Synergistic induction of HSP40 and HSC70 in the mouse hippocampal neurons after cerebral ischemia and ischemic tolerance in gerbil hippocampus
Abstract
An ischemia-induced gene was screened using a differential display technique in mouse transient forebrain ischemia. One of the ischemia-responsive clones was found to encode mouse hsp40. HSP40 has a critical regulatory function in the HSC70 ATPase activity. Expression of hsp40 mRNA was low in the nonischemic mouse hippocampus, but it was significantly upregulated 4 hr after ischemia by Northern blot analysis. In situ hybridization analysis revealed hsp40 mRNA induction in the neuron. HSP40 protein expression was also enhanced in the pyramidal and dentate granular neurons from 2 to 4 days after ischemia. The temporal expression and distribution profile of HSC70 protein was similar to that of HSP40, and both proteins were colocalized in ischemic hippocampal neurons. In the gerbil transient forebrain ischemia model, both HSP40 and HSC70 proteins were expressed strongly in ischemia-resistant CA3 neurons and dentate granule cells 1 day after 5 min ischemia, but were not expressed in vulnerable CA1 neurons. However, both proteins were in parallel expressed in the tolerance-acquired CA1 neurons. Based on the current observation that both HSP40 and HSC70 proteins were synergistically expressed in the ischemia-resistant and tolerance-acquired neurons, cochaperone HSP40 may play a significant role against postischemic neuronal response and lead to cell survival through interaction with simultaneously induced HSC70. © 2002 Wiley-Liss, Inc.
Transient forebrain ischemia leads to selective neuronal death in vulnerable areas. In this area, many genes were induced and some of these genes may contribute to delayed neuronal death, while other genes may be protective against ischemic insult and facilitate cell survival (Akins et al., 1996; Chen et al., 1996; Kinoshita et al., 1997; Nogawa et al., 1997; Velier et al., 1999). Moreover, induction of certain genes has been suggested to be associated with ischemic tolerance phenomenon and a candidate for neuronal protection (Kato et al., 1995; Sommer et al., 1995; Ide et al., 1999). However, the molecular mechanisms underlying ischemic neuronal death and ischemic tolerance are not fully understood. To help clarify the mechanisms underlying neuronal degeneration or protection, identification of an ischemia-responsive gene may be significant. Recently, we identified a ischemia-responsive gene, irp94, using a rat forebrain ischemia model with the differential display method (Yagita et al., 1999). A mouse model of forebrain ischemia may be more suitable for screening ischemia-responsive genes than a gerbil or rat model, because we can take full advantage of using the mouse EST database programs and preparing transgenic mice for further investigation.
In the present study using forebrain ischemia model of the mouse (Kitagawa et al., 1998) and the mRNA differential display technique, we isolated the mouse hsp40 cDNA clone as an ischemia-responsive protein. Evidence is accumulating that HSP40 is closely associated with HSC70 (Ohtsuka and Hata, 2000). HSP40 enhances HSC70 ATPase activity by an approximate 7-fold increase (Minami et al., 1996), and furthermore, combined expression of HSP70 and HSP40 is effective in reducing aggregate formation and providing cellular protection in cultured neurons (Kobayashi et al., 2000). In the normal brain, both HSP40 and HSC70 proteins are constitutively expressed and colocalized at postsynaptic structures (Suzuki et al., 1999). In ischemic brain, acceleration of hsc70 gene expression was observed in tolerance-acquired hippocampal neurons (Aoki et al., 1993). Although a previous study showed upregulation of hsp40 mRNA in the postischemic rat brain with quantitative polymerase chain reaction (PCR; Paschen et al., 1998), the precise expression and the spatial distributions of hsp40 mRNA and HSP40 protein and its relationship with HSC70 protein expression in the postischemic brains have not been examined. Therefore, we investigated hsp40 mRNA and HSP40 protein expression together with HSC70 protein expression in the ischemic mouse hippocampus. Furthermore, using the gerbil transient forebrain ischemia model, we examined the relationship between the expression of both HSP40 and HSC70 and selective neuronal vulnerability or ischemic tolerance (Kitagawa et al., 1990; Kirino et al., 1991), because the gerbil model was well established for delayed neuronal death and ischemic tolerance.
MATERIALS AND METHODS
Animal Model
The experimental protocol has been approved by the Institutional Animal Care and Use Committee of Osaka University Graduate School of Medicine. Mouse transient forebrain ischemia was induced by two-vessel occlusion and reperfusion as previously described (Kitagawa et al., 1998). Adult male C57BL/6 mice (Charles River Inc., Yokohama, Japan) weighing 25–30 g were used in this study. Mice were allowed free access to food and water before surgery. General anesthesia was induced with 4.0% halothane and maintained with 1.0% halothane by means of an open face mask. Both common carotid arteries (CCA) were exposed and occluded with microaneurysm clips for 15 min and then reperfused. A probe for Laser Doppler Flowmetry (LDF; Advance Laser Flowmetry, model ALF-21, Advance Co., Ltd., Tokyo, Japan) was attached to the intact skull, 3.5 mm right of the bregma. Cortical microperfusion was continuously monitored by LDF and recorded before and after occlusion. Only mice that showed less than 10% of baseline cortical microperfusion during the first 1-min bilateral CCA occlusion were used. A metal plate type thermometer with a diameter of 3.0 mm was also attached to the skull over the right cerebral cortex to measure skull temperature. Body and skull temperature were maintained at 36.0–37.0°C with a heat lamp before and during the operation, and monitored after recirculation. For gerbils, transient forebrain ischemia was induced by bilateral carotid artery occlusion under anesthesia with 1.0% halothane by means of an open face mask. Bilateral CCA were exposed and occluded with microaneurysmal clips for 2 min or 5 min and then reperfused. For the experiment of ischemic tolerance, gerbils were pretreated with 2-min ischemia and subsequently subjected to 5-min ischemia 4 days later as described previously (Kitagawa et al., 1990). Body temperature was maintained at 36.0–37.0°C with a heat lamp before and during the operation, and monitored after recirculation.
Differential Display Analysis
Differential display analysis was performed as previously described (Liang and Pardee, 1992). After recirculation periods of 4 hr, 8 hr, 1 day, 2 days, and 4 days (n= 4 for each), mice were killed and the hippocampus was quickly separated, and frozen in liquid nitrogen. The total RNA was isolated using ISOGEN (Nippon Gene, Tokyo, Japan) reagent. Total RNA (4.0 μg) was then reverse-transcribed with reverse transcriptase (Toyobo, Osaka, Japan) in the presence of oligo dT primers (Nippon Gene) for 60 min at 37°C. The cDNAs obtained from the nonischemic (sham) or ischemic (4 hr, 24 hr after 15-min ischemia) hippocampi were subjected to 30 cycles of PCR with 10-mer arbitrary primers with a setting of 94°C for 30 sec, 40°C for 2 min, 72°C for 45 sec, and 72°C for 5 min. PCR products were identified by 5.0% polyacrylamide gel electrophoresis. After staining with GEL-STER (Takara, Tokyo, Japan), the cDNA bands differentially displayed were recovered and reamplified by PCR using the corresponding primers. Reamplified cDNA fragments were subcloned into pGEM-T easy vector (Promega, Madison, WI) and used as probes for Northern hybridization. Plasmid DNA sequencing of cloned fragments was performed using the ABI PRISM dye terminator cycle sequencing core kit (Applied Biosystems, Foster, CA) and analyzed using an ABI 310 Genetic Analyzer (Applied Biosystems). The resulting sequence information was compared with GenBank™ and EST databases at the National Center for Biomedical Information using the BLAST computer program.
Northern Blot Analysis
Total RNA was isolated from the hippocampus of each group. Twenty micrograms of RNA samples from each group were electrophoresed on 1.0% formaldehyde-agarose gels and transferred onto Hybond N membranes (Amersham Pharmacia Biotech Ltd., Buckinghamshire, U.K.). The Rediprime DNA labeling system (Amersham Pharmacia Biotech) was used to label mouse hsp40 cDNA fragment with [α-32P] dCTP. The membranes were hybridized with each probe and all hybridization procedures were performed according to the manufacture's recommendations as described previously (Ohtsuki et al., 1996). As controls, cDNA fragments for glyceraldehyde-3-phosphate dehydrogenase (G3PDH) were used.
cDNA Library Screening
The cDNA library screening was performed by the standard method. The cDNA library of the mouse brain constructed with λZAPII (Stratagene, LaJolla, CA) was screened with the 32P-labeled cDNA probe by colony hybridization. The hsp40 cDNA was isolated from the mouse brain cDNA library and sequenced using an automatic DNA sequencer 310 (Applied Biosystems).
In Situ Hybridization
We performed almost all procedures as described previously (Ohtsuki et al., 1996; Yagita et al., 1999). A 450-bp fragment was cut out and then inserted into pGEM-T easy plasmid (Promega). The plasmid was linearized with NcoI or SpeI, and then in vitro transcription and digoxigenin labeling were performed to prepare sense or antisense RNA probes, respectively, using the digoxigenin RNA labeling kit (Boehringer Mannheim, Mannheim, Germany). Frozen coronal sections of mouse brains including the hippocampus 4 hr after reperfusion were treated with 2.5 μg/ml proteinase K at 37°C for 15 min, treated with 0.2 M HCl for 20 min to quench endogenous alkaline phosphatase, and acetylated with 0.1 M triethanolamine-HCl (pH 8.0)/0.25% acetic anhydride for 13 min at room temperature. After prehybridization for 20 min at 55°C with 50% formamide, 10% dextran sulfate, 1× Denhardt's solution, 20 mM Tris-HCl (pH 8.0), 300 mM NaCl, 0.2% sarcosine, and 0.02% transfer RNA, digoxigenin-labeled probes were hybridized to the brain sections at 55°C for 16 hr. The brain sections were washed in 5 × standard sodium citrate (SSC) at room temperature for 20 min and in 50% formamide/2 × SSC at 65°C for 1 hr. Anti-digoxigenin antibody conjugated to alkaline phosphatase (Boehringer Mannheim) was reacted at a 1:500 dilution, and the other color reaction was carried out with 4-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate.
Western Blot Analysis
Nonischemic (sham) or ischemic (4 hr, 1, 2, 4, 7, and 14 days after ischemia, n = 4 for each) hippocampi were separated from the brains of both mouse and gerbils, and each specimen was homogenized in Laemmli's buffer consisting of 62.5 mmol/l Tris-HCl pH 6.8, 2% sodium dodecyl sulfate, 10% glycerol, and 2% 2-mercaptoethanol. An equal amount of protein (10 μg) was separated on 10% or 12.5% polyacrylamide gels and electrophoretically transferred to polyvinilidene difluoride membranes (Millipore, Bedford, MA). The membranes were washed in phosphate-buffered saline (PBS), pH 7.4, and blocked in 5% skimmed milk (Meiji Co. Ltd., Tokyo, Japan) in phosphate-buffered saline containing 0.2% Tween 20 (T-PBS) overnight at 4°C. Anti-HSP40 polyclonal antibody (1:10,000, Stressgen, Victoria, Canada) and anti-HSC70 monoclonal antibody (1:5,000, Stressgen) were used as the first antibodies. Horseradish peroxidase-conjugated goat anti-rabbit IgG (Amersham Pharmacia Biotech.) or goat anti-rat IgG both at 1/2,000 dilution were used as the second antibodies. Peroxidase activity was detected using an enhanced chemiluminescence method (ECL, Amersham Pharmacia Biotech.). The densitometric value of each point was normalized to the value in the control group.
Immunohistochemistry
One day after the last ischemia, the mice or gerbils were killed and perfusion-fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) under deep pentobarbital anesthesia. Each brain was removed and postfixed in the same fixative overnight at 4°C, and cryoprotected in 30% sucrose solution. We prepared 8-μm sections using a cryostat, thaw-mounting them on silane-coated glass slides (Matsunami, Tokyo, Japan) and storing them at −80°C until use. Each section was air-dried and postfixed using 4% paraformaldehyde solution in 0.1 mol/L phosphate-buffer for 15 min at room temperature. After washing with PBS, pH 7.4, all sections were treated with 0.6% H2O2, 10% methanol in PBS to quench the endogenous peroxidase and blocking 2% appropriate nonimmunized serum in PBS containing 0.2% Triton X-100. Immunoreaction was performed overnight at 4°C with the following antibodies: rabbit polyclonal antibody against human HSP40 (1:2,000, Stressgen), rat monoclonal antibody against mouse HSC70 (1:4,000, Stressgen). HSP40 antibody was detected with the PAP system and HSC70 antibodies were detected with the ABC Vector Elite kit (Vector, Burlingame, CA). The peroxidase reaction was carried out by incubation with diaminobenzidine and hydrogen peroxide. For double immunostaining of HSP40 and HSC70, mouse brain sections were co-incubated with two primary antibodies and then detected by fluorescein isothiocyanate (FITC)- and rhodamine-labeled secondary antibodies. Immunostained sections were analyzed using the Zeiss confocal laser scanning microscope system (LSM410, Zeiss, Germany).
Histologic Examination
For histologic analysis, 12 gerbils were used. Four sham-operated animals were used as controls. For induction of tolerance, eight gerbils were subjected to 2 min of ischemia (n = 4) or sham operation (n=4), and 4 days later they were subjected to 5 min of ischemia. Four days after the last ischemia, gerbils were decapitated and their brains were promptly removed, divided into coronal sections and fixed in 5% acetic acid in ethanol at 4°C for 4 hr and embedded in paraffin. Coronal 5-μm sections corresponding to the stereotactic planes 1.4– 1.6 mm caudal to the bregma were stained with hematoxylin-eosin.
Data Analysis
In all experiments, the mean ± S.D. is reported. Statistical comparisons among groups were determined using one-way analysis of variance (ANOVA) with the Bonferroni/Dunn post hoc test (Stat View, Abacus Concepts, Berkeley, CA).
RESULTS
Differential display techniques using 150 primer pairs initially identified one hundred PCR products. These products were then reamplified, sequenced, and cloned into a plasmid vector. One of these clones was obtained as a fragment amplified 4 hr and 24 hr after 15 min ischemia. Northern blots revealed that this message was upregulated 4 hr after ischemia, and remained elevated 24 hr after ischemia (Fig. 1A). After screening the mouse brain library, one positive 1.3-kb clone was obtained. Sequence analysis revealed that this clone was a human hsp40 homolog and that the open reading frame encoded a polypeptide of 340 amino acids. Later this clone was found to be identical to mouse hsp40 by Gene Bank data analysis.
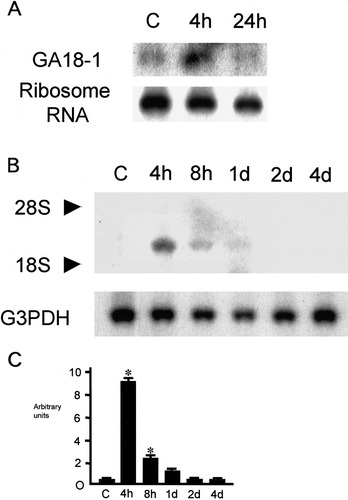
Identification of ischemia-induced mRNA in the mouse hippocampus. A: Northern blot demonstrates that the candidate mRNA (GA18-1) expression was enhanced after ischemia for 15 min and reperfusion for 4 hr. 28S ribosomal RNA was used as the control. Each lane contained 10 μg of total RNA from the hippocampus. Lane C, sham; lanes 4h and 24h indicate 4 hr and 24 hr after reperfusion, respectively. B: Northern blot for mouse hsp40 demonstrates the temporal profile of mRNA expression following 15 min of ischemia. Transcription of hsp40 mRNA was enhanced 4 to 8 hr after reperfusion. Lane C, sham; lanes 4h, 8h, 1d, 2d, and 4d indicate 4 hr, 8 hr, 1 day, 2 days, and 4 days after reperfusion, respectively. Each lane contained 10 μg of total RNA from the hippocampus. 28S and 18S indicate the location of 28S and 18S ribosomal RNA used as markers of mRNA size. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was used as the control. C: Summary of relative hsp40 mRNA level changes. Values are means ± S.D. (bars). *P< 0.05 compared with the control group.
hsp40 mRNA Expression in Mouse Brain After Transient Forebrain Ischemia
Northern blot analysis revealed that the level of hsp40 mRNA expression was low in the sham-operated hippocampus. After a transient 15 min ischemia and reperfusion, hsp40 mRNA expression was sharply enhanced from 4 hr to 8 hr, and gradually declined thereafter (Fig. 1B,C). To examine the localization of hsp40 mRNA in the hippocampus, in situ hybridization was performed using a cRNA probe. At 4 hr after a transient 15 min ischemia, hsp40 mRNA expression was upregulated in the pyramidal cell layer of the CA1∼CA3 region and the granular cell layer of the dentate gyrus (Fig. 2B).
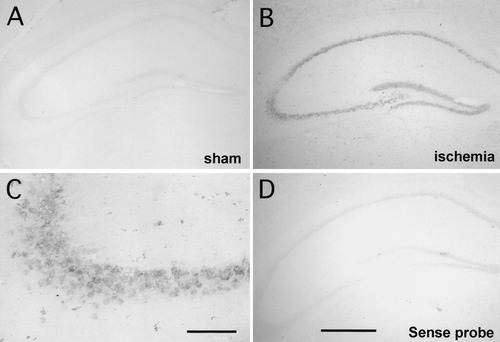
Expression of hsp40 mRNA in the hippocampus 4 hr after transient forebrain ischemia. In situ hybridization for hsp40 demonstrates that mRNA signals were mainly detected in the pyramidal cell layers of the CA1–CA3 sector and granule cell layers of the dentate gyrus. A: Antisense probe (sham). B: Antisense probe (ischemia). C: Magnification of the CA3 region shown in B. D: Sense probe (ischemia). Scale bars =500 μm in A,B,D; 50 μm in C.
Synergistic Induction of HSP40 and HSC70 Protein in the Mouse Hippocampus After Transient Forebrain Ischemia
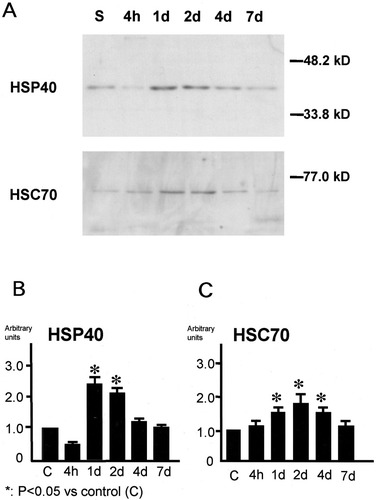
In Western blotting, HSP40 protein expression was enhanced from 2 to 7 days (Fig. 3A,B), and HSC70 protein expression was enhanced from 1 to 4 days (Fig. 3A,C). Temporal profiles of both proteins' expression were very similar as shown in the densitometric analysis.
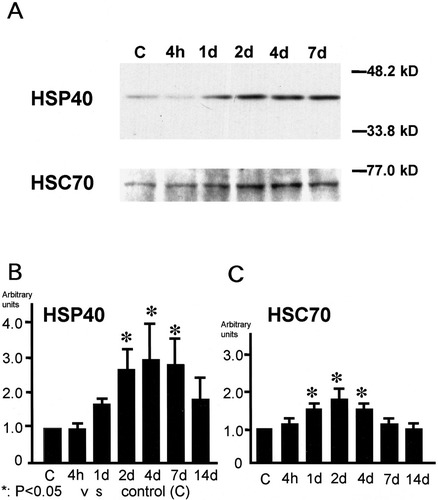
Immunoblotting of HSP40 and HSC70 in the ischemic mouse hippocampus. A: Western blotting for HSP40 and HSC70 protein expression following 15 min of ischemia. Each lane contained 10 μg of total protein from an ischemic hippocampus. B,C: Densitometric analysis of immunoblotting of HSP40 (B) and HSC70 (C). The level of HSP40 protein was significantly increased from 2 days to 7 days, and the level of HSC70 protein was also significantly increased from 1 day to 4 days compared with the control group. *P < 0.05.
In immunohistochemistry, HSP40 immunoreactivity was very weak throughout the whole hippocampus in sham-operated mice (Fig. 4A). Two days after reperfusion, HSP40 expression was upregulated in all hippocampal neurons (Fig. 4B). The signal in CA3 pyramidal neurons and granule cells of the dentate gyrus was stronger than that of the CA1 sector. The induction of HSC70 immunoreactivity 2 days after ischemia (Fig. 4D) showed a similar pattern to that of HSP40. HSC70 was also strongly expressed in the CA3 and dentate granular neurons.
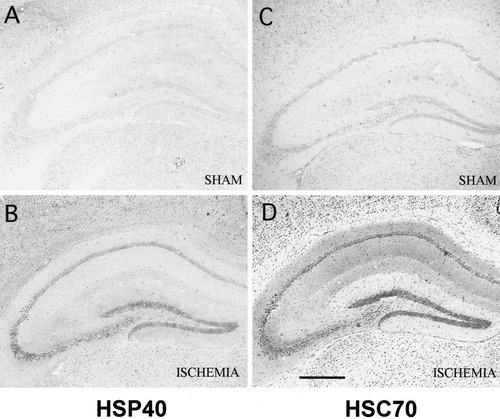
Distribution of immunoreactive HSP40 (A,B) and HSC70 (C,D) in the mouse hippocampus after 15 min of ischemia. Brain sections were prepared from sham operated mice (A,C), and from mice 2 days after reperfusion (B,D). Both HSP40 and HSC70 signals were upregulated in the every hippocampal neurons after ischemia. Scale bar = 500 μm.
To examine a coexpression of HSP40 and HSC70, double immunofluoresence of HSP40 and HSC70 was used with confocal microscopy. Because the signal for HSP40 was very weak in normal condition, brain sections prepared from ischemic gerbils were used for double immunofluorescence. HSP40 was clearly colocalized with HSC70 in the hippocampal neurons 2 days after ischemia (Fig. 5C).
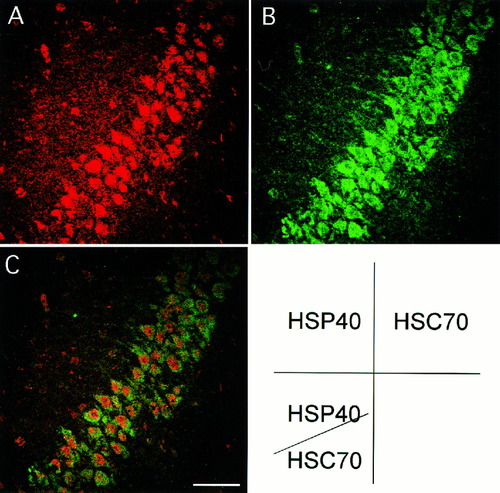
Double immunofluoresence staining of HSP40 and HSC70 in the CA3 sector of mice 2 days after ischemiaA: HSP40 (Rhodamine). B: HSC70 (fluorescein isothiocyanate, FITC). C: Combination of HSP40 (Rhodamine) and HSC70 (FITC). Colocalization of HSP40 and HSC70 was observed in the ischemia-resistant CA3 neurons. Scale bar = 50 μm.
Synergistic Induction of HSP40 and HSC70 Protein in the Resistant and Tolerant Neurons in the Gerbil Hippocampus After Ischemia
Expression of HSP40 and HSC70 protein after cerebral ischemia was also confirmed in gerbil models by both Western blotting and immunohistochemistry (Fig. 6). In Western blotting, the level of HSP40 proteins in the hippocampus was increased from 1 to 2 days after ischemia for 5 min, and HSC70 protein expression was enhanced from 1 to 4 days after ischemia (Fig. 6). The level of both proteins returned to the baseline level 7 days later. In immunohistochemistry, both proteins showed little immunoreaction in sham-operated gerbils (Fig. 7A and E). The levels of both HSP40 and HSC70 proteins were upregulated in the CA3 neurons 1 day after ischemia for 2 min (Fig. 7B and F) or 5 min (Fig. 7C and G), but neither proteins were induced in the vulnerable CA1 neurons. However, both HSP40 and HSC70 were synergistically induced in the CA1 neurons 1 day after 5 min ischemia when gerbils were pretreated with 2 min ischemia 4 days earlier (Fig. 7D and H). While 5 min of ischemia resulted in delayed neuronal death in the CA1 neurons (Fig. 7I), two min of ischemia induced tolerance in the CA1 neurons against subsequent lethal (5 min) ischemic insult (Fig. 7J).
Immunoblotting of HSP40 and HSC70 in the ischemic gerbil hippocampus. A: Western blotting for HSP40 and HSC70 protein expression. Each lane contained 10 μg of total protein extracted from an ischemic hippocampi. Each lane indicates the period of reperfusion. B,C: Densitometric analysis of immunoblotting of HSP40 (B) and HSC70 (C). Level of both HSP40 and HSC70 protein was significantly increased after ischemia for 5 min in gerbil hippocampi. *P < 0.05.
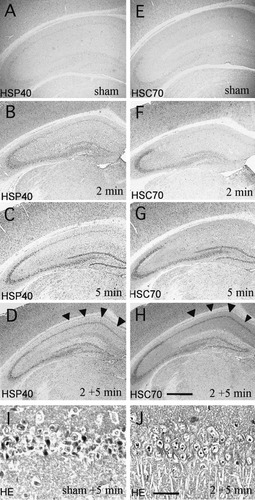
Distribution of immunoreactivity for HSP40 (A–D) and HSC70 (E–H) in the ischemic gerbil hippocampi. Brain sections were prepared from sham-operated gerbils (A,E), from gerbils 1 day after 2 min ischemia (B,F), 1 day after 5 min ischemia (C,G), 1 day after 5 min ischemia pretreated with 2 min ischemia 4 days before (D,H). A single 2 min ischemia caused only weak expression of HSP40 and HSC70 in the CA3 and dentate gyrus (B,F). HSP40 and HSC70 proteins were induced strongly in the CA3 pyramidal neurons and granule cells of the dentate gyrus 1 day after 5 min ischemia, while little induction of both proteins were found in the vulnerable CA1 neurons (C,G). On the other hand, tolerance-acquired CA1 neurons in the gerbils pretreated with 2 min ischemia 4 days earlier, expressed substantial amounts of both HSP40 and HSC70 after 5 min ischemia (arrowheads in D,H). Hematoxylin-eosin staining in the CA1 neurons 4 days after 5 min ischemia with sham-operation (I) and pretreatment (J). The majority of CA1 neurons died after 5 min ischemia without pretreatment (I); however, most CA1 neurons survived after 5 min ischemia when they were pretreated with 2 min ischemia 4 days before (J). Scale bars = 500 μm for A–H; 50 μm for I,J.
DISCUSSION
In the present study, we isolated the mouse hsp40 gene as an ischemia-responsive protein, using the forebrain ischemia model and the mRNA differential display technique. HSP40 was first detected in human HeLa cell (Ohtsuka et al., 1993). One of the major roles of the HSP40 could be the assistance of HSP70 chaperone activity by binding to HSC70 (Frydman et al., 1994; Minami et al., 1996), and, therefore, HSP40 was called cochaperone. A novel chaperone system with HSP40, HSP70, and HSP104 is now recognized (Glover and Lindquist, 1998). A previous study showed HSP40 was constitutively but weakly expressed in the normal hippocampus (Suzuki et al., 1999). Although the previous quantitative PCR technique showed an induction of hsp40 mRNA from 2 hr to 24 hr in the hippocampus after transient forebrain ischemia by four vessel occlusion in rats (Paschen et al., 1998), information about HSP40 protein expression and its association with HSC70 after cerebral ischemia has not been obtained. In the current study, we showed the gene and protein expression of hsp40 after cerebral ischemia and the synergistic induction of both HSP40 and HSC70 protein in the postischemic hippocampus. HSP40 protein seemed to be localized in the nucleus after ischemia (Fig. 5); however, further studies will be required to clarify the ischemia-induced translocation into the nucleus. Because previous studies demonstrated that HSP40 was localized in the cytoplasm of cultured neurons and it translocated into the nucleus after heat shock (Hattori et al., 1993; Terada et al., 2000), nuclear translocation of HSP40 after ischemia may be important for neuronal survival after ischemic insult. It remains unclear why HSP40 protein expression reached to the peak a few days after the maximum induction of HSP40 mRNA (Figs. 1 and 3); however, previous studies also showed a similar pattern of mRNA and protein expression for other HSP genes after cerebral ischemia (Vass et al., 1988; Nowak, 1991; Nishi et al., 1993). A recent study showed that hsp40 mRNA was regulated by the transcription factor of heat shock factor 1 (HSF1; Hata et al., 1996). Since HSF1 was activated in the rat brain during cerebral ischemia or after heat shock (Higashi et al., 1995), hsp40 and hsc70 alike may be regulated by HSF1 in the ischemic hippocampus, but the precise mechanisms of transcription would need further clarification.
The expression of several stress proteins after cerebral ischemia has been intensively studied during the past two decades. Among them, an inducible isoform of HSP70 was the most intensively examined (Nowak and Jacewicz, 1994). In contrast, there are only a few studies of the protein expression of the constitutive isoform of HSP70 family, HSC70, after cerebral ischemia. HSC70 mRNA and protein are constitutively and abundantly expressed in the brain (Manzerra et al., 1993; Foster et al., 1995). Although it was shown that the level of HSC70 protein remained unchanged after heat shock in the rabbit brain (Manzerra et al., 1993), previous studies have shown upregulation of HSC70 mRNA in the CA1–CA3 sector after transient ischemia in gerbils using in situ hybridization and Northern blotting (Nowak et al., 1990; Kawagoe et al., 1993; Abe et al., 1993). In contrast, Harrub and Nowak (1998) and Bertrand et al.(2000) showed a decrease or no significant change in HSC70 immunoreactivity in the CA1 neurons after ischemia. We observed 2- to 3-fold increases in HSC70 content by Western blotting in both mouse and gerbil hippocampi after ischemia. Since bilateral CCA occlusion for 15 min in the mice resulted in only scattered cell death in the CA1, CA2, CA3, and CA4 (Yang et al., 2000), most cells expressing both HSP40 and HSC70 48 hr after ischemia are resistant neurons against ischemia. However, in gerbils, CA1 neurons are highly vulnerable after 5 min ischemia. In our immunohistochemical study, both HSP40 and HSC70 immunoreactivity decreased in the CA1 neurons after 5 min ischemia in gerbils. The findings of HSC70 in the CA1 sector are in apparent agreement with those of previous studies (Harrub and Nowak, 1998; Bertrand et al., 2000). However, both proteins were synergistically expressed in the ischemia-resistant CA3 neurons and in tolerant CA1 neurons when gerbils were pretreated with 2 min ischemia 4 days earlier. Upregulation of HSC70 protein in tolerant CA1 neurons is in agreement with the finding that hsc70 mRNA expression was accelerated in tolerant CA1 neurons (Aoki et al., 1993). For understanding the functional role of HSP40/HSC70 coexpression, it would be necessary to investigate the expression of several other genes such as death-related Bax gene in these cells, because it was shown that Bax protein was induced in the CA1 neurons after transient forebrain ischemia (Krajewski et al., 1995).
Evidence is accumulating that heat shock proteins are protective against ischemic brain damage from studies using hsp70 transgenic mice and hsp70 gene transfer experiments (Yenari et al., 1998; Rajdev et al., 2000). However, the interaction of several stress proteins may be much more important than the level of specific HSP such as HSP70 for neuronal survival after ischemic insult, because recent studies emphasized the crucial role of the interaction of several stress proteins for molecular chaperone activity (Glover and Lindquist, 1998). A recent study also showed that the chaperone activities of HSC70 and HSP40 were modulated by HSP105 or HSP104 (Glover et al., 1998; Yamagishi et al., 2000). We cloned the HSP110 family gene, irp94, in the rat brain after transient forebrain ischemia (Yagita et al., 1999). Under ischemic stress conditions, these chaperone systems may regulate protein maturation and refold denatured proteins, and may finally lead to facilitate cell survival. Therefore, future studies will be needed to clarify the association and functional role of HSP40, HSC70, and HSP110 in the ischemic neurons.
Acknowledgements
The authors thank Ms. Y. Imaeda and Ms. R. Morimoto for their secretarial assistance.




