Overexpression of nPKC θ is inhibitory for agrin-induced nicotinic acetylcholine receptor clustering in C2C12 myotubes
Abstract
Protein kinase C (PKC) activity has been implicated in nicotinic acetylcholine receptor (nAChR) cluster disruption but the specific PKC isoforms involved have not been identified. We first tested whether phorbol esters, which activate PKCs, regulate agrin-induced nAChR clustering in C2C12 cells. We found that extended phorbol ester treatment (6 hr) increased nAChR clustering by two-fold. This increase correlated in time with downregulation of PKCs, as indicated by the disappearance of cPKC α, suggesting that the presence of PKCs is inhibitory for maximal nAChR clustering. To address the question whether nPKC θ, a specific PKC isoform restricted in expression to skeletal muscle and localized to neuromuscular junctions, regulates agrin-induced nAChR cluster formation we overexpressed an nPKC θ -green fluorescent protein (GFP) fusion protein in C2C12 myotubes. The number of nAChR clusters was significantly reduced in nPKC θ-GFP compared to GFP overexpressing myotubes at less-than-maximal clustering concentrations of agrin. These data indicate that nPKC θ activity inhibits nAChR cluster formation. To examine whether nPKC θ activation by phorbol esters regulates agrin-induced nAChR clustering, we treated overexpressing myotubes overnight with maximal agrin concentrations followed by phorbol esters for 1 hr. Phorbol ester treatment reduced preexisting nAChR cluster numbers in nPKC θ-GFP compared to GFP-overexpressing myotubes, suggesting that stimulating nPKC θ activity disrupts nAChR clusters in the presence of maximal clustering concentrations of agrin. Together these findings, that nPKC θ activity inhibits agrin-induced nAChR cluster formation and disrupts preexisting agrin-induced nAChR clusters, suggest that nPKC θ activity is inhibitory for agrin function. © 2002 Wiley-Liss, Inc.
Synapse formation at the neuromuscular junction involves the redistribution of nicotinic acetylcholine receptors (nAChRs) on the muscle cell surface. Many of the biochemical mechanisms responsible for concentrating nAChRs at synaptic endplates in skeletal muscle have been identified and characterized extensively (reviewed by Sanes and Lichtman, 2001; Huh and Fuhrer, 2002). The reverse process of destabilizing nAChRs from the synaptic apparatus is comparatively less understood. During development, prenatal aneural nAChR aggregates are disrupted in an innervation-dependent manner (Lin et al., 2001; Yang et al., 2001) and postnatal skeletal muscle polyneuronal synaptic withdrawal is accompanied by the dissolution of synaptic nAChR aggregates (Balice-Gordon and Lichtman, 1993). Redistribution of aggregated nAChRs occurs during synaptic remodeling, denervation, and reinnervation of skeletal muscle (Moody-Corbett and Cohen, 1982; Kuromi and Kidokoro, 1984; Wigston, 1989). The biochemical mechanisms that mediate these processes of nAChR aggregate reorganization have not yet been elucidated.
Protein kinase C (PKC) activity was first implicated in nAChR redistribution at the neuromuscular junction based on experiments in which nAChR clusters on cultured chick myotubes were dispersed by exposure to phorbol esters, which activate PKC (Bursztajn et al., 1988). Spontaneous nAChR clusters in myotubes were dispersed completely within 3 hr after addition of phorbol esters. Similarly, nAChR clusters induced by the application of embryonic chick brain extract to chick myotubes were also found to disperse within hours after phorbol ester treatment (Ross et al., 1988). Dispersal of nAChR clusters could not be thoroughly accounted for by reduced nAChR expression, suggesting that a PKC-mediated phosphorylation event was responsible for dispersal.
Clustering of nAChR can be induced by agrin, an extracellular matrix proteoglycan produced by motor neurons (Nitkin et al., 1987; Rupp et al., 1991). Certain isoforms of agrin indirectly activate a muscle-specific receptor tyrosine kinase (MuSK) that triggers nAChR clustering via an unelucidated signal transduction pathway involving tyrosine phosphorylation of the nAChR β subunit (Wallace et al., 1991; Ferns et al., 1996; Glass et al., 1996). Phorbol ester treatment accelerates disruption of agrin-induced nAChR clusters in chick myotubes whereas pre- or co-treatment of chick myotubes with phorbol esters inhibits agrin-induced nAChR cluster formation and nAChR tyrosine phosphorylation (Wallace, 1988; Wallace et al., 1991). These results suggest that PKC activity compromises agrin function but the underlying mechanism remains unclear.
Recent studies in cultured mouse myotubes innervated in vitro provide evidence that PKC activity regulates nAChR distribution in mammalian neuromuscular synapses. Phorbol ester treatment of mouse myotubes innervated with spinal cord neurites caused a reduction in postsynaptic endplate potentials (Lanuza et al., 2000). This diminished synaptic efficacy was attributed to the loss of nAChRs from synaptically functional nAChR aggregates. In addition, inhibition of PKC activity in vivo prevented normal nAChR dispersal associated with axon withdrawal, suggesting that PKC activity regulates postnatal synaptic nAChR disappearance in neonatal rat muscle (Lanuza et al., 2002).
PKC activity is mediated by multiple isoforms that are members of a protein serine/threonine kinase family. The distinct tissue-specific expression pattern and intracellular targeting of PKC isoforms are thought to confer isozyme physiological specificity (Newton, 1996; Mochly-Rosen and Gordon, 1998). To advance our understanding of the role of PKC activity in synaptic nAChR reorganization, we sought to establish whether a particular PKC isoform, nPKC θ, is involved in regulating nAChR distribution. Our focus on this isoform evolves from our previous demonstrations that nPKC θ, which is highly expressed in adult rat skeletal muscle, is localized to neuromuscular junctions and that skeletal muscle denervation in vivo selectively downregulates nPKC θ levels (Hilgenberg and Miles, 1995; Hilgenberg et al., 1996). Together these observations suggest that nPKC θ plays a functional role in the neuromuscular junction.
To explore the role of nPKC θ in nAChR distribution we created an expression construct consisting of nPKC θ fused to green fluorescent protein (nPKC θ -GFP)(Miles and Wagner, 2000). We demonstrate that overexpression of nPKC θ-GFP both inhibits the formation of and disrupts agrin-induced nAChR clusters in C2C12 myotubes. These results support the hypothesis that nPKC θ activity modulates nAChR distribution in the neuromuscular junction. Understanding PKC isoform specificity of expression and function may be essential to explaining how PKC activity regulates nAChR distribution in the neuromuscular junction.
METHODS AND MATERIALS
Cell Culture
C2C12 or COS cells were passaged in DMEM growth medium supplemented with 20% and 10% fetal calf serum (Fisher Scientific, Suwanee, GA, and Gibco BRL, Rockville, MD, respectively). Confluent C2C12 cells were induced to differentiate by shifting to DMEM supplemented with 2% horse serum. Phorbol 12-myristate 13-acetate (PMA; Alexis Biochemicals, San Diego, CA) was dissolved in dimethylsulfoxide (DMSO; Sigma, St. Louis MO).
Expression Constructs and Transfection
Agrin was obtained by collecting the culture medium from COS cells transfected with a plasmid encoding a truncated secreted isoform of agrin containing the 8-amino acid insert at the C terminus alternative splice Z site (Agrin8)(Ferns et al., 1993; Hoch et al., 1994). The agrin plasmid was generously provided by Dr. Z. Hall. Preparation of the expression construct for nPKC θ-GFP has been described previously (Miles and Wagner, 2000). Mouse nPKC θ cDNA was a generous gift from Dr. S. Osada (Osada et al., 1992); pEGFP was obtained from Clontech (Palo Alto, CA). C2C12 myoblasts or COS cells were transfected using 3 μl Fugene (Roche Molecular Biochemicals, Indianapolis, IN) and 1.5 μg DNA (0.2 μg/μl) per 35-mm dish according to the manufacturer's instructions.
Analysis of nAChR Clusters
C2C12 myotube cultures were incubated for 1 hr with rhodamine-conjugated α bungarotoxin (Rh-α-btx) (1 μg/ml, Molecular Probes, Eugene, OR). Cultures were washed and fixed with 4% paraformaldehyde in PBS for 1 hr. The number of nAChR clusters per random 1,000× field of view were counted using a fluorescence microscope (Fig. 1). Transfected myotubes, overexpressing either nPKC θ-GFP or GFP, were incubated with Rh-α-btx as described above and analyzed individually. Randomly selected green fluorescent myotubes were measured along their entire length. The number of nAChR clusters greater than 3 μm was counted on each myotube and the lengths of nAChR clusters were measured along the longest axis. To control for differences in myotube lengths, nAChR cluster numbers are reported as clusters per 100 μm myotube. Statistical analysis on differences in the number of nAChR clusters between transfected populations of myotubes was conducted using Poisson regression, with log (myotube length) as an offset variable. Analysis of nAChR average cluster lengths was conducted using mixed linear models, with treatment as a fixed factor and myotube identity as a random factor.
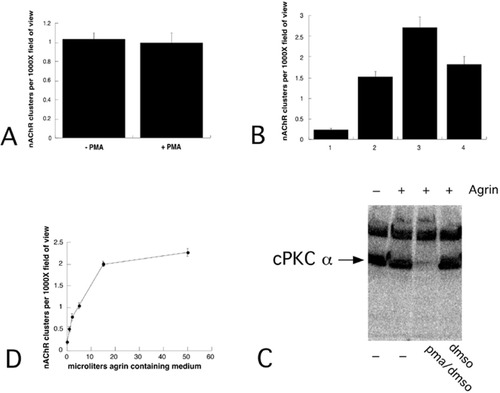
Long term phorbol ester treatment enhances nAChR clustering in C2C12 cells. A: C2C12 myotubes were treated with a half maximal clustering concentration of agrin (5 μl, see D) overnight and then incubated with 50 nM PMA as indicated for 1 hr. Rh-α-btx was added at the same time as PMA. The number of nAChR clusters per 1,000× field of view was counted. B: C2C12 myotubes were treated with maximal clustering concentration of agrin (50 μl) overnight (2–4). PMA (50 nM) (3) or DMSO (0.01% final concentration) (4) was added for 6 hr. Rh-α-btx was added during the last hour and cultures were treated as described above. C: C2C12 myotubes were treated in parallel to those described in (B). After 6 hr cultures were solubilized and proteins in the soluble fraction were separated by SDS-PAGE and transferred to nitrocellulose. The blots were then exposed to anti-cPKC α antiserum. D: C2C12 myotubes were treated overnight with the indicated supernatant volumes from COS cells overexpressing a secreted agrin fragment. Clusters of nAChR were counted as described in Materials and Methods.
Immunoprecipitation Kinase Assay
Transfected COS cells were solubilized in 20 mM Tris pH 7.6, 0.5% NP40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM Na3VO4, 10 mM benzamidine, 10 mM phenylmethylsulfonylfluoride, 50 μg/ml leupeptin and centrifuged at 6,000 × g; nPKC θ was immunoprecipitated from supernatants using anti-nPKC θ antiserum followed by Protein A-Sepharose. Protein A-Sepharose pellets were washed in kinase assay buffer containing 20 mM HEPES pH 7.2, 137 mM NaCl, 5.4 mM KCl, 0.3 mM NaH2PO4, 0.4 mM KH2PO4, 10 mM MgCl2, 5 mM EGTA, 2.5 mM CaCl2. Pellets were incubated with 50 μl 32P-ATP (100 μM at 500 cpm/pmol) and 100 μm PKC substrate peptide (Bachem #H-9375, San Carlos, CA) in kinase assay buffer at 30°C for 30 min. Assay supernatants were spotted onto Whatman P81 ion exchange chromatography paper, which was then washed with 75 mM phosphoric acid. Radioactivity incorporated into substrate peptide was quantitated by scintillation counting; background cpm in parallel samples lacking substrate peptide were subtracted from each kinase reaction.
Immunoblot Analysis
Mouse hindlimb skeletal muscle was prepared as described previously (Hilgenberg et al., 1996). Immunoprecipitated proteins were subjected to SDS-PAGE and then transferred electrophoretically to nitrocellulose membranes. The membranes were incubated overnight at 4°C in Blotto buffer containing 50 mM Tris pH 7.4, 200 mM NaCl, 0.2% Tween-20 and 5% nonfat dry milk (w/v) to block nonspecific binding. nPKC θ was detected using S22 antiserum raised against the C-terminus 17 amino acids of mouse nPKC θ (Hilgenberg and Miles, 1995) and cPKC α was detected using monoclonal anti-cPKC α antiserum (UBI, Lake Placid, NY) followed by a secondary antibody. Primary or secondary antiserum was recognized using 125I-Protein A (NEN, Boston, MA). Radioactivity was detected using a PhosphorImager and analyzed using ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
RESULTS
Effect of Phorbol Ester Treatment on Agrin-Induced nAChR Clustering in C2C12 Myotubes
To examine the role of endogenous PKC activity in regulating agrin-induced nAChR clustering in mouse C2C12 myotubes, we treated cultures overnight with a half-maximal clustering concentration of agrin (Fig. 1D) and then added 50 nM PMA. No differences in nAChR cluster numbers were detected between phorbol ester treated and control cultures after 1 hr (Fig. 1A). nAChR clustering on myotubes treated with the culture medium from mock-transfected COS cells was equal to that of untreated myotubes (data not shown) and no effect of DMSO on nAChR clustering was observed. Prolonged treatment with phorbol esters for 6 hr, however, increased agrin-induced nAChR clusters by two-fold in C2C12 myotubes (Fig. 1B). This result was surprising because prolonged exposure to phorbol esters has been shown to reduce nAChR expression approximately 15% in primary rat myotubes (Miles et al., 1994). Prolonged exposure to phorbol esters is also known to downregulate PKC (Goode et al., 1995). To determine if the observed increase in nAChR clustering correlates with decreased PKC levels, we measured cPKC α levels in myotubes treated overnight with agrin followed by a 6-hr exposure to phorbol esters. We selected cPKC α to document total phorbol ester-sensitive PKC downregulation because its expression is readily detectable in C2C12 myotubes (Miles and Wagner, 2000). cPKC α disappeared from cultures treated for 6 hr with phorbol esters, indicating that PKCs were downregulated and suggesting that their reduced levels may be a factor in increased nAChR clustering (Fig. 1C). Although these results suggest that PKC is involved in regulating nAChR clustering, they do not identify the PKC isoforms responsible for the observed effects of phorbol esters.
Agrin-Induced nAChR Cluster Formation in C2C12 Myotubes Overexpressing nPKC θ-GFP
Two PKC isoforms, cPKC α and nPKC θ, have been shown to be highly expressed in mammalian skeletal muscle (Osada et al., 1992; Hilgenberg and Miles, 1995). We reported previously that nPKC θ expression in rat skeletal muscle is regulated postnatally, both developmentally and transsynaptically, and that nPKC θ is localized to neuromuscular junctions (Hilgenberg and Miles, 1995; Hilgenberg et al., 1996).
To test the hypothesis that nPKC θ activity regulates agrin-induced nAChR clustering we sought an experimental system in which to compare high and low levels of nPKC θ activity. Because nPKC θ expression is low in C2C12 myotubes, as seen in noninnervated primary myotubes, we chose to overexpress this enzyme in these cells (Hilgenberg et al., 1996; Miles and Wagner, 2000). To study cells overexpressing nPKC θ we used an expression construct encoding an nPKC θ-GFP fusion protein that can be visualized directly to indicate overexpressing myotubes (Miles and Wagner, 2000). To examine whether nPKC θ-GFP overexpressing myotubes cluster nAChRs in response to agrin we applied maximal agrin clustering concentrations to overexpressing myotubes overnight. Because the transfection efficiency for this PKC isoform is less than 30%, individual transfected myotubes were analyzed. No difference in nAChR cluster numbers on myotubes overexpressing nPKC θ-GFP compared to GFP alone could be detected in cultures treated overnight with high agrin concentrations (50 μl; see Fig. 1D, Fig. 2). Spontaneous nAChR cluster numbers were not affected by overexpression of nPKC θ-GFP (data not shown). These results indicate that overexpression of this PKC isoform does not disable the agrin-induced nAChR clustering mechanism and that nAChR expression is not affected significantly by nPKC θ-GFP overexpression.
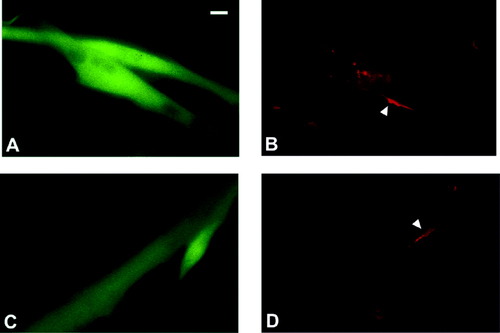
Agrin-induced nAChR clustering in C2C12 myotubes overexpressing nPKC θ-GFP or GFP. C2C12 myoblasts were transfected with an expression construct for nPKC θ-GFP (A, B) or pEGFP (C, D). After 48 hr the cultures were shifted to differentiation medium. Day 6 after plating, cultures were exposed overnight to 50 μl agrin supernatant. The cultures were then incubated with Rh-α-btx for 1 hr, rinsed and fixed. Overexpressing myotubes were visualized by epifluorescence microscopy and photographed for either green (A,C) or red (B,D) fluorescence in the same field of view. Arrows indicate nAChR clusters. Scale bar = 3 μm.
To explore the hypothesis that nPKC θ modulates agrin-induced nAChR clustering, we applied less-than-maximal agrin concentrations overnight (2 and 5 μl; see Fig. 1D) to C2C12 myotubes overexpressing nPKC θ-GFP or GFP. At less-than-maximal agrin concentrations, significantly fewer nAChR clusters per 100 μm myotube length formed on nPKC θ-GFP compared to GFP overexpressing myotubes (Table I) without affecting average nAChR cluster lengths. These results suggest that nPKC θ activity inhibits agrin-induced nAChR cluster formation.
| Agrin (μl) | Expression vector | Clusters per 100 μm myotube | na | 95% Confidence interval | Average cluster length (μm) |
|---|---|---|---|---|---|
| 2 | nPKC θ-GFP | 0.5 | 20 | 0.26–0.95 | 4.0 |
| 2 | GFP | 1.2 | 21 | 0.7–2.25 | 4.0 |
| P = 0.036 | |||||
| 5 | nPKC θ-GFP | 0.9 | 37 | 0.7–1.3 | 5.2 |
| 5 | GFP | 1.5 | 37 | 1.3–1.9 | 5.3 |
| P = 0.038 | |||||
| 15 | nPKC θ-GFP | 1.48 | 28 | 1.1–2.0 | 4.3 |
| 15 | GFP | 1.80 | 22 | 1.4–2.4 | 4.8 |
| P = 0.274 |
- a Number of myotubes. Data from one of two experiments with similar results.
Kinase Activity of nPKC θ-GFP
To rule out the possibility that weak nPKC θ-GFP catalytic activity underlies the observation that nAChR clustering is affected by nPKC θ -GFP overexpression only when agrin signaling is less than saturating, we assayed nPKC θ-GFP kinase activity. We transfected COS cells with an expression construct encoding nPKC θ-GFP or wild-type nPKC θ, immunoprecipitated the exogenous enzymes using an antiserum directed against nPKC θ and compared their ability, as well as the ability of endogenous nPKC θ immunoprecipitated from mouse skeletal muscle, to phosphorylate a substrate peptide. Both wild-type and nPKC θ-GFP displayed kinase activity in approximate proportion to their expression levels and this kinase activity was in the range of endogenous enzyme (Fig. 3A,B). This result indicates that the nPKC θ-GFP fusion protein is catalytically active.
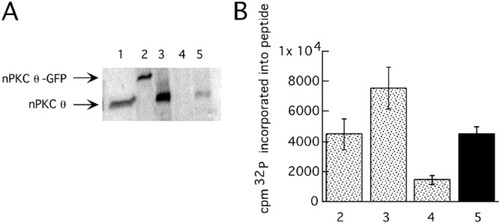
nPKC θ-GFP is catalytically active. A: COS cells were transfected with expression constructs for nPKC θ-GFP (lane 2) or nPKC θ (lane 3) or not transfected (lane 4). The COS cells were solubilized and nPKC θ -GFP or nPKC θ was immunoprecipitated with anti-nPKC θ antiserum. Mouse skeletal muscle was solubilized and an amount equal to that loaded in lane 1 was immunoprecipitated using anti-nPKC θ antiserum (lane 5). The immunoprecipitates were subjected to SDS- PAGE and immunoblotted using anti-nPKC θ antiserum. B: Transfected COS cell cultures or mouse skeletal muscle membrane fractions were treated in parallel with those described in (A). The immunoprecipitates were instead incubated with substrate peptide and 32P-ATP. Radioactivity incorporated into the substrate peptide was quantitated after subtracting counts obtained in the absence of substrate peptide in each case.
Activation of Overexpressed nPKC θ-GFP Disrupts Agrin-Induced nAChR Clusters
To test whether phorbol ester activation of nPKC θ-GFP regulates nAChR clusters in the continued presence of maximal agrin concentrations, overexpressing myotubes were treated with a maximal clustering concentration of agrin (50 μl) overnight followed by phorbol esters for 1 hr. Phorbol ester treatment of nPKC θ-GFP overexpressing myotubes reduced the number of nAChR clusters per 100 μm myotube compared to GFP overexpressing myotubes by more than half (Table II, Fig. 4). No differences were detected in the average cluster length because the majority of clusters are in the range of 3 μm before treatment with phorbol esters and are not counted at shorter lengths (Table II). These findings indicate that in the presence of saturating concentrations of agrin, activation of overexpressed nPKC θ-GFP activity disrupts preexisting nAChR clusters.
| Expression vector | PMAa | Clusters per 100 μm myotube | nb | 95% Confidence interval | Average cluster length (μm) |
|---|---|---|---|---|---|
| nPKC θ-GFP | − | 1.5 | 31 | 1.2–1.9 | 5.0 |
| GFP | − | 1.8 | 30 | 1.4–2.3 | 4.1 |
| P = 0.344 | |||||
| nPKC θ-GFP | + | 0.6 | 29 | 0.5–0.9 | 4.2 |
| GFP | + | 2.1 | 31 | 1.6–2.6 | 4.4 |
| P = 0.0001 |
- Data from one of four experiments with similar results.
- a 50 nM, 30 min exposure.
- b Number of myotubes.
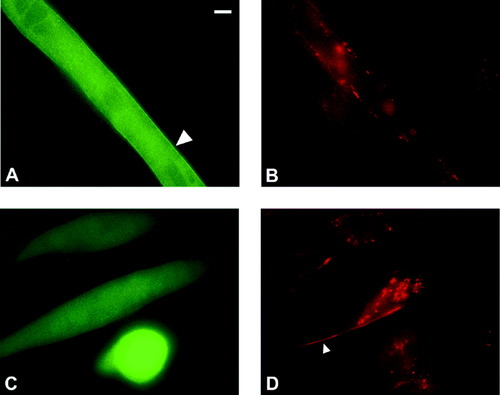
Activation of nPKC θ-GFP with phorbol esters disrupts agrin-induced nAChR clusters. C2C12 myoblasts were transfected with an expression construct for nPKC θ-GFP (A,B) or pEGFP (C,D) and were differentiated into myotubes as described previously. Cultures were incubated overnight with 50 μl agrin and treated with 50 nM PMA for 30 min. Myotubes were labeled with Rh-α-btx for 1 hr. Scale bar = 3 μm. Large arrow indicates translocated nPKC θ -GFP. Small arrow indicates nAChR cluster. Quantitative analysis of these results is shown in Table II.
DISCUSSION
PKC activity has been shown to regulate agrin-induced nAChR clustering in chick and innervated mammalian myotubes. We sought to determine if endogenous PKC activity in noninnervated C2C12 myotubes regulates nAChR clustering by treating cultures with phorbol esters. Our results show that short-term treatment with phorbol esters, which stimulates PKC activity, does not affect nAChR clustering whereas long-term treatment with phorbol esters, which both stimulates PKC activity and downregulates PKC levels, enhances agrin-induced nAChR clustering by two-fold. Because increased nAChR clustering is concomitant with the disappearance of PKCs from cultures, one possible interpretation is that the presence of PKCs inhibits maximal nAChR clustering. These observations in C2C12 cells treated with phorbol esters stand in contrast to primary chick myotubes where phorbol ester treatment disrupts nAChR clusters within a few hours (Bursztajn et al., 1988; Ross et al., 1988). Perhaps these disparate findings between noninnervated primary chick and mammalian C2C12 myotubes reflect species differences in the regulation of nAChR clustering by PKC activity. It is plausible that PKC isoform expression and function differs between chick and mammalian skeletal muscle and that this may underlie the diverse responses to phorbol esters with respect to nAChR clustering.
Exposure of innervated mammalian myotubes to phorbol esters has been shown to rapidly disrupt synaptic nAChR aggregates (Lanuza et al., 2000). Based on our previous observations that nPKC θ is localized synaptically in skeletal muscle and that postnatal development and innervation increase nPKC θ expression (Hilgenberg and Miles, 1995; Hilgenberg et al., 1996), Lanuza et al. (2000) hypothesized that nPKC θ kinase activity is responsible for the reduction of synaptic nAChRs seen in their studies. The experiments here were designed to address directly the question whether nPKC θ activity regulates nAChR clustering.
To assess the effect of nPKC θ activity on nAChR clustering we overexpressed nPKC θ in C2C12 myotubes. In a previous study, we overexpressed a chimeric protein composed of nPKC θ linked to GFP and demonstrated that this fusion protein is stable throughout myogenesis and translocates in response to phorbol ester treatment of C2C12 myoblasts (Miles and Wagner, 2000). In these studies we also demonstrate that nPKC θ-GFP has catalytic activity in the range of wild-type enzyme. These results validate using nPKC θ-GFP as a tool to explore nPKC θ function in C2C12 myotubes.
We find that at less-than-maximal agrin concentrations, overexpression of nPKC θ-GFP in C2C12 myotubes inhibits agrin-induced nAChR cluster formation compared to cells overexpressing GFP alone. The finding that nPKC θ overexpression inhibits agrin-induced nAChR clustering when agrin signaling is attenuated suggests that the extent of nAChR clustering is a function of the relative strengths of agrin and nPKC θ signaling. In the neuromuscular junction, this may mean that nPKC θ inhibits clustering when agrin levels are low or when a yet-to-be identified first messenger stimulates nPKC θ activity. Neurotrophins that interact with TrkB receptors have also been found to inhibit agrin-induced nAChR cluster formation only at less-than-maximal agrin concentrations in chick myotubes (Wells et al., 1999). Given that PKCs act downstream of activated TrkB receptors, perhaps TrkB receptor stimulation activates nPKC θ in skeletal muscle (Zirrgiebel et al., 1995).
Overexpression of nPKC θ has been used to reveal distinct physiological functions for this PKC isoform but in some cases these effects were only observed after nPKC θ-overexpressing cells were exposed to phorbol esters (Baier-Bitterlich et al., 1996). We examined whether phorbol esters, in the presence of maximal agrin concentrations, stimulated nPKC θ activity to regulate nAChR clustering in C2C12 myotubes overexpressing nPKC θ-GFP. Significantly fewer nAChR clusters were present on nPKC θ-GFP compared to GFP overexpressing myotubes 1 hr after phorbol ester treatment, indicating that at maximal agrin concentrations nPKC θ can be stimulated to disrupt nAChR clusters. Taken together with the inhibitory effects of nPKC θ on the formation of agrin-induced nAChR clusters, these data suggest that nPKC θ activity opposes agrin function and destabilizes the association of nAChRs to the synaptic apparatus.
Agrin-induced nAChR clustering is the consequence of an enhanced linkage between nAChRs and the cytoskeleton. Although the exact nature of this tether has not been elucidated, protein tyrosine kinase activity is clearly involved. Agrin, interacting indirectly with the MuSK receptor tyrosine kinase, increases nAChR β subunit tyrosine phosphorylation and regulates cytoskeletal anchoring and clustering of nAChRs (Wallace, 1992; Borges and Ferns, 2001). Inhibition of protein tyrosine phosphatase activity enhances nAChR clustering (Wallace, 1995) and microinjection of a protein tyrosine phosphatase disperses nAChR clusters in muscle cells (Dai and Peng, 1998). Evidence that src protein tyrosine kinase activity enhances nAChR cytoskeletal anchoring has been reported (Mohamed et al., 2001; Smith et al., 2001).
The mechanism by which nPKC θ destabilizes nAChR clusters is not known but based on the observations cited above and the results reported here, it is likely to involve an intersection of protein tyrosine kinase/phosphatase and PKC signaling pathways. For example, in mast cells, antigen-induced nPKC θ activation causes nPKC θ to become tyrosine phosphorylated, which then enhances its ability to associate with src (Liu et al., 2001). Perhaps a similar interaction occurs between nPKC θ and protein tyrosine kinases/phosphatases intrinsic to agrin signaling that mutually modulates catalytic activity and thereby regulates nAChR clustering.
Another mechanism by which nPKC θ activity might regulate nAChR clustering is through direct phosphorylation of the nAChR on serine residues. nAChR serine phosphorylation could counteract tyrosine phosphorylation and release nAChRs from the cytoskeleton. In chick myotubes, increased PKC-mediated phosphorylation of three sites on nAChR δ subunits correlates with nAChR cluster dispersal (Nimnual et al., 1998). In rat primary myotubes, nAChRs are basally highly phosphorylated on serine residues and are a likely substrate for PKC phosphorylation because phorbol ester treatment increases nAChR γ and δ subunit phosphorylation (Miles et al., 1994). Serine phosphorylation of nAChRs has yet to be examined in innervated mammalian myotubes where nPKC θ is elevated and phorbol ester treatment disrupts nAChR synaptic localization (Hilgenberg et al., 1996; Lanuza et al., 2000).
Specific PKC isoform activity has been implicated recently in the redistribution of neurotransmitter receptors in the central nervous system as a consequence of specific interactions between PKC, its substrate neurotransmitter receptor and a scaffolding protein (Perez at al., 2001). To understand fully how PKC regulates neurotransmitter distribution at the neuromuscular junction it may be necessary to consider the specific targeting and activation of PKC isoforms, as well as specific substrate phosphorylation events.
Acknowledgements
We thank Dr. J. Weeden from the SUNY Brooklyn Office of Scientific Computing for assistance with the statistical analysis of clustering data.




