Protective effect of apolipoprotein E against ischemic neuronal injury is mediated through antioxidant action
Abstract
Recent studies have demonstrated that apolipoprotein E (APOE) deficiency worsened neuronal injuries after transient focal and global cerebral ischemia. However, the molecular mechanism underlying the protective effect of APOE remains uncertain, even though several mechanisms, including excitotoxicty, free radicals, and apoptosis, have been cited as causes of selective neuronal vulnerability in cerebral ischemia. In the present study, we first compared the vulnerability of cultured neurons prepared from APOE-knockout mice upon exposure to glutamate, hydrogen peroxide, and staurosporine. No significant difference in cell viability was observed after exposure to glutamate or staurosporine between APOE-deficient and wild-type mice. However, exposure to hydrogen peroxide significantly increased the level of cell death in APOE-deficient mice compared with that in wild-type mice. After transient forebrain ischemia for 12 min, APOE-deficient mice showed more neuronal death than wild-type mice. Pretreatment of APOE-deficient mice with vitamin E for 2 months markedly reduced neuronal death caused by ischemia. The results suggest that APOE exerted its neuroprotective effect against ischemia through its antioxidant action but not through mitigation of glutamate toxicity or blocking of apoptosis. © 2002 Wiley-Liss, Inc.
Previous studies have shown that apolipoprotein E (APOE) deficiency worsened histological outcome after transient focal (Laskowtiz et al., 1997) and global (Horsburgh et al., 1999; Sheng et al., 1999) cerebral ischemia and that intraventricular infusion of APOE ameliorated neuronal damage in the caudate nucleus and hippocampal CA2 sector in APOE-deficient mice but not in wild-type mice (Horsburgh et al., 2000). Furthermore, APOE4 transgenic mice developed larger infarcts than APOE3 transgenic mice in a focal cerebral ischemia model (Sheng et al., 1998). These studies supported a protective effect of APOE against ischemic neuronal damage; however, the molecular mechanism underlying the APOE-mediated protection remains unclear. Ischemic neuronal vulnerability after recovery from energy failure may be composed of several factors (Dirnagl et al., 1999). Glutamate toxicity has been believed for more than 10 years to play an essential role in calcium influx into neuronal cytoplasm (Rothmann and Olney, 1986; Hossman, 1994). Subsequent calcium overload has been believed to be the most important factor triggering cell death (Choi, 1995).
Oxidative stress generated by ischemia–reperfusion has also been considered important even before the advent of the concept of excitotoxity (Chan, 2001). Recent studies using transgenic animals, overexpressing superoxide dismutase, supported the involvement of free radicals in ischemic neuronal damage after ischemia–reperfusion (Chan et al., 1998; Kawase et al., 1999). Finally, apoptosis has been examined intensively over the past several years (Linnik et al., 1993; Nitatori et al., 1995; Schulz et al., 1999), although the presence of apoptosis is still controversial (Petito et al., 1997; Colbourne et al., 1999). Other mechanisms, such as inflammation (Kochanek and Hallenbeck, 1992; Mabuchi et al., 2000) and microcirculatory disturbance (del Zoppo, 1994; Kitagawa et al., 1998a), may also contribute to ischemic neuronal vulnerability. In the present study, we tried to clarify which pathway, among glutamate toxicity, oxidative stress, and apoptosis, was effectively blocked by APOE, using cultured neurons prepared from APOE-knockout mice and an in vivo model of transient forebrain ischemia.
Materials and Methods
Animals
Animals used in the present study were fed standard laboratory chow and given free access to water prior to surgery. All experimental procedures were approved by the Institutional Animal Center Use Committee of the Osaka University Graduate School of Medicine. APOE-knockout mice, originally produced by Zhang et al. (1992), were purchased from the Jackson Laboratory (Bar Harbor, ME) and back-crossed to wild-type C57BL/6 mice (Charles River Inc., Yokohama, Japan) to have genetic backgrounds of the homozygote and wild-type mice. After mating of heterozygotes, we selected the homozygous and wild-type mice by polymerase chain reaction (PCR) amplification of genomic DNA extracted from tails.
Neuron–Glia Mixed Culture
After mating pairs of homozygous mice and of wild-type mice separately, pregnant APOE-knockout and wild-type female mice were used. Primary neuronal cultures containing the hippocampus and cerebral cortex from 15–17 day mouse fetuses were obtained as described previously by Chen and Mattson (1994). Cells were dissociated with papain (Papain Dissociation System; Worthington, Freehold, NJ) and plated onto six-well plates (Falcon, Becton Dickinson and Company, Franklin Lakes, NJ) coated with polyethylenimine at a density of approximately 5.0 × 105 cells/dish in high-glucose Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, Tokyo, Japan) containing 10% fetal calf serum (FCS; Sigma), 100 IU penicillin/ml, and 100 mg of streptomycin sulfate/ml during the first 2 days. Then, the cultures were maintained in Neurobasal Medium (Gibco BRL, Life Technologies, Rockville, MD) containing B27 supplement (Gibco BRL) for the next 3–5 days, and the medium was changed to Neurobasal Medium with B27 supplement but without antioxidants 24 hr before glutamate, hydrogen peroxide, or staurosporine treatment. These cultures contained not only neurons but also astrocytes; the latter constituted approximately 15–20% of the cell population by 7 days.
After 6 or 7 days in culture, cells were treated with glutamate (50 or 100 μM) for 15 min, hydrogen peroxide (5 or 10 μM) for 15 min, or staurosporine (30 or 100 nM) for 1 hr. All chemicals were added directly to the medium. The incubation medium was then changed completely to that without antioxidants. Cell viability was assessed using six-well chambers for each group. Quantitative assessment of neuronal injury was accomplished by measuring lactate dehydrogenase (LDH) activity in the medium 24 hr after exposure to glutamate, hydrogen peroxide, or staurosporine using the Cytotoxicity Detection Kit (Boehringer Manheim, Manheim, Germany). The genotype was again confirmed by immunoblotting of the cell extract using an antibody against APOE (Chemicon, Temecula, CA).
For immunocytochemistry for microtubule-associated protein 2 (MAP2), cells were cultured in two-chamber glass slides and incubated as described above. Twenty-four hours after exposure to glutamate, hydrogen peroxide, or staurosporine, the cells were fixed immediately in 4% paraformaldehyde (PFA) for 15 min and permeabilized with 0.01% Triton X-100. Cells were then incubated with monoclonal anti-MAP2 antibody (1:100; Sigma) for 1 hr at room temperature. The slides were next washed in three changes of phosphate-buffered saline, incubated for 1 hr in a 1:200 dilution of fluorescein isothiocyanate (FITC)-labeled secondary antibody, and evaluated using a confocal microscope. The number of MAP2-positive neurons was counted in a field of 0.01 mm2.
Transient Forebrain Ischemia
All homozygous and wild-type mice used for transient forebrain ischemia were mature males age at 12–16 weeks weighing 26.6 ± 1.5 g (homozygous; n = 30) and 24.1 ± 2.1 g (wild-type; n = 30), respectively. Fifteen mice in each group were fed a chow diet (CE2; Clea Japan Inc., Osaka, Japan) supplemented with vitamine E (2 g/kg) for 2 months before surgery. Transient forebrain ischemia was created by bilateral common carotid artery (BCCA) occlusion as described previously (Kitagawa et al., 1998b). Each mouse was anesthetized with 2.0% halothane, and anesthesia was maintained with 0.5% halothane by means of an open face mask. A polyacrylamide column for measurement of cortical perfusion by laser Doppler flowmetry (LDF; Unique Medical) was attached to the intact skull with dental cement, 3.5 mm lateral to the bregma. A metal plate-type thermometer was also attached to the skull over the parietal cortex to record skull temperature. Body and skull temperatures were monitored and maintained at 36.0–37.5°C and 35.0–36.5°C, respectively, using a heat lamp. Both common carotid arteries were exposed and occluded with aneurysmal clips. We selected mice that showed less than 12% of baseline cortical microperfusion as measured by LDF during BCCA occlusion for 1 min, based on our previous findings that no patent posterior communicating artery existed on either side, if a mouse showed less than 12% of baseline cortical microperfusion during BCCA occlusion (Kitagawa et al., 1998b). Twenty-four wild-type and twenty-two APOE-knockout mice met the criteria during the first 1 min and were subjected to extended BCCA occlusion for additional 11 min without interruption. Cortical microperfusion by LDF and body and skull temperature were monitored until 15 min of reperfusion. After discontinuation of halothane anesthesia, each mouse was allowed to recover for 2 hr in a separate chamber, where ambient temperature was maintained at 35°C to prevent hypothermia, and they were kept at room temperature afterward. Seven days later, each mouse was killed by an overdose of pentobarbital, and the whole brain was carefully removed and fixed for histological examination by immersion into the alcohol/5% acetic acid solution for 5 hr at 4°C before dehydration and embedding in paraffin, as described previously (Kitagawa et al., 1998b). Tissue sections encompassing the dorsal hippocampus, 5 mm caudal from the frontal pole according to the mouse brain atlas, were examined after staining with hematoxylin-eosin or cresyl violet. For semiquantitative evaluation, the degree of damage was assessed in the CA1–CA3 sector by percentage of damaged cells (Fig. 1): grade 0, no cell damage visible; grade 1, <50% of cells damaged; grade 2, >50% of cells damaged. The length (in millimeters) of the CA1–CA3 sector with each degree of damage was measured, and the mean histological score was calculated as described previously (Kitagawa et al., 1998b) by the following formula: (1 × length with grade 1 + 2 × length with grade 2)/(total length from the CA1 to the CA3 sector).
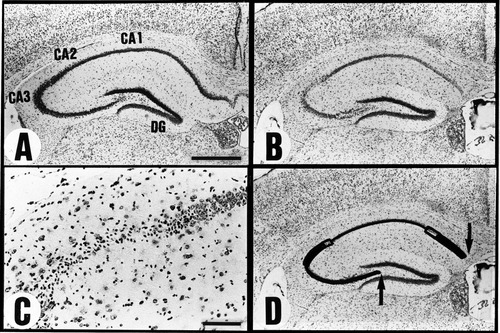
Semiquantitative evaluation of hippocampal injury. A: Normal mouse hippocampus. B: Ischemic mouse hippocampus after transient bilateral carotid occlusion for 12 min. C: Higher magnification of the lateral segment of CA1, CA2, and part of CA3 in B. Note cell loss in the CA2 and CA3 sectors. D: Diagram showing histological grading of B. The thick line denotes the segment with grade 2 damage (>50% of cells are damaged), and the open boxes indicate the segments with grade 1 damage (<50% of cells are damaged); the thin line shows the segment with grade 0 damage (intact). The distance between the two arrows denotes the length from the CA1 to the CA3 sector. Scale bar in A = 0.5 mm for A,B,D; bar in C = 0.1 mm.
Statistical Analysis
The results were expressed as mean ± SD. Statistical analysis was performed by one-way ANOVA, followed by Scheffe's test. P < 0.05 was considered statistically significant.
Results
Cell Cultures
Western blot analysis of cell extract from wild-type mice showed a 34 kDa band. There was no band in the knockout mice. After medium change, percentage release of LDH after 24 hr was 19.3% ± 1.9% in wild-type mice (n = 6) and 22.6% ± 0.7% in APOE-knockout mice (n = 4), and no significant difference was observed between them (Fig. 2). No significant difference in cell viability was found after exposure to either glutamate (at 50 μM, percentage release of LDH: 44.4% ± 10.1% in wild-type mice, n = 6, and 43.6% ± 9.4% in APOE-knockout mice, n = 6; at 100 μM, 64.4% ± 7.7% in wild-type mice, n = 6, and 65.2% ± 3.2% in APOE-knockout mice, n = 6) or staurosporine (at 30 nM, percentage release of LDH: 50.8% ± 10.4% in wild-type mice, n = 5, and 49.6% ± 12.1% in APOE-knockout mice, n = 5; at 100 nM, 70.5% ± 5.5% in wild-type mice, n = 5, and 74.5% ± 2.0% in APOE-knockout mice, n = 5). However, exposure to hydrogen peroxide caused significant worsening of neuronal injury in APOE-knockout mice (percentage release of LDH: 46.3% ± 9.4% at 5 μM, and 74.3% ± 8.0% at 10 μM, n = 5 each) compared with that in wild-type mice (34.4% ± 8.4% at 5 μM, and 42.2% ± 10.6% at 10 μM, n = 5 each). The number of MAP2-positive neurons decreased after exposure to glutamate, hydrogen peroxide, and staurosporine compared with that after medium change alone in both wild-type and APOE-knockout mice (Fig. 3). The degree of decrease in MAP2-positive cells after exposure to glutamate and hydrogen peroxide was similar in both types; however, significant decrease in MAP2-positive cells was observed after exposure to hydrogen peroxide in APOE-knockout mice from in wild-type mice (Fig. 3).
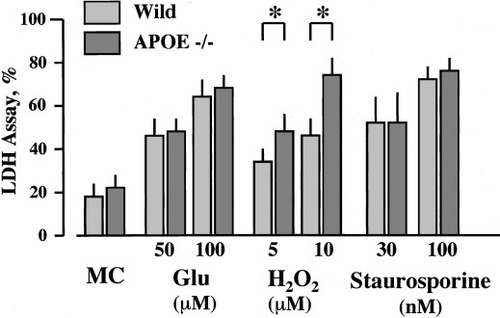
Neuronal cell death assessed by LDH release 24 hr after exposure to glutamate (Glu, 50 or 100 μM) or hydrogen peroxide (H2O2, 5 or 10 μM) for 15 min or staurosporine (30 or 100 nM) for 1 hr in the primary culture prepared from wild-type and APOE-knockout mice. *P < 0.05 between the two types of mice. MC, medium change only.
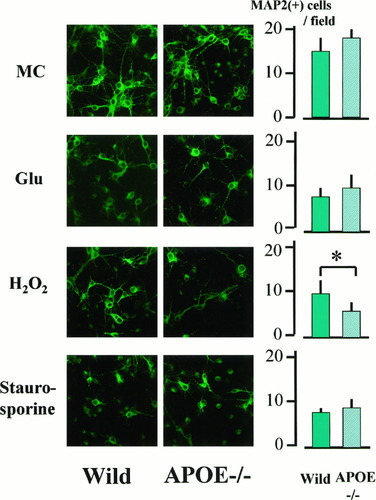
Immunocytochemistry for MAP2 in cultured neurons from wild-type and APOE-knockout mice. MC, medium change only; Glu, glutamate treatment (100 μM). Cytoplasms and processes of cultured cells are MAP2-positive in neurons prepared from both types of mice. The right panels show the number of MAP2-positive neurons in the field of 0.01 mm2. The number of MAP2-positive neurons decreased similarly after exposure to glutamate and staurosporine (100 nM) in both wild-type and APOE-knockout mice, but more marked reduction of MAP2-positive neurons was observed in APOE-knockout mice after exposure to hydrogen peroxide (10 μM). *P < 0.05 between two types of mice.
Hippocampal Injury After Transient Forebrain Ischemia
Residual cortical microperfusion during BCCA occlusion was about 5% of the baseline, but cortical microperfusion jumped to 70–130% of the baseline after reperfusion in both wild-type and APOE-knockout mice with or without vitamin E supplementation. Body and skull temperature were maintained similarly between two groups. One of twenty-four wild-type and three of twenty-two APOE-knockout mice died during reperfusion. In the normal-chow group, APOE-knockout mice showed a higher degree of neuronal damage after ischemia than wild-type mice (Fig. 4). After vitamin E supplementation, only scattered cell death in the CA2 was observed in both types. In the normal-chow group, the mean histological score for wild-type mice (0.052 ± 0.071; n = 12) was significantly less than that for APOE-knockout mice (0.232 ± 0.229; n = 9; P < 0.02; Fig. 5). However, vitamin E supplementation markedly reduced ischemic injury in APOE-knockout mice (0.020 ± 0.033, n = 10; P < 0.01 vs. normal-chow group; Fig. 5).
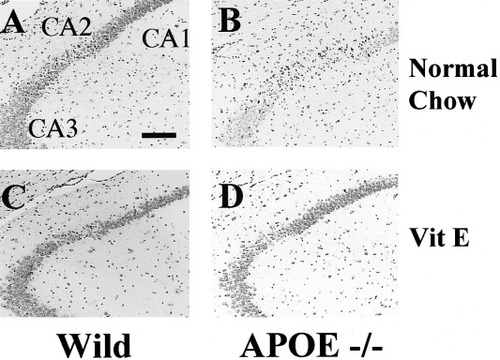
Hippocampal injury after ischemia in wild-type and APOE-knockout mice fed with normal chow or vitamin E supplementation. In the wild-type mouse fed normal chow, cell loss was observed in the CA2 sector, but, in the APOE-knockout mouse fed normal chow, extensive cell loss occurred in the CA2 and CA3 sector. In both types of mice with vitamin E supplementation, scattered cell loss was observed in the CA2 sector. VitE, vitamin E supplementation. Scale bar = 50 μm.
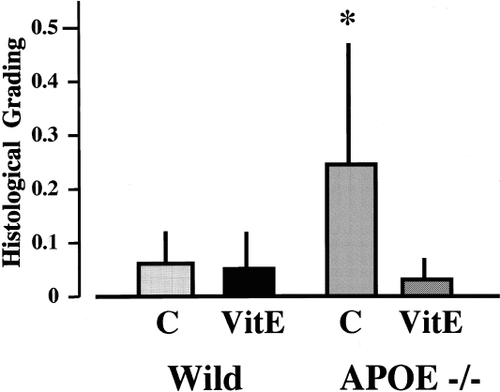
Semiquantitatve assessment of ischemic neuronal damage in the hippocampus after transient global ischemia for 12 min. The histological grade was calculated by dividing the integration of each grading and its length by the total length of the CA1–CA3 sector. There was no difference in wild-type mice after VitE supplementation, but neuronal damage seen in APOE-knockout mice with normal chow was markedly reduced by vitamin E supplementation. C, normal chow; VitE, vitamin E supplementation. *P < 0.02 vs. the other three groups.
Discussion
APOE protein was found to be expressed in reactive astrocyte, degenerating neurons, and macrophages after cerebral ischemia (Hall et al., 1995; Kida et al., 1995; Horsburgh and Nicoll, 1996; Ishimaru et al., 1996; Ali et al., 1996; Kitagawa et al., 2001), and several studies have supported the protective role of endogenous APOE against ischemic brain injury by using APOE-knockout mice (Laskowitz et al., 1997; Sheng et al., 1999; Horsburgh et al., 1999). However, the mechanism inducing the neuroprotective effect of APOE remains unclear. Selective neuronal vulnerability has been extensively investigated, where excitotoxicity, oxidative stress, and apoptosis have been considered crucial or very important. Therefore, we used the primary culture system to examine which insult was more toxic to cultured neurons derived from APOE-knockout mice after exposure to glutamate, hydrogen peroxide, or staurosporine.
Staurosporine is an inhibitor of protein kinase A and can induce apoptosis in cultured neurons (Koh et al., 1995). Our study clearly demonstrated that cultured neurons derived from APOE-knockout mice were more vulnerable after exposure to hydrogen peroxide. The lack of difference between APOE-knockout and wild-type mice after exposure to glutamate was in agreement with Lendon et al. (2000), who demonstrated no effect of endogenous APOE on neuronal cell death caused by exposure to N-methyl-D-aspartic acid. However, experiments with transgenic mice or gene transfer with the human APOE isoform in APOE-knockout mice will be required to confirm the effect of APOE deficiency.
All previous experiments showing protective effect of APOE against cerebral ischemia used ischemia–reperfusion models (Laskowitz et al., 1997; Sheng et al., 1999; Horsburgh et al., 1999), where more oxygen radicals occurred compared with permanent occlusion models (Peters et al., 1998). Because the antioxidant action and the differential ability of different APOE isoforms to bind 4 hydroxynonenal have been shown for APOE protein in vitro (Miyata and Smith, 1996; Pedersen et al., 2000), we next examined the role of APOE as an antioxidant in cerebral ischemia in vivo. Because Ramassamy et al. (2001) have demonstrated the reduction of α-tocopherol in the hippocampus of APOE-knockout mice, we also examined the effect of vitamin E supplementation on ischemic neuronal damage in APOE-knockout and wild-type mice. Vitamin E supplementation has been shown to enhance the antioxidative activity in many organs, including brain, and is one of the most commonly used strategies to prevent free radical-mediated pathology in the brain (Hara et al., 1990) and atherosclerotic vessels (Pratico et al., 1998). After transient global ischemia for 12 min, APOE-knockout mice fed normal chow showed more pronounced damage in the hippocampal neurons compared with wild-type mice. This finding was in agreement with the findings in previous studies (Sheng et al., 1999; Horsburgh et al., 1999). Furthermore, vitamin E supplementation markedly mitigated the degree of neuronal damage after ischemia in APOE-knockout mice. Because of the minimal extent of neuronal damage in wild-type mice, we could not assess the effect of vitamin E supplementation in wild-type mice; however, our results support the notion that the difference in the degree of neuronal damage between wild-type and APOE-knockout mice could likely be ascribed to more susceptibility to oxygen radicals in the latter mice.
Although we did not assess lipid peroxidation or levels of oxidative stress in neurons from wild-type and APOE-knockout mice, the finding by Lomnitski et al. (2000) of increased levels of intracellular iron in APOE-knockout mice after head injury supported increased vulnerability to oxygen stress in APOE-knockout mice. Distinct alterations in phospholipid metabolism and phosphoinositide hydrolysis in brains of APOE-knockout mice (De Sarno and Jope, 1998; Lomnitski et al., 1999) may contribute to the effect of APOE deficiency on oxygen stress. In conclusion, our in vitro and in vivo studies demonstrated that the protective effect of APOE previously reported for several ischemia models was mainly ascribable to its antioxidant action.
Acknowledgements
The authors thank Mr. Nobuo Katsube of Ono Pharmaceutical Company for technical assistance, and Miss R. Morimoto and Miss S. Imoto for secretarial assistance. The present study was supported in part by a Grant-in-aid for Scientific Research on Priority Areas (A) in Japan.




