of spontaneous excitatory activity results mainly from α1-adrenoceptor stimulation.
Effect of selective adrenergic receptor agonists.
Next, we examined the effect of relatively selective α1-, α2-, and β-adrenoceptor agonists, i.e., cirazoline, clonidine, and isoproterenol respectively, all tested at 10 μM. α1- and α2-adrenoceptor stimulation elicited an inhibitory effect on spontaneous excitatory activity. In the presence of cirazoline and clonidine, burst frequency was 60.6% ± 5.7% (n = 6) and 78% ± 6.5% (n = 4) of control frequency, respectively (Fig. 2a,b,d). By contrast, β-adrenoceptor stimulation elicited an increase in spontaneous excitatory activity. The application of isoproterenol enhanced burst frequency to 174% ± 23% (n = 5) of control (Fig. 2c,d). α-Adrenoceptor activation did not significantly affect burst amplitude, whereas β-adrenergic agonist decreased burst amplitude slightly but significantly to 89.5% ± 3.4% of control (Fig. 2d). As illustrated in Figure 2c, the stimulatory effects of isoproterenol were still observed long after wash out. We then examined whether α-adrenoceptor-mediated inhibition of sEPSC resulted from an indirect activation of GABAB-receptors via the stimulation of endogenous GABA release. Indeed, GABAB-receptor activation elicits a strong inhibition of excitatory activity at hippocampal synapses (Thompson, 1994). The stimulation of GABA release could mediate an indirect inhibition of sEPSC via the activation of GABAB-receptors, even under blockade of GABAA-receptors as was performed in these experiments. In the presence of the GABAB-receptor antagonist CGP 55845 (1 μM), cirazoline (10 μM), and clonidine (10 μM) reduced burst frequency to 60% ± 7.6% (n = 5) and 48% ± 13% (n = 4), respectively (Fig. 3). Burst frequency was expressed as percentage of control frequency measured in the presence of CGP 55845. The depressant effect of α1- and α2-receptor activation on excitatory synaptic transmission was still observed under these conditions. Therefore, the inhibitory effect mediated by α1- and α2-receptor agonists is independent of GABAB-receptor activation. It has to be mentioned that by itself CGP 55845 induced a significant enhancement of sEPSC frequency (159% ± 18%, n = 11, 1 μM; and 153% ± 23%, n = 4, 10 μM). This confirms that native GABAB-receptors can be activated by endogenously released GABA to depress excitatory transmission tonically.
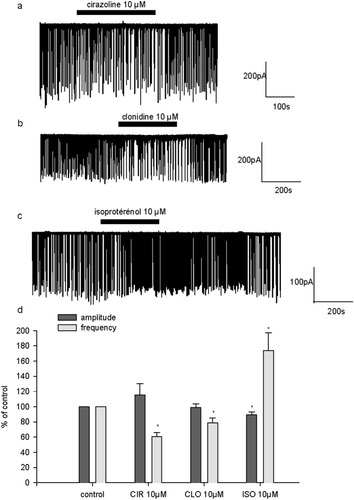
Effects of selective adrenoceptor agonists on excitatory spontaneous postsynaptic transmission. a: Single example illustrating the effect of the α1 agonist cirazoline (CIR; 10 μM) in a 17 DIV neuron. b: Single example illustrating the effect of the α2-receptor agonist clonidine (CLO; 10 μM) in a 19 DIV neuron. c: Single example illustrating the effect of the β-receptor agonist isoproterenol (ISO; 10 μM) in a 14 DIV neuron. d: Pooled data of the effect of adrenergic agonists CIR (n = 6), CLO (n = 4), and ISO (n = 5) on sEPSC frequency and amplitude. On this graph, sEPSC frequency and amplitude are expressed as a percentage of control frequency and amplitude, which were 0.6 ± 0.2 Hz and 292.5 ± 65.3 pA for CIR, 0.8 ± 0.1 Hz and 177.3 ± 23.2 pA for CLO, and 0.2 ± 0.05 Hz and 261.3 ± 56.9 pA for ISO, respectively. Data are means ± SEM. *P < 0.05.
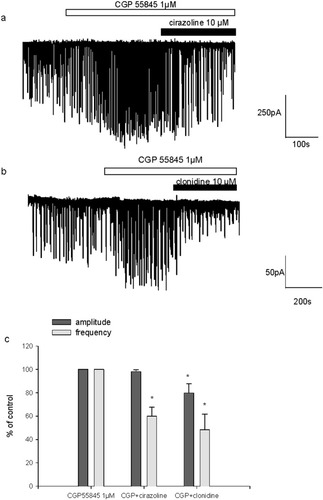
Effect of α1- and α2-adrenoceptors activation on sEPSC under GABAB-receptor blockade. a: Representative continuous recording of sEPSC illustrating the remaining inhibitory effect of cirazoline (10 μM) in the presence of CGP 55845 (1 μM) on bursting activity in an 18 DIV neuron. b: Single example illustrating the persistence of the depressant effect of clonidine (10 μM) on sEPSC in the presence of CGP 55845 (1 μM) in a 23 DIV neuron. Note the excitatory effect of CGP 55845 in both examples. c: Pooled data obtained in five cells with CGP 55845 + cirazoline and in four cells with CGP 55845 + clonidine. Data are means ± SEM and are expressed as a percentage of control frequency and amplitude measured in the presence of CGP 55845, which were 1.4 ± 0.2 Hz and 164 ± 17.4 pA for experiments performed with cirazoline and 0.7 ± 0.2 Hz and 127.5 ± 13.1 pA for experiments performed with clonidine. *P < 0.05.
Actions of adrenergic agonists on spontaneous excitatory synaptic transmission in the presence of TTX.
We next examined the possibility of a direct effect of NA on spontaneous excitatory synaptic transmission. For this purpose, mEPSCs were recorded in the presence of TTX (500 nM) and picrotoxin (50 μM). The frequency of mEPSCs was decreased by the application of NA (1, 10, and 100 μM) in a concentration-dependent manner (Fig. 4). NA induced a robust reduction of mEPSC frequency (70% ± 6%, n = 4, 1 μM; 46% ± 7%, n = 6, 10 μM; and 36% ± 12%, n = 3, 100 μM). On average, mEPSC amplitude was not altered by NA (89% ± 10%, n = 4, 1 μM; 105% ± 2.3%, n = 6, 10 μM; and 94% ± 4.3, n = 3, 100 μM; Fig. 4b). Therefore, the inhibitory effect of NA on spontaneous excitatory synaptic transmission is still observed in the presence of TTX.
Effect of NA and selective adrenoceptor agonists on miniature excitatory postsynaptic currents (mEPSC) recorded in the presence of TTX (500 nM). a: Single example illustrating the inhibitory effect of NA on mEPSC in a 14 DIV cultured hippocampal neuron. b: Plot of pooled data on the effect of NA at 1 μM (n = 4), 10 μM (n = 6), and 100 μM (n = 3) on the frequency and amplitude of mEPSC. Data are expressed as a percentage of control frequency and amplitude, which were 0.6 ± 0.1 Hz and 15 ± 2 pA for experiments performed with 1 μM NA, 0.5 ± 0.2 Hz and 18.7 ± 2.7 pA for experiments performed with 10 μM NA, and 0.5 ± 0.3 Hz and 15.7 ± 3 pA for experiments performed with 100 μM NA, respectively. Data are means ± SEM. c: Single example illustrating the effect of the α1 agonist clonidine (CLO; 10 μM) in a 19 DIV neuron. d: Single example illustrating the effect of the α2-receptor agonist cirazoline (CIR; 10 μM) in a 20 DIV neuron. e: Single example illustrating the effect of the β-receptor agonist isoproterenol (ISO; 10 μM) in a 14 DIV neuron. f: Pooled data of the effect of adrenergic agonists CIR (n = 6), CLO (n = 6), and ISO (n = 5) on mEPSC frequency and amplitude. On this graph, mEPSC frequency and amplitude are expressed as percentages of control frequency and amplitude, which were 0.9 ± 0.1 Hz and 23.3 ± 4 pA for CIR, 0.9 ± 0.2 Hz and 19.1 ± 6.9 pA for CLO, 0.7 ± 0.2 Hz and 31.8 ± 7.2 pA for ISO, respectively. Data are means ± SEM. *P < 0.05.
Next we tested the effects of adrenergic agonists on mEPSC. The adrenergic agonists used, i.e., clonidine, cirazoline, and isoproterenol, at 10 μM, modified the frequency and amplitude of miniature events (Fig. 4c–f). α-Adrenoceptor stimulation elicited an inhibitory effect on miniature excitatory activity. In the presence of cirazoline and clonidine, mEPSC frequency was 67.5% ± 11.2% (n = 6) and 43.8% ± 9.5% (n = 6) of control frequency, respectively (Fig. 4c,d,f). By contrast, β-adrenoceptor stimulation elicited an increase in mEPSC occurrence. The application of isoproterenol enhanced mEPSC frequency to 145.6% ± 12% (n = 5) of control (Fig. 4e,f). α1- and α2-adrenoceptor activation did not affect mEPSC amplitude significantly. Indeed, in the presence of cirazoline and clonidine, mEPSC amplitude was 93% ± 6% (n = 6) and 91% ± 10% (n = 6), respectively, as expressed in percentage of control amplitude. β-Adrenergic agonist increased mEPSC amplitude slightly but significantly to 117.2% ± 6.3% of control (Fig. 4f). Therefore, the effect of adrenoceptor agonists on mEPSC is similar to that observed on sEPSC.
Adrenergic Regulation of Spontaneous Inhibitory Synaptic Transmission
Effect of NA.
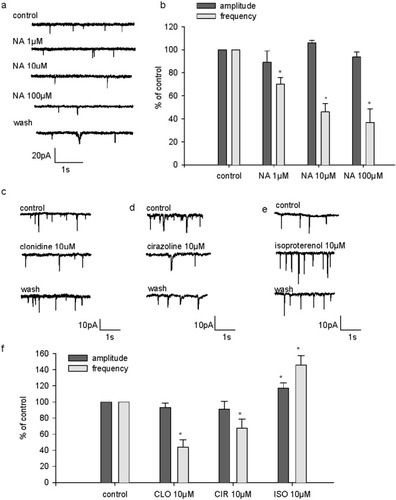
sIPSCs were recorded at a holding voltage of 0 mV in the presence of increasing concentrations of NA (1, 10, and 100 μM; Fig. 5). In four of eight cells, an increase of sIPSC appearance was observed with 1 and 10 μM concentrations (Fig. 5a). By contrast, an inhibition was recorded in the remaining cells (four of eight) at these concentrations (Fig. 5b). In all the cells tested, NA had an inhibitory effect at 100 μM (Fig. 5a,b). In the cells in which NA displayed an excitatory effect, sIPSC frequency was enhanced to 332% ± 53% of control at 1 μM and to 226% ± 40% at 10 μM. In these cells, 100 μM NA reduced sIPSC frequency to 67% ± 8% of control (Fig. 5c). In the cells in which NA displayed only an inhibitory effect, sIPSC frequency was decreased to 45% ± 9% of control at 1 μM and to 33% ± 12% at 10 μM. In these cells, 100 μM NA reduced sIPSC frequency to 12% ± 6% of control (Fig. 5d). Therefore, NA at 1 and 10 μM had a dual effect on sIPSC occurrence depending on the cell recorded.
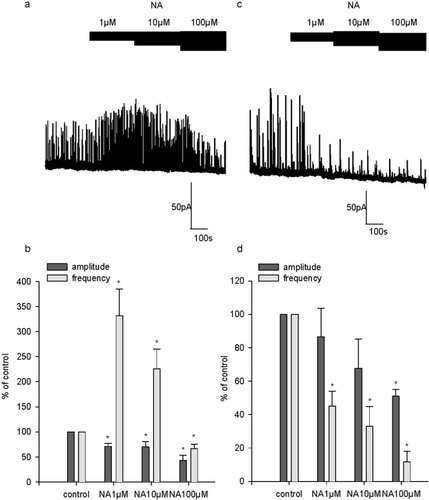
Effect of NA on sIPSC. a: Representative recording of sIPSC obtained from a 15 DIV cultured hippocampal neuron illustrating the excitatory action of NA at 1 μM and 10 μM, but not at 100 μM, on sIPSC, which was observed in 50% of the cells recorded. b: Graph plotting the pooled data obtained in cells where 1 and 10 μM NA had an excitatory effect on sIPSC. The frequency and amplitude of sIPSC are expressed as a percentage of control frequency and amplitude, which were 0.2 ± 0.1 Hz and 25.5 ± 3.3 pA for NA at 1 μM, 0.1 ± 0.015 Hz and 31.7 ± 8.4 pA for NA at 10 μM, and 0.1 ± 0.015 Hz and 35 ± 11.5 pA for NA at 100 μM, respectively. c: Single example illustrating the inhibitory action of NA at 1 μM, 10 μM, and 100 μM on sIPSC in a 15 DIV neuron, which was observed in 50% of the cells recorded. d: Pooled data on the inhibitory effect of NA on frequency and amplitude of sIPSC. Data are expressed as percentages of the control frequency and amplitude, which were 0.9 ± 0.5 Hz and 55 ± 20.1 pA for NA at 1 μM, 0.5 ± 0.2 Hz and 35 ± 8.4 pA for NA at 10 μM, and 0.7 ± 0.5 Hz and 42 ± 18 pA for NA at 100 μM. Data are means ± SEM. *P < 0.05.
Effect of adrenoceptor agonists.
We next examined the effect of the selective adrenoceptor agonists cirazoline, clonidine, and isoproterenol, all at 10 μM, on sIPSC. Cirazoline and clonidine both had an inhibitory effect on spontaneous inhibitory transmission as they decreased sIPSC frequency to 41% ± 13% (n = 5) and to 50% ± 7% (n = 7) of control, respectively (Fig. 6a,b,d). By contrast, isoproterenol increased sIPSC frequency to 136% ± 7% (n = 4; Fig. 6c,d). Cirazoline elicited a weak but significant decrease of sIPSC amplitude: On average, sIPSC amplitude was 59.2% ± 6% of control (Fig. 6d).
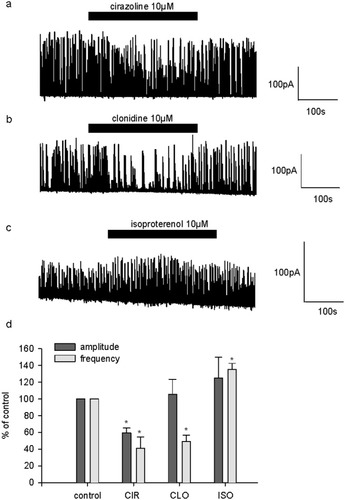
Effects of selective adrenoceptor agonists on sIPSC. a: Single example illustrating the effect of the α1-agonist cirazoline (CIR; 10 μM) in a 17 DIV neuron. b: Single example illustrating the effect of the α2-receptor agonist clonidine (CLO, 10 μM) in a 22 DIV neuron. c: Single example illustrating the effect of the β-receptor agonist isoproterenol (ISO, 10 μM) in a 15 DIV neuron. d: Pooled data on the effect of adrenergic agonists CIR (n = 5), CLO (n = 7), and ISO (n = 4) on sIPSC frequency and amplitude. On this graph, sIPSC frequency and amplitude are expressed as a percentage of control frequency and amplitude, which were 0.5 ± 0.1 Hz and 103.8 ± 11.7pA for CIR, 1 ± 0.2 Hz and 133.6 ± 36.1 pA for CLO, and 0.4 ± 0.1 Hz and 28.5 ± 5.3 pA for ISO, respectively. Data are means ± SEM. *P < 0.05.
Characterization of the receptors involved in NA's effect on sIPSC.
Because β-adrenoceptor activation had a stimulatory effect on sIPSC occurrence, we examined whether the potentiating effect of NA at 1–10 μM observed in 50% of the cells was mediated by the activation of β-adrenoceptors and whether the inhibitory effect in the remaining 50% was due to α1-adrenoceptor stimulation. For this purpose, 1 μM NA was applied. When a stimulating effect on sIPSC was detected, the β-adrenoceptor antagonist propranolol (1 μM) was further applied (Fig. 7a). Conversely, if an inhibitory effect was observed, the α1-adrenoceptor antagonist HEAT (1 μM) was applied (Fig. 7c). In six of twelve cells, 1 μM NA increased sIPSC occurrence and on average enhanced sIPSC frequency to 170.4% ± 15% of control. In these cells, 1 μM propranolol reversed this effect. Indeed, under these conditions, sIPSC frequency was 60% ± 12% of control (Fig. 7b). By itself, 1 μM propranolol had no effect on spontaneous inhibitory activity. Indeed, in the presence of 1 μM propranolol alone, sIPSC frequency and amplitude were 103.2% ± 1.2% and 97% ± 13% of control, respectively (n = 4; data not shown). Therefore, propranolol unveils the inhibitory effect of NA in these cells. In four of twelve cells, NA (1 μM) had an inhibitory action on sIPSC and decreased sIPSC frequency to 60% ± 4% of control (Fig. 7c,d). The application of HEAT (1 μM) elicited a reversal of this inhibition; under these conditions, mean sIPSC frequency was 105% ± 8% of control (Fig. 7d). It has to be mentioned that, in two of twelve cells recorded, NA (1 μM) had no detectable effect on sIPSC.
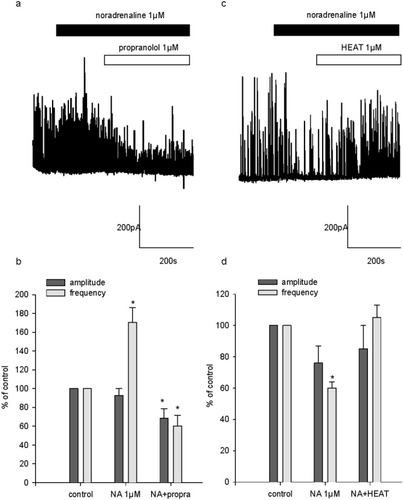
Characterization of the excitatory and inhibitory effects of NA on sIPSC. a: Representative recording of sIPSC in a 34 DIV cultured hippocampal neuron illustrating the excitatory effect of NA (1 μM) on bursting activity and the reversal by the β-adrenergic antagonist propranolol (1 μM). b: Pooled data obtained from six cells. On this graph, sIPSC frequency and amplitude are expressed as a percentage of control frequency and amplitude, which were 1.4 ± 0.5 Hz and 206.5 ± 88.1 pA, respectively. Data are means ± SEM. c: Single example illustrating the reversal of the inhibitory effect of NA (1 μM) by α1-adrenoceptor blockade with HEAT (1 μM) in a 18 DIV cultured hippocampal neuron. d: Pooled data obtained from four cells. On this graph, sIPSC frequency and amplitude are expressed as percentages of control frequency and amplitude, which were 0.8 ± 0.4 Hz and 166 ± 19 pA, respectively. Data are means ± SEM. *P < 0.05.
Actions of adrenergic agonists on spontaneous inhibitory synaptic transmission in the presence of TTX.
mIPSCs were recorded at a holding voltage of 0 mV by including TTX (500 nM) in the perfusate (Fig. 8). Under these conditions, NA at 1, 10, and 100 μM induced a strong reduction of mIPSC mean frequency (56% ± 9% of control, n = 4, 1 μM; 44% ± 10% of control, n = 5, 10 μM; and 30% ± 10% of control, n = 3, 100 μM). On average, mIPSC amplitude was not altered by NA (94% ± 4%, n = 5, 10 μM; and 90% ± 10%, n = 3, 100 μM; Fig. 8a,b). Therefore, the inhibitory effect of NA on spontaneous inhibitory synaptic transmission is TTX independent.
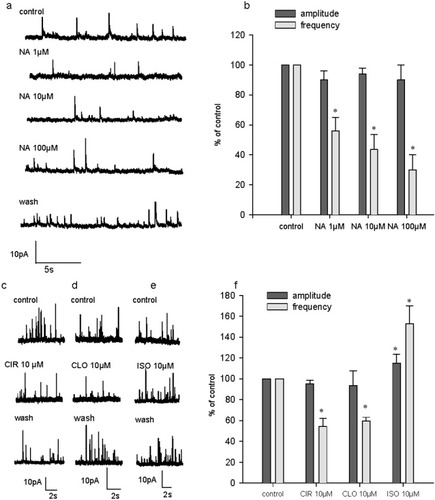
Effect of NA and selective adrenoceptor agonists on miniature inhibitory postsynaptic currents (mIPSC) recorded in the presence of TTX (500 nM). a: Single examples illustrating the inhibitory effect of NA of mIPSC, in a 16 DIV culture hippocampal. b: Plot of pooled data on NA at 1 μM (n = 4), 10 μM (n = 5), and 100 μM (n = 3) for the frequency and amplitude of mIPSC. Data are expressed as a percentage of control frequency and amplitude, which were 1.25 ± 0.8 Hz and 35 ± 4 pA for experiments performed with 1 μM NA, 0.5 ± 0.1 Hz and 61.8 ± 16.1 pA for experiments performed with 10 μM NA, and 0.4 ± 0.1 Hz and 31 ± 9 pA for experiments performed with 100 μM NA, respectively. Data are means ± SEM. c: Single example illustrating the effect of the α1 agonist cirazoline (CIR; 10 μM) in an 18 DIV neuron. d: Single example illustrating the effect of the α2-receptor agonist clonidine (CLO; 10 μM) in an 18 DIV neuron. e: Single example illustrating the effect of the β-receptor agonist isoproterenol (ISO; 10 μM) in a 14 DIV neuron. f: Pooled data on the effect of adrenergic agonists CIR (n = 5), CLO (n = 4), and ISO (n = 3) on mIPSC frequency and amplitude. On this graph, mIPSC frequency and amplitude are expressed as percentages of control frequency and amplitude, which were 1.6 ± 0.3 Hz and 25 ± 3.6 pA for CIR, 1.1 ± 0.5 Hz and 22 ± 2.2 pA for CLO, 0.4 ± 0.1 Hz and 16.7 ± 1.7 pA for ISO, respectively. Data are means ± SEM. *P < 0.05.
The application of selective adrenoceptor agonists also modulated mIPSC occurrence. Indeed, cirazoline and clonidine induced a decrease of mIPSC frequency to 54% ± 8% (n = 4) and 60% ± 3.5% (n = 5) of control, respectively (Fig. 8c,d,f). Isoproterenol increased mIPSC frequency to 153% ± 17% (n = 3) of control (Fig. 8e,f). Mean mIPSC amplitude was not modified in the presence of cirazoline or clonidine (94% ± 14% and 90% ± 3% of control amplitude, respectively), but isoproterenol significantly enhanced it to 115% ± 9% of control (Fig. 8f).
DISCUSSION
The exogenous application of NA potently modulates both excitatory and inhibitory spontaneous synaptic transmission in cultured hippocampal neurons. NA application mediates a concentration-dependent inhibition of sEPSC. This effect is still observed in the presence of TTX. Insofar as only mEPSC frequency is significantly affected in the presence of NA, the inhibitory effect of exogenous NA likely results from a direct presynaptic modulation. This depressant action of NA involves the stimulation of α1-adrenoceptor stimulation. This is supported by the reversal of the inhibition by the selective antagonist HEAT and further confirmed by the inhibitory effects of the α1-adrenoceptor agonist cirazoline on sEPSC and mEPSC. Although α2-receptor stimulation with the selective agonist clonidine also exerts a depressant action on spontaneous synaptic transmission, the inhibitory effect of NA does not seem to involve these receptors. Similarly, although the β-adrenergic agonist isoproterenol enhances both sEPSC and mEPSC occurrence, NA itself does not elicit any stimulatory effect on spontaneous excitatory synaptic transmission. This is not the case when one considers GABA-mediated inhibitory synaptic transmission. Indeed, although subtype-selective adrenoceptor agonists display effects on inhibitory spontaneous synaptic transmission similar to those observed on spontaneous excitatory synaptic transmission, NA has two opposite actions on GABA-mediated synaptic transmission. In 50% of the cells recorded, we observed that NA at 1 and 10 μM strongly enhanced sIPSC appearance, likely via the stimulation of β-adrenergic receptors as evidenced by the reversal of NA effect by the β-adrenoceptor antagonist propranolol. In the remaining 50% of cells recorded, NA application elicited a depressant effect on sIPSC via the stimulation of α1-adrenergic receptors. Interestingly, this stimulatory action of NA on sIPSC was never observed in the presence of TTX; NA elicits only an inhibitory effect on mIPSC occurrence, and this independently of the cell recorded. Therefore, this excitatory effect of NA on GABA-releasing cells may be due to the selective activation of somatodendritic β-adrenoceptors, although β-adrenergic agonist elicits a direct presynaptic enhancement of TTX-independent spontaneous synaptic transmission. This also suggests that β-adrenoceptor-mediated enhancement of synaptic transmission should involve both pre- and postsynaptic mechanisms. We found, in support of this hypothesis, that isoproterenol enhances miniature event occurrence as evidenced by a large increase in miniature frequency accompanied by a small but consistent enhancement of miniature mean amplitude. In the hippocampus, inhibitory cells sensitive to β-adrenergic excitation have been identified. Indeed, the application of NA in the micromolar concentration range excites type I hilar neurons, which inhibit granule dentate cells, and this effect is mimicked by isoproterenol (Bijak and Misgeld, 1995). Therefore, the cultured hippocampal neurons network should include a subset of GABA-releasing cells bearing a larger proportion of β- than of α-adrenoceptors and in which NA exerts mainly excitatory effects.
We have recently reported that, in a mature network of cultured hippocampal neurons, adrenergic receptors were, to some extent, tonically activated likely by a not yet accurately identified adrenergic ligand that enhances TTX-dependent excitatory synaptic transmission (Aubert et al., 2001). The exogenous application of NA does not further increase this tonic excitation; no enhancement of synaptic transmission is observed with NA, except in some GABA-releasing cells. The exogenous application of NA results mainly in the activation of presynaptic α1-receptors mediating a direct inhibition of synaptic transmission. Therefore, except in some GABA-releasing cells, there is a discrepancy between the effects of exogenously applied NA reported here and those elicited by the putative endogenous adrenergic ligand (Aubert et al., 2001). One possibility is that NA has a higher affinity for inhibitory α1-presynaptic receptors than for somatodendritic receptors mediating the excitatory effect of the endogenous ligand. Therefore, the direct stimulation of adrenoceptors with NA completely overwhelms the excitatory action of this ligand.
Although the inhibitory effects of α1-adrenoceptor stimulation on sEPSC seem to involve a direct presynaptic action, the possibility of an indirect inhibition via the release of an inhibitory transmitter was examined. In this respect, we have studied the possible stimulation of GABA release by α1-adrenoceptor stimulation. Indeed, GABA is the principal inhibitory neurotransmitter in the hippocampus. Although experiments were carried out in the presence of picrotoxin to eliminate GABAA-receptor-mediated synaptic events, the activation of GABAB-receptors could mediate a strong depressant effect on sEPSC, as indicated in the review of Thompson (1994). In addition, NA may induce an increase of GABA-mediated synaptic transmission via the stimulation of α1-receptors, as evidenced in hippocampal (Bergles et al., 1996) and in cortical (Kawaguchi and Shindou, 1998) GABA-releasing cells. GABAB-receptor activation elicits a strong inhibition on sEPSC occurrence, which can be reversed by the selective antagonist CGP 55845 (data not shown). We find here that GABAB-receptors are tonically activated under these conditions, in that, by itself, CGP 55845 enhances sEPSC frequency. This is in good agreement with a recent report showing that GABAB-receptors can be tonically activated in hippocampal neurons when a rhythmic activity takes place (Scanziani, 2000). However, the inhibitory effect of α-adrenoceptor stimulation is retained even in the presence of GABAB antagonist. This indicates that α1-adrenergic-mediated inhibition of sEPSC is independent of GABA release. We have also examined the possibility that adrenoceptor-mediated effects on sIPSC were due primarily to indirect actions on sEPSC. Indeed, we have recently demonstrated that, in cultured hippocampal neurons, GABA-releasing cells are tonically excited by endogenous glutamate (Vignes, 2001). Therefore, one can expect that a manipulation of excitatory transmission leads to an alteration of inhibitory transmission. However, in the presence of glutamate receptor antagonists, adrenoceptor agonists retain their action on sIPSCs (data not shown). Thus, the effects of adrenoceptor stimulation on GABAergic transmission are direct and independent of glutamate-mediated tonic excitation of GABA-releasing cells.
It has been demonstrated that, in the hippocampus, the noradrenergic system originating from the locus coeruleus could interfere with epileptic activity. Indeed, NA release leads mainly to an inhibition of this activity. This antiepileptic action is also reported from in vitro models of hippocampal epilepsy, obtained by GABAA-receptor blockade, for instance (O'Donovan, 1999). Indeed, it has been demonstrated that, in hippocampal slices, NA may have both anticonvulsant and proconvulsant effects by acting on α- and β-adrenoceptors, respectively (Mueller and Dunwiddie, 1983). Although β-adrenoceptor stimulation is always associated with an enhancement of this activity, the inhibitory effect of NA may be due to the activation of either α1 or α2, according to the preparation and brain area. For instance, α2-receptor stimulation mediates the antiepileptic activity of NA in the basolateral amygdala (Stoop et al., 2000), whereas α1-receptors mediate it in the CA3 area of the hippocampus (Rutecki, 1995). With our model, we observed that NA mediates only inhibitory effects on this epileptic-like activity, as shown by a decrease of the bursting activity obtained under GABAA antagonism. This effect involves only the stimulation of α1-receptors, α2-receptors not being activated, just as in the CA3 area of the hippocampus (Rutecki, 1995). Therefore, the network of cultured hippocampal neurons mimics, at least partly, the synaptic circuitry occurring within the hippocampal CA3 area. It has been suggested that the antiepileptic effect of NA could be due to a decrease of glutamate release via the activation of α-adrenoceptors (Scanziani et al., 1993) and/or to an enhancement of GABA release via the excitation of inhibitory interneurons by α1-receptors (Bergles et al., 1996; Sirviö and McDonald, 1999). Our data suggest that NA may have antiepileptic actions directly by the stimulation of presynaptic α1-receptors on glutamatergic terminals and/or also indirectly by the activation of somatodendritic β-adrenoceptors located on GABA-releasing cells. Therefore, the stimulation of β-adrenoceptors on interneurons could contribute to the antiepileptic effect of β-adrenoceptor agonists observed in hippocampal epilepsy models, such as in the penicillin model (Ferraro et al., 1994).
CONCLUSIONS
The stimulation of all adrenoceptor subtypes, i.e., α1, α2, and β, in cultured hippocampal neurons potently alters spontaneous synaptic transmission, including excitatory (glutamatergic) and inhibitory (GABAergic) transmission. This confirms the functionality of adrenoceptors whose encoding mRNAs have already been detected (Aubert et al., 2001). Interestingly, the activation of these receptors elicits the same kind of effect on both excitatory and inhibitory spontaneous transmission, and this in the absence or in the presence of TTX. Indeed, α1- and α2-receptor activation inhibited, whereas β-receptor stimulation increased, the occurrence of all spontaneous events recorded with and without TTX. This tends to indicate that these receptors control both glutamate and GABA release via a direct presynaptic effect. NA has a mainly inhibitory effect on synaptic transmission, likely via the activation of presynaptic α1-adrenoceptors, although it mediates stimulatory effects in a subset of GABA-releasing cells via β-adrenoceptor stimulation. Overall, these data provide evidence that adrenoceptors, via their different subtypes, mediate a larger spectrum of regulatory mechanisms for synaptic transmission than a simple tonic activation of excitatory activity in the cultured rat hippocampal neuron network and, thus, may influence hippocampal epileptic activity.
Acknowledgements
We are grateful to Dr. Janique Guiramand for helpful comments on the manuscript and to Marie-France Bézine Lopez, Gisèle Roch, and Francis Malaval for their invaluable help in the preparation of hippocampal cultures. A.C. is the recipient of a grant from Unilever.




