Monocyte recruitment and myelin removal are delayed following spinal cord injury in mice with CCR2 chemokine receptor deletion
Abstract
The inflammatory response initiated after spinal cord injury (SCI) is characterized by the accumulation of macrophages at the impact site. Monocyte chemoattractant protein-1 (MCP-1) is a strong candidate for mediating chemotaxis of monocytes to the injured nervous system. To help in defining the role of MCP-1 in inflammation after SCI, we evaluated the time course of macrophage accumulation for 2 weeks following a midthoracic spinal cord contusion injury in mice lacking CCR2, a principal receptor for MCP-1. Mice with a deletion of CCR2 resulted in significantly reduced Mac-1 immunoreactivity restricted to the lesion epicenter at 7 days postinjury. The regions devoid of Mac-1 immunoreactivity corresponded to areas of reduced myelin degradation at this time. By 14 days postinjury, however, there were no differences in Mac-1 staining between CCR2 (+/+) and CCR2 (–/–) mice. Analyses of mRNA levels by RNase protection assay (RPA) revealed increases in MCP-1 as well as MCP-3 and MIP-2 mRNA at 1 day postinjury compared with 7 day postinjury. There were no differences in chemokine expression between CCR2-deficient mice and wild-type littermate controls. The CCR2-deficient mice also exhibited reduced expression of mRNA for chemokine receptors CCR1 and CCR5. Together, these results indicate that chemokines acting through CCR2 contribute to the early recruitment of monocytes to the lesion epicenter following SCI. © 2002 Wiley-Liss, Inc.
Traumatic injury to the mammalian spinal cord results in immediate physical disruption of the spinal cord parenchyma, followed by a sequence of secondary events, which extend the region of tissue destruction beyond the primary injury site (Balentine, 1978; Schwab and Bartholdi, 1996). Several lines of evidence suggest that inflammation is one of the principal mediators of the secondary injury cascade, with macrophages contributing profoundly to the pathogenesis of spinal cord injury (SCI; Blight, 1985,HB: Blight, 1992; Dusart and Schwab, 1993; Perry et al., 1993; Popovich et al., 1997, 1999; Zhang et al., 1997). To begin to elucidate the complex effects of the inflammatory response in SCI, it is important to identify the signals that mediate macrophage accumulation at the site of injury.
A primary mechanism for selective leukocyte infiltration after injury or disease is the differential expression of chemokines (chemoattractive cytokines; Glabinski et al., 1995; Adams and Lloyd, 1997; Ransohoff, 1997; Rollins, 1997). Traumatic injury in the central nervous system (CNS) has been consistently associated with enhanced expression of the β-chemokine, CCL2, commonly referred to as monocyte chemoattractant protein-1 (MCP-1; Ransohoff et al., 1993; Glabinski et al., 1995, 1996; Berman et al., 1996; Ransohoff, 1997; Hausmann et al., 1998; McTigue et al., 1998; Lee et al., 2000). MCP-1 mRNA expression in the CNS has been localized most frequently to reactive astrocytes (Glabinski, 1998), but it may also be produced by microglia (Gourmala et al., 1997; Simpson, 1998) and neurons (Coughlan et al., 2000). There are additional murine MCPs that exhibit high homology to MCP-1, with overlapping functional characteristics in vitro. However, recent evidence from pharmacological and murine knockout studies suggests that MCP-1 mediates important and nonredundant actions in CNS inflammation (Ghirnikar et al., 1998; Huang et al., 2001).
In the mouse, the major monocyte receptor for MCP-1 is CCR2 (Boring et al., 1996; Charo, 1999). In addition to MCP-1, CCR2 also binds other β-chemokines, including MCP-2, MCP-3, MCP-4, and MCP-5 (Ben-Baruch et al., 1995; Garcia-Zepeda et al., 1996; Gong et al., 1997; Sarafi et al., 1997). Thus, the CCR2 receptor represents an attractive target for examining the functional role of this closely related family of chemokines. Genetic deletion of CCR2 results in the impaired recruitment of macrophages in several models of injury and disease, including experimental peritoneal inflammation (Boring et al., 1997; Kurihara et al., 1997; Kuziel et al., 1997), apolipoprotein E-related atherosclerosis (Boring et al., 1998), and Listeria monocytogenes infection (Kurihara et al., 1997). CCR2-deficient mice also exhibit altered inflammatory responses in nervous tissue, including decreased susceptibility to experimental autoimmune encephalomyelitis (Fife et al., 2000; Izikson et al., 2000) and impaired recruitment of macrophages and removal of myelin debris following sciatic nerve injury (Siebert et al., 2000). Together, these results implicate CCR2 as a principal mediator of early macrophage recruitment in tissue inflammation. In the present study, we investigated the macrophage response to SCI in CCR2-deficient mice and wild-type controls. The results demonstrate that chemokines acting through CCR2 play an important role in the early phase of monocyte recruitment and myelin phagocytosis in SCI.
Materials And Methods
Animals
The generation of CCR2 (–/–) mice by homologous recombination has been described previously (Boring et al., 1997). One line was maintained by crossing CCR2 (–/–) mice onto a C57Bl/6 background for eight generations. A second line was produced on a hybrid C57Bl/6 × 129S4/SvJae background. Pairs of heterozygous breeders were obtained from Dr. Charo's laboratory, and the colony was maintained in pathogen-free housing at the Ohio State University vivarium for the course of these studies. The mice used for spinal cord injury were F2 progeny of heterozygous pairs from the C57Bl/6 line or the hybrid line or heterozygous crosses from the two lines (Hesselgesser and Horuk, 1999; Steward et al., 1999). Littermate CCR2 (–/–) and CCR2 (+/+) pairs were used when available, and mice from all three breeding lines were included in each experimental group to minimize any effects of background genes. Genotypes were determined by PCR analysis of tail samples collected at weaning and confirmed at the time of tissue harvest (Boring et al., 1997). An additional 18 adult female C57Bl/6 mice (Taconic Farms, Germantown, NY) were used to evaluate the time course of microglia/macrophage activation and infiltration after SCI in this strain.
SCI
All animal surgery and postoperative care was performed in accordance with the Ohio State University Institutional Animal Care and Use Committee, using methods described in detail previously (Jakeman et al., 2000; Ma et al., 2001). The mice were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg), and a laminectomy was performed at the T9 vertebral level. By using an electromagnetic SCI injury device (ESCID), a contusion injury was produced by a single, rapid displacement of the exposed dorsal surface of the spinal cord for a distance of 0.5 mm (10 msec peak displacement duration). This level of injury is considered to be of moderate severity and produces immediate hind limb paralysis, followed by incomplete recovery over the first 2 weeks postinjury. After injury, the muscle and overlying skin were sutured, and the mice were treated with topical antibiotic and hydrated with lactated Ringer's solution.
Tissue Preparation
The mice received an overdose of ketamine/xylazine anesthesia at 1, 3, 7, or 14 days postinjury (dpi). For histology and immunohistochemistry, the mice were perfused transcardially with 0.1 M phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in PBS. The fixed spinal cords were dissected from the T1–L1 vertebral levels, and the tissue blocks were postfixed for 2 hr and then transferred to 0.2 M phosphate buffer overnight at 4°C. Tissues were cryoprotected in 30% sucrose and frozen on dry ice. For analysis of chemokine and chemokine receptor mRNA levels, the entire spinal cords (C1–2–L5 vertebrae) were snap frozen in liquid nitrogen.
Histopathology
Frozen spinal cord blocks were embedded in TissueTek (OCT compound; Fisher Scientific, Pittsburgh, PA). Serial 10-μm-thick cryostat sections through the entire injury site were mounted on Superfrost Plus slides (Fisher Scientific). All analyses were performed by an investigator who was blinded with regard to experimental groups. Tissues from animals in each group were mounted on the same slides and processed simultaneously.
Lesion epicenter.
One complete set of evenly spaced sections (100 μm apart) was stained with Luxol fast blue (LFB) to identify myelinated white matter regions. For each specimen, the lesion epicenter was defined as the tissue section with the smallest cross-sectional area of LFB staining in the peripheral rim (Behrmann et al., 1992; Jakeman et al., 2000; Ma et al., 2001).
Mac-1 immunohistochemistry.
For immunohistochemical detection of activated microglia and macrophages, adjacent slide series were stained with a monoclonal rat anti-mouse antibody raised against complement receptor CR3 (Mac-1; Serotec Ltd., Oxford, United Kingdom; 1:200). Biotinylated rabbit anti-rat antibody (Vector, Burlingame, CA; 1:400) was applied overnight and then stained using Elite ABC (Vector) and the chromagen diaminobenzidine (Vector). At 7 and 14 dpi, macrophages and microglia coalesce and cannot be distinguished and counted. Therefore, macrophage activation/infiltration was quantified using proportional area measurements, with a computer-assisted image analysis program as described previously (MCID; Imaging Research Inc., Toronto, Ontario, Canada; Popovich et al., 1997; Ma et al., 2001). The cross-sectional area of the spinal cord tissue section was drawn on digitized, spatially calibrated images (Sony 970 color CCD). Images from equally spaced sections at 100 μm intervals surrounding the epicenter were digitized, and the optical density detection threshold was adjusted to differentiate positive staining from background. Proportional area was computed as the fraction of the area of positive staining divided by the total cross-sectional area of the tissue section.
Oil red O.
Myelin phagocytosis was analyzed from sections stained with Oil red O (Fisher Scientific) and counterstained with Mayer's hematoxylin (Zymed, South San Francisco, CA) to identify cell nuclei (Chester et al., 1971). Oil red O staining was measured using the MCID by determining the proportional area of an 0.18 mm2 sampling region positioned in the center of the tissue sections that was occupied by the staining in the red range of the color spectrum (digital threshold settings set by hue and intensity of staining).
RNA Preparation and RNase Protection Assay
Mouse chemokine and chemokine receptor mRNA expression was measured at 1 and 7 dpi by RNase protection assay (RPA) following in vitro transcription (PharMingen, San Diego, CA). Total cellular RNA was prepared from frozen spinal cord tissue using Trizol extraction (Gibco BRL, Gaithersburg, MD). A total of 20 μg of total RNA was used per sample. The chemokine probe set was a gift from Dr. Iain L. Campbell at the Scripps Research Institute (Asensio and Campbell, 1997). The chemokine receptor probe set was obtained from PharMingen (MCR-5). Protected fragments labeled with 32P were visualized and quantified by autoradiography using a PhosphorImager (Molecular Dynamics, Sunnyville, CA).
Statistical Analysis
Histological outcome measures from CCR2 (+/+) and CCR2 (–/–) specimens were compared using the unpaired Student's t-test. Two-way ANOVA was used to compare chemokine or chemokine receptor message across days and genotype. All analyses were peformed using GraphPad Prism3 software (GraphPad Software, Inc., San Diego, CA).
Results
Time Course of Microglia/Macrophage Reactions After Contusion Injury in C57Bl/6 Mice
To determine the time course of macrophage accumulation in the mouse contusion injury model, a qualitative study of Mac-1 immunoreactivity was performed in injured C57Bl/6 mice. Mac-1 immunostaining revealed the distribution of reactive microglia, monocytes, and macrophages at 1, 3, 7, and 14 dpi. At 1 dpi, small, irregularly shaped, Mac-1+ cells were few and were most prominent within the spinal gray matter regions (Fig. 1A,B). At 3 dpi, the number and size of Mac-1+ cells were greater, both within the gray matter region and in surrounding white matter (Fig. 1C). Many of the stained cells had an enlarged, rounded phagocytic morphology, especially in the ventral gray matter region and at the interface between the region of damaged and residual white matter (Fig. 1D). By 7 dpi, Mac-1 immunoreactivity was intense throughout the lesion epicenter (Fig. 1E). Large, round macrophage profiles occupied most of the cross-sectional area of the spinal cord (Fig. 1F). Within the center region, staining was associated with large groups of cells. By 14 dpi, the Mac-1-stained region occupied the entire central area of all specimens. Immunostaining was most prominent at the interface between the residual rim of white matter and the original gray matter region of the spinal cord (Fig. 1G). In the center of the lesion, the intensity of Mac-1 immunoreactivity was less than that seen at 7 dpi and was associated with large, round cells and distributed throughout the surrounding extracellular matrix (Fig. 1H). The cells within the peripheral tissue rim exhibited irregular morphology characteristic of chronically reactive microglia (Fig. 1I). We chose, based on these results, 1, 7, and 14 dpi for further analyses encompassing the initiation, peak, and chronic periods of the macrophage response in the CCR2 (+/+) and CCR2 (–/–) mice.
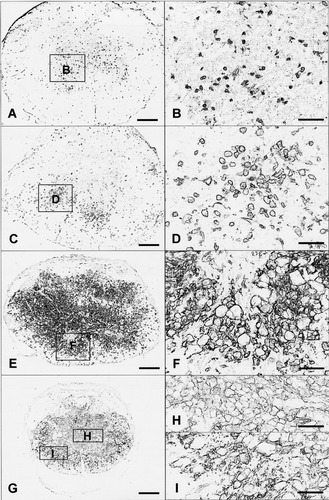
Representative photomicrographs of Mac-1 immunoreactivity at the lesion epicenter of C57Bl/6 mice at 1 (A,B), 3 (C,D), 7 (E,F), and 14 (G–I) days after the 0.5 mm contusion injury. Small, irregularly shaped Mac-1+ cells were few and were most prominent within the spinal gray matter regions at 1 dpi (A,B). Round phagocytic profiles are found within the gray matter region and surrounding white matter at 3 dpi (C,D). By 7 dpi, large, round macrophages occupied most of the cross-sectional area of the spinal cord with intense Mac-1 immunoreactivity (F). By 14 dpi, the intensity of Mac-1 staining was less than that seen at 7 dpi and was associated with large, round cells in the center of the lesion (H). Reactive microglia profiles can be found within the peripheral tissue rim (I). Scale bars = 200 μm in A,C,E,G; 50 μm in B,D,F,H,I.
Mac-1+ Immunoreactivity Is Reduced at 7 dpi in CCR2 (–/–) Mice
Proportional area measures of Mac-1-stained tissues (stained area/total cross-section tissue area) were made in the section closest to the lesion epicenter as defined in adjacent sections stained with LFB (see Materials and Methods). At 1 dpi, the distribution and morphology of Mac-1+ cells were as described above (see also Fig. 2A,B). There were no differences in the proportional area between CCR2 (+/+) and CCR2 (–/–) specimens at this time (Fig. 2C). By 7 dpi, Mac-1 immunoreactivity was evident throughout the lesion epicenter of CCR2 (+/+) specimens (Fig. 2D). In contrast, the epicenter sections of CCR2 (–/–) mice at 7 dpi contained large regions that were devoid of Mac-1 staining, particularly in the area corresponding to the location of normal gray matter (Fig. 2E). Mac-1 distribution and cell morphology in the peripheral rim of these specimens were similar in CCR2 (+/+) and CCR2 (–/–) specimens. The total cross-sectional tissue area was not different between the two groups at any time. However, the reduction in Mac-1-positive staining in the center of the lesion was great enough to result in a significant decrease in the proportional area of Mac-1 immunoreactivity for the entire section (Fig. 2F). Although Mac-1 staining extended for over 1 mm in each direction, the decreased Mac-1 staining in CCR2 (–/–) mice was highly localized to the lesion epicenter and 0.2 mm in the caudal direction (Fig. 3). By 14 dpi, there were no differences in Mac-1 staining between the CCR2 (+/+) and CCR2 (–/–) animals (Fig. 2G–I).
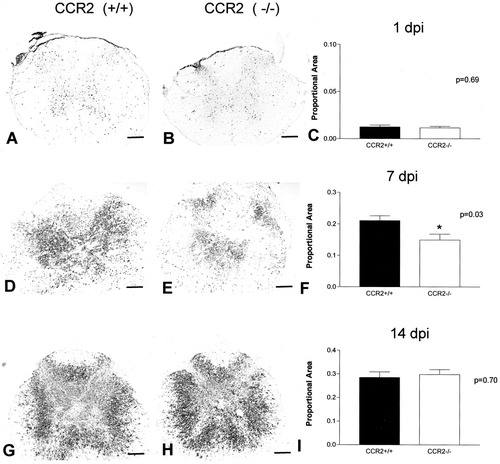
Representative photomicrographs and quantitative analysis of Mac-1 distribution and proportional area measures at the lesion epicenter at 1 dpi (A–C), 7 dpi (D–F), and 14 dpi (G–I). Macrophage density was reduced at the injury epicenter in specimens from CCR2 (–/–) mice obtained at 7 dpi (t-test; *P < 0.05) but not at 1 or 14 dpi. The effect was due to a reduction in Mac-1 immunoreactivity at the center of the lesion site (E). Scale bars = 200 μm.
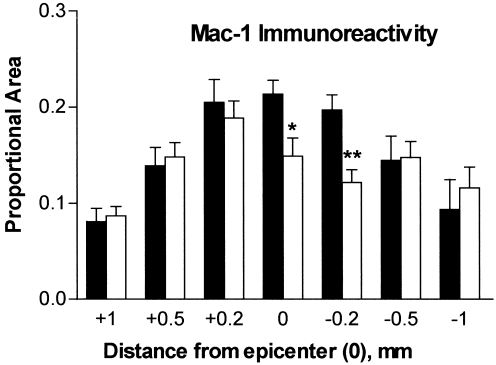
Distribution of Mac-1 proportional area measures at intervals spanning the rostrocaudal extent of the lesion at 7 dpi. Solid bars correspond to CCR2 (+/+) specimens and open bars correspond to CCR2 (−/−) specimens. Differences in Mac-1 immunoreactivity were restricted to the center of the lesion and 0.2 mm caudal to the epicenter (t-test; *P < 0.05, **P < 0.01).;1>
Reduced Mac-1 Immunoreactivity Is Associated With Reduced Myelin Degradation
Macrophages are important for removal of cellular and myelin debris after injury (Blight, 1985). At 7 dpi, LFB myelin staining was found within the peripheral tissue rim in both CCR2 (+/+) and CCR2 (–/–) specimens. However, the CCR2 (–/–) animals also exhibited regions within the lesion center that stained densely with LFB, whereas only small patches of similar staining were observed in the CCR2 (+/+) controls (Fig. 4A,B). Close microscopic analysis of this LFB-stained region within the gray matter revealed a staining pattern (more densely stained) different from that of the spared peripheral white matter, suggestive of myelin debris that was not completely degraded. This region of dense LFB stain corresponded with those areas that were devoid of Mac-1 immunoreactivity (Fig. 4C–H). The CCR2 (–/–) mice also showed significantly less oil red O staining in the lesion center than the CCR2 (+/+) mice at this time. Oil red O stains myelin degradation products, indicating reduced phagocytic activity by macrophages at the lesion epicenter (Figs. 4I–J, 5). By 14 dpi, the center of the lesion was devoid of LFB but exhibited oil red O staining in both CCR2 (+/+) and CCR2 (–/–) mice.
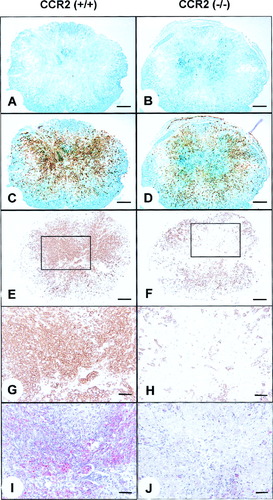
Digital micrographs taken from the injury epicenter at 7 dpi. Representative sections stained with LFB myelin stain (blue; A–D), Mac-1 immunoreactivity (brown; C–H), and oil red O/hematoxylin (I,J). The dual staining in C and D was obtained by superposing images of LFB and Mac-1 stain using digital imaging. A,C,E: CCR2 (+/+) specimen contains Mac-1 immunoreactivity throughout the lesion epicenter, in areas devoid of LFB. B,D,F: Reduced Mac-1 immunoreactivity corresponds to greater residual LFB stain and absence of oil red O reaction in the epicenter of a CCR2 (–/–) specimen. G,H: Higher magnification of area outlined in E and F, to illustrate paucity of Mac-1 staining in the central region. I,J: Oil red O from sections adjacent to G and H reveals active macrophages surrounding myelin debris in the CCR2 (+/+) specimen only. Scale bars = 200 μm in A–F; 50 μm in G–J.
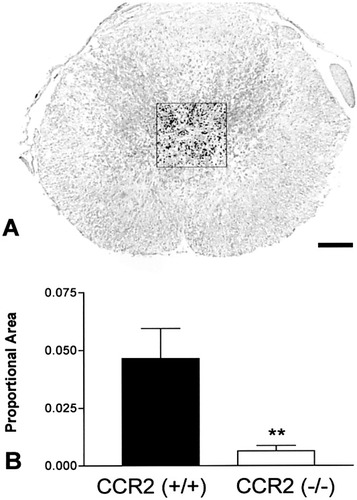
Quantitative analysis of oil red O staining in the center of the lesion. A: Representative photomicrograph illustrating the region selected for analysis from a CCR2 (+/+) specimen (see Fig. 3I for example). Staining in the red spectrum range was established as the target for analysis (black overlay in A). A fixed area of 0.18 mm2 from the middle of the epicenter section was used for analysis, as shown. B: Oil red O stain was reduced in CCR2 (–/–) specimens (n = 9) compared with CCR2 (+/+) specimens (n = 8); t-test, **P < 0.01.
Chemokine and Chemokine Receptor mRNA Expression in Contused Mouse Spinal Cord
The expression of selected α-chemokine (MIP-2, IP-10) and β-chemokine (species MCP-1, MCP-3, MIP-1α, MIP-1β, and C10) mRNA was determined by RPA of spinal cord tissue obtained at 1 and 7 dpi (Fig. 6A). MCP-1, MCP-3, and MIP-2 were increased at 1 dpi compared with 7 dpi. Modest increases were observed for MIP-1α, IP-10, and C10. These data are similar to those reported after spinal contusion injury in rats (McTigue et al., 1998). There was no significant difference in the pattern or magnitude of chemokine message expression between CCR2 (+/+) and CCR2 (–/–) animals at either time (Fig. 7).
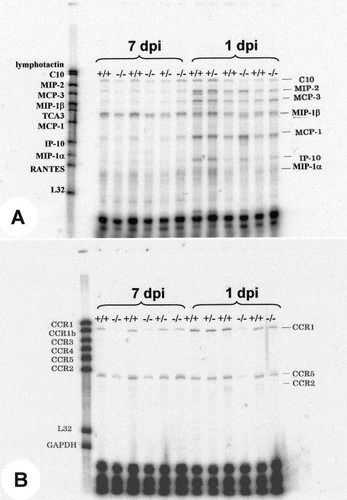
Sample images from RPA using probe sets to identify chemokine (A) and chemokine receptor (B) mRNAs. Total RNA extracted from one spinal cord (20 μg) was loaded per lane.
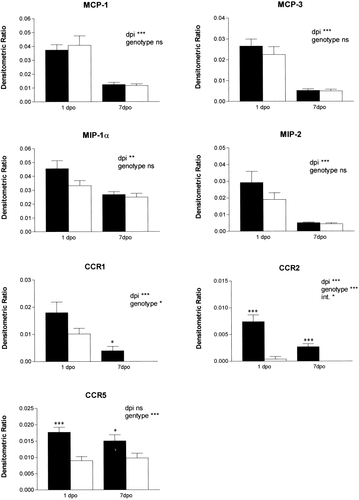
Quantitative analysis of chemokine and chemokine receptor mRNA expression obtained from RPA. Bars represent mean ± SEM of the densitometric ratio of protected species, calculated relative to L32 density. Solid bars represent CCR2 (+/+); n = 7 at 1 dpi; n = 5 at 7 dpi. Open bars are CCR2 (–/–); n = 12 at 1 dpi, n = 9 at 7 dpi. Two-way ANOVA was used to determine the effect of dpi and genotype on group means (key at upper right in each panel; int., interaction effect). Asterisks above each pair of bars represent the results of t-tests to compare densitometric ratio for each chemokine at 1 or 7 dpi: *P < 0.05, **P < 0.01, ***P < 0.001.
Chemokine receptor mRNA expression was also regulated after injury (Fig. 6B). In wild-type mice, CCR1 and CCR2 mRNA levels were increased at 1 dpi relative to 7 dpi. CCR2 (–/–) mice had no detectable CCR2 mRNA. In addition, the CCR2 (–/–) mice had significantly reduced levels of CCR1 and CCR5 receptor mRNA at both 1 and 7 dpi compared with the CCR2 (+/+) mice (Fig. 7).
Discussion
The interaction of MCP-1 with CCR2 is a critical signaling event in a number of models of inflammatory disease or trauma. In the present study, we investigated the role of CCR2 in the inflammatory response following spinal cord contusion injury in mice. CCR2 depletion impaired the recruitment of monocytes and the degradation of myelin at the impact site at 7 days following SCI. The results demonstrate an important role of this mouse chemokine receptor in the early phase of monocyte recruitment to the injured spinal cord.
Spinal cord contusion results in rapid activation of microglia and astrocytes and the recruitment of neutrophils to the site of impact within hours of the initial injury (Dusart and Schwab, 1993; Carlson et al., 1998). This response is followed by the accumulation of monocytes and macrophages at the injury epicenter, beginning about 24 hr postinjury and reaching a peak at approximately 7 days in the rat (Popovich et al., 1997; Streit et al., 1998). The time course of Mac-1 immunoreactivity in C57Bl/6 mice has shown for the first time a similar temporal sequence of macrophage accumulation following SCI. Microglia/macrophage staining reached maximal activation at 7 days postinjury, and this decreased in intensity by 14 dpi. Neutrophil and macrophage recruitment in this injury is a highly regulated process that is correlated with the expression of selective chemokines. Peak expression of mRNA for the α-chemokines GRO-α and IP-10, precedes neutrophil invasion, whereas expression of the β-chemokines MCP-1 and MCP-5 precedes the peak appearance of monocytes and macrophages (McTigue et al., 1998; Streit et al., 1998; Lee et al., 2000).
MCP-1 is a potent monocyte chemotactic mediator and is the predominant β-chemokine in models of CNS injury that result in a macrophage-rich inflammatory response. In addition to SCI, increased MCP-1 expression occurs in response to cortical stab wounds or cryolesions (Berman et al., 1996; Grzybicki et al., 1998; Hausmann et al., 1998), lysophosphatidylcholine injection leading to focal demyelination (Ousman and David, 2000), focal ischemia (Gong et al., 1997), or deafferentation (Muessel et al., 2000; for review see Ransohoff, 1997). Peripheral nerve injury also induces a selective expression of MCP-1 (Ransohoff, 1997; Toews et al., 1998; Coughlan et al., 2000). We have shown here that contusion injury in the mouse evokes a robust increase in MCP-1, MCP-3, and MIP-2 expression at 1 day postinjury relative to 7 days postinjury and a more modest increase in MIP-1α at this time. Thus, MCP-1 and MCP-3 represent likely candidates for modulating monocyte/macrophage recruitment to the injured mouse spinal cord.
MCP-1 evokes chemotaxis of monocytes through CCR2 (Kuziel et al., 1997; Dzenko et al., 2001). The present results demonstrate that CCR2 is required for the initial phase of monocyte recruitment at the epicenter of a contusion lesion. The results are similar to those reported following sciatic nerve injury in CCR2 (–/–) mice, which demonstrated reduced macrophage recruitment and myelin phagocytosis during the first week postinjury (Siebert et al., 2000). As seen in the peripheral nerve injury model, the effect of CCR2 deletion after SCI was transient, and the reduction in macrophage accumulation and myelin phagocytosis was restored by 2 weeks postinjury. Thus, the effect of CCR2 deletion is a delayed recruitment of phagocytic cells to the lesion site. These results suggest that, in the absence of CCR2, MCP-1 might act through other non-CCR2 receptors, such as CCR1 and CCR5, to accumulate macrophages to the lesion site with a slower time course. Alternatively, additional signals are able to recruit phagocytic macrophages to the center of the injury site and the surrounding tissues. Such signals might include other β-chemokines acting on macrophages or microglia, such as MCP-3, MIP-1α, and MIP-1β. These chemokines have also shown to be capable of binding to CCR1 and CCR5 (Murphy et al., 2000). However, the definitive role of these receptors in mediating inflammatory responses is not clear from receptor depletion studies. For example, CCR5 knockouts have been shown to exhibit reduced leukocyte recruitment into the CNS after Cryptococus infection (Huffnagle et al., 1999) but were not responsible for macrophage accumulation after sciatic nerve axotomy (Siebert et al., 2000). CCR1-deficient mice showed exacerbated nephrotoxic nephritis, with increased accumulation of macrophages (Topham et al., 1999). Other possibilities are that other cytokines and growth factors that are up-regulated after SCI, such as interleukin (IL)-1, tumor necrosis factor-α, and granulocyte-macrophage colony-stimulating factor, can induce microglial and monocyte activation and inflammation (Bartholdi and Schwab, 1997; Streit et al., 1998). In the lysophosphatidylcholine model of demyelinating disease, for example, treatment at the injection site with specific antibodies to each of these cytokines resulted in partial suppression of macrophage recruitment, whereas robust suppression was observed following infusion of a cocktail of all four cytokines (Ousman and David, 2001).
The effects of CCR2 deletion on macrophage accumulation were restricted to the center of the impact site. The very localized effect is consistent with the known heterogeneous nature of CNS macrophages. These cells are derived from two distinct sources, including the endogenous tissue microglia and circulating hematogenous monocytes. The immunocytochemical marker Mac-1 cannot distinguish these two populations of macrophages. However, a recent study has employed a bone marrow chimeric rat model to define the regional distribution of peripherally derived macrophages and central (microglial) macrophages after contusion SCI (Popovich and Hickey, 2001). The two cell types are distinguished in the chimeric rats by unique immunocytochemical cell surface markers derived from the different rat strains. Using this approach, the authors showed that, by 1–3 days after a contusion, macrophage markers were associated primarily with host microglia, and, by 7 days postinjury, blood monocytes were the predominant source of macrophages at the injury center, but virtually all of the macrophages in the surrounding white matter and rostral and caudal segments of the injured spinal cord were derived from the microglia of the chimera hosts. Taken together with our current findings, these results strongly support the interpretation that CCR2 mediates the recruitment of hematogenous monocytes to the injury site.
The role of monocytes and the prominent macrophage response in SCI remain enigmatic, in part because of the functional diversity of these cells. On one hand, macrophages play a beneficial role in axonal regeneration by facilitating the removal of cellular debris (Scheidt et al., 1986; Avellino et al., 1995; Zeev-Brann et al., 1998) and by secreting extracellular matrix molecules and antiinflammatory cytokines (Nathan, 1987). The delayed infiltration of macrophages in the CNS relative to the periphery may contribute to the failure of regeneration after CNS injury (Perry et al., 1987; George and Griffin, 1994; Rapalino et al., 1998; Zeev-Brann et al., 1998). The findings in this study that reduced Mac-1 immunoreactivity at the epicenter at 7 days was associated with reduced oil red O staining in CCR2-deficient mice suggest a primary role of monocyte-derived macrophages in phagocytosis at this time.
However, macrophages within the CNS may also be deleterious to spared tissue following SCI. These cells produce neurotoxic molecules, including quinolinic acid (Popovich et al., 1994; Blight et al., 1995) and nitric oxide (Grzybicki et al., 1998; Yamanaka et al., 1998), which can contribute to secondary or bystander damage to tissues and cells that survive the initial wave of necrotic cell death (Blight, 1992). Systemic treatments that reduce the inflammatory response to SCI, including bolus infusions of the glucocorticoid, methylprednisolone, and the antiinflammatory cytokine IL-10, have been reported to result in improved tissue sparing and improved recovery in models of spinal contusion injury (Behrmann et al., 1994; Bethea et al., 1999). The depletion of peripheral monocytes during the first week postinjury has been shown to enhance tissue sparing and promote functional recovery in guinea pig and rat models of SCI (Blight, 1994; Popovich et al., 1999). Thus, macrophages participate in a wide variety of functions in the injured CNS, which both facilitate and interfere with recovery after SCI. In the present study, we found no significant differences in tissue sparing as a result of CCR2 deletion (data not shown). These data suggest that other mechanisms of macrophage recruitment are compensatory with regard to tissue sparing and functional recovery. However, recent studies suggest that cellular events associated with SCI may extend for several weeks after injury in rats and mice (Watanabe et al., 1999; Ghirnikar et al., 2001; Sroga et al., 2001). For example, using an MCP-1 antagonist with treatment to 7 days delayed the migration of macrophages into the lesion but did not affect degeneration (Ghirnikar et al., 2000); however, continuous treatment with the broad-spectrum antagonist vMIPII up to 21 days was shown to promote tissue survival and provide a growth-permissive environment (Ghirnikar et al., 2001). Therefore, long-term (>14 days) studies may reveal more striking effects of delayed monocyte recruitment on tissue sparing and functional outcome after injury.
The present study has shown for the first time the direct involvement of the β-chemokine receptor CCR2 in inflammation after spinal cord contusion. The results indicate that chemokines acting through CCR2 are necessary during the early phase of macrophage recruitment following SCI. However, the effects mediated by CCR2 following SCI are complex, reflecting the heterogeneity of cellular responses to chemokines and other intercellular signaling molecules after traumatic SCI.
Acknowledgements
This work was supported by the Spinal Cord Research Foundation of the Paralyzed Veteran's Adminstration (L.B.J.; SCRF 1892) and the National Institutes of Health (RMR-NS32151, IFC-HL52773). We thank Drs. Phillip Popovich, Dana McTigue, and Bradford Stokes for helpful discussions and criticism. P. Walters, Z. Guan, C. Schneider, and P. Wei provided expert technical assistance.




