Selective CC chemokine receptor expression by central nervous system-infiltrating encephalitogenic T cells during experimental autoimmune encephalomyelitis
Abstract
Experimental autoimmune encephalomyelitis (EAE) is a CD4+ T cell disease of the central nervous system (CNS) characterized by mononuclear cell infiltration, demyelination, and paralysis. Recent studies describing the relationship of chemokine expression with development of clinical disease have led to the hypothesis that distinct chemokine receptors corresponding to specific ligands are expressed by CNS-infiltrating antigen-specific encephalitogenic T cells as well as host-derived bystander T cells and monocytes. In an effort to study encephalitogenic T cell chemokine receptor expression, we examined CC chemokine receptor expression from resting, activated, and CNS-isolated CD4+ T cells. CCR1, CCR2, CCR3, CCR5, CCR6, CCR7, and CCR8 mRNA is expressed by normal CD4+ T cells. In vitro activated T cells expressed CCR1, CCR2, CCR3, CCR5, CCR6, CCR7, and CCR8 mRNA as well as CCR4. After EAE induction, CCR1 mRNA was expressed by donor-derived encephalitogenic and host-derived CD4+ T cells isolated only from CNS and not from spleen. In vivo neutralization of the CCR1 ligand, macrophage inflammatory protein-1α (CCL3), resulted in less encephalitogenic CD4+ T cell CNS infiltration. These results demonstrate the importance of CC chemokine receptor expression by CD4+ encephalitogenic T cells for CNS infiltration and subsequent disease development. J. Neurosci. Res. 66:705–714, 2001. © 2001 Wiley-Liss, Inc.
Experimental autoimmune encephalomyelitis (EAE) is a CD4+ Th1 cell-mediated inflammatory demyelinating disease of the central nervous system (CNS) that serves as a model for the human demyelinating disease multiple sclerosis (MS) (Hohlfeld, 1997). EAE can be induced in SJL mice by immunization with proteolipid protein (PLP) or the immunodominant encephalitogenic peptide sequence 139–151 (PLP139–151) emulsified in complete Freund's adjuvant (CFA) (Tuohy et al., 1992). Alternatively, EAE can be adoptively transferred to normal recipient mice by antigen-activated PLP139–151-specific T cells (Whitham et al., 1991; Kuchroo et al., 1992). Immunohistological analysis of mononuclear cell infiltration in the CNS has revealed that antigen-specific and nonspecific CD4+ and CD8+ T cells as well as macrophages constitute the recruited cell population, with little or no polymorphonuclear cell infiltration (Hickey et al., 1983). The pathogenic mechanisms of disease development include Ag-specific T cell activation and Th1 differentiation (Segal and Shevach, 1996), followed by transmigration into tissue compartments, including the CNS (Hickey et al., 1991).
The entry of activated T cells into tissue compartments, including the CNS, is a process governed by both integrin-mediated adhesions as well as chemokine-mediated migration. VLA-4 (Yednock et al., 1992), LFA-1 (Kobayashi et al., 1995), and ICAM-1 (Morrissey et al., 1996) have all been demonstrated as important adhesion molecules regulating the pathogenesis of EAE. In addition, several chemokine-specific parameters have recently been implicated in the migration of lymphocytes into the CNS and the subsequent development of EAE. Anti-MIP-1α (CCL3) and anti-MCP-1 (CCL2) treatment has been shown to inhibit the acute and relapsing phases of disease, respectively (Kennedy et al., 1998). Mice deficient for CCR2 failed to develop EAE as a result in defective monocyte trafficking to the CNS (Fife et al., 2000), and mice deficient for CCR1 expression developed an attenuated form of EAE (Rottman et al., 2000). CCR5 expression does not appear to play a role in the development of EAE, in that mice deficient for this receptor developed disease comparable to that in control mice (Tran et al., 2000).
EAE studies using chemokine receptor knockout mice have demonstrated a role for CC chemokine receptor expression by macrophages in the development of clinical disease (Fife et al., 2000); however, encephalitogenic T cell chemokine receptor expression during ongoing EAE has not been reported. In the present study, we investigated chemokine receptor expression on normal, activated, and CNS tissue-derived infiltrating T cells during EAE induction in an effort to understand the mechanism of mononuclear cell trafficking and accumulation during the development and progression of autoimmune encephalomyelitis.
MATERIALS AND METHODS
Mice
Female SJL mice (H-2s) were purchased from Harlan Sprague Dawley (Indianapolis, IN). Congenic SJL.Thy1a mice (H-2s; Skundric et al., 1993) were received from Dr. Harley Tse (Wayne State University, Detroit, MI) and subsequently bred and maintained at Northwestern University Medical School (Chicago, IL). Mice were 6–7 weeks old at the initiation of the experiment and were maintained on standard laboratory chow and water ad libitum. Animals were housed under specific pathogen-free, barrier facility conditions. Animal care was provided in accordance with the Northwestern University and NIH guidelines.
Antigens
PLP139–151 (HSLGKWLGHPDKF) was purchased from Peptides International (Louisville, KY). The amino acid composition was verified my mass spectrometry and purity (>98%) was assessed by HPLC.
Priming of Donor Lymphocytes, Cell Culture, and Transfer of EAE
Donor SJL.Thy1a mice were primed by s.c. immunization of 50 μg of PLP139–151 emulsified in CFA containing 4 mg/ml Mycobacterium tuberculosis (Difco, Detroit, MI) Seven days later, draining lymph node cells were pooled and cultured in vitro for 72 hr in complete Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY) containing 5 × 10–5 M 2-ME (Gibco), 2 mM L-glutamine (Gibco), 100 U/ml penicillin (Gibco), 100 μg/ml streptomycin (Gibco), 0.1 M nonessential amino acids (Gibco), and 10% fetal calf serum (FCS; Hyclone, Logan, UT) at 6 × 106 cells/ml in the presence of 50 μg/ml PLP139–151. Cells were incubated at 37°C in a humidified atmosphere containing 7.5% CO2. The cells were harvested after 72 hr in culture and washed, and 5 × 106 viable T cell blasts were transferred i.v. to normal SJL recipients. Subsequent to cell transfer, mice were evaluated for the development of EAE.
Clinical Evaluation
Adoptive EAE was induced by the transfer of 5 × 106 in vitro-stimulated PLP139–151-specific T cell blasts from PLP139–151 peptide-primed mice. Individual animals were observed daily and graded according to their clinical severity as follows: grade 0, no abnormality; grade 1, limp tail; grade 2, limp tail and hind limb weakness (waddling gait); grade 3, partial hind limb paralysis; grade 4, complete hind limb paralysis; grade 5, death. A relapse was scored when a mouse developed additional neurological deficits (≥1 clinical grade) following a period of stabilization or improvement.
Isolation of CNS-Infiltrating Mononuclear Cells, Peripheral Blood Mononuclear Cells, Lymph Node Cells, and Splenocytes
Mice were anesthetized with methoxyflurane (Schering-Plough, Union, NJ) and perfused through the left ventricle with ∼60 ml of phosphate-buffered saline (PBS). Spinal cords were extruded by flushing the vertebral canal with PBS and rinsed in PBS. Prior to perfusion, ∼500 μl blood was collected from the left ventricle using a 25 gauge needle and a heparinized 1 cc syringe (0.1 ml of 100 U/ml; Sigma Chemical Co., St. Louis, MO). Lymph nodes and spleens were removed from the same mice and placed in Hank's balanced salt solution (HBSS; Gibco). Tissues were forced through 100-mesh stainless steel screens to give a single cell suspension. Red blood cells (RBCs) in the spleen cell preparations were lysed by hypotonic shock in Tris-NH4Cl, and the cells were washed and resuspended in HBSS. CNS mononuclear cells were isolated by centrifugation (500g) at 24°C over a 30%/70% discontinuous Percoll (Pharmacia, Piscataway, NJ) gradient. Cells were collected from the 30%/70% interface, washed in HBSS, and resuspended in isotonic buffered saline (IBS; Baxter Diagnostics, McGaw Park, IL) containing 0.1% NaN3 (Sigma Chemical Co.) and 0.2% donkey serum (Jackson Immunoresearch Laboratories, West Grove, PA).
Antibodies
Monoclonal antibodies to murine CD90.1 (clone HIS51), CD90.2 (clone 53-2.1), CD3 (clone 145-2C11), CD4 (clone RM4-5), and CD16/32 (clone 2.4G2; anti-mouse FcRγII/III) were purchased from PharMingen (San Diego, CA). Monoclonal antibodies to murine F4/80 were purchased from Caltag Laboratories (Burlingame, CA). Polyclonal goat antibodies to murine CCR1, -2, -3, -4, and -5 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal donkey anti-goat F(ab′)2 PE antibodies were purchased from Jackson Immunoresearch Laboratories. Isotype control antibodies were purchased from PharMingen and R&D Systems (Minneapolis, MN). Anti-CCL3 was generated as previously described (Karpus et al., 1995).
Flow Cytometry
Cells (0.5–1 × 106) were incubated with CD16/32, anti-mouse FcRγII/III, for 15 min at 4°C in IBS to block Fc-mediated binding. Cells were washed in IBS, followed by incubation with specific polyclonal goat antibodies to murine CCR1, -2, -3, and -4 at a predetermined optimal concentration for 15 min at 4°C. As a control, parallel populations of cells were incubated in the presence of isotype-matched control antibodies (R&D Systems). Cells were washed twice in IBS, followed by incubation with polyclonal donkey anti-goat F(ab′)2 PE antibodies in combination with directly conjugated CD90.1 fluorescein isothiocyanate (FITC), CD90.2 APC, and CD4 PerCP or F4/80 FITC for 15 min at 4°C in the dark. Cells were washed and resuspended in 0.5 ml IBS. Data collection and analysis were performed on a FACSCalibur (Becton Dickinson, San Jose, CA) flow cytometer using Cellquest software with 5 × 104 events/analysis.
Cell Sorting
Splenocytes and infiltrating mononuclear cells were isolated from the CNS as described above and were incubated with CD16/32 at 1 × 106 cells/ml for 15 min at 4°C in IBS to block Fc-mediated binding. Cells were washed in IBS, followed by incubation with directly conjugated CD90.1 FITC and CD4 PE monoclonal antibodies for 15 min at 4°C in the dark at a predetermined optimal concentration. Cells were washed twice in IBS and resuspended in IBS at final concentration of 1 × 106 cells/ml for the CNS and 1 × 107 cells/ml for the spleen cells. CD4+CD90.1+ and CD4+CD90.1– cells were sorted from the CNS and spleen cell preparations using an EPICS Elite cell sorter (Beckman Coulter, Fullerton, CA). Cells were collected in HBSS supplemented with 2% FCS, pelleted, lysed in 4 M guanidine isothiocyanate (Gibco), and stored at –70°C for RNA isolation. After cell sorting, an aliquot of cells from each tube was analyzed for percentage purity by flow cytometry. All sorted populations had ≥98% purity.
Chemokine Receptor RT-PCR
Total RNA was isolated from the sorted cells using the RNeasy kit following the manufacturer's suggested protocol (Qiagen, Valencia, CA). Total RNA was quantitated by measuring A260/A280 absorbance. RNA was DNAse treated using RQ1 RNAse-free DNAse following the manufacturer's protocol (Promega, Madison, WI). Equal amounts of total RNA were used for the synthesis of cDNA using the FirstStrand cDNA synthesis kit from Clonetech (Palo Alto, CA) following the manufacturer's suggested protocol. PCR was performed using a Perkin Elmer 9600 thermocycler (Norwalk, CT). PCR products were visualized by ethidium bromide agarose gel electrophoresis using a 2% agarose gel (Gibco). Glucose-3-phosphate dehydrogenase (G3PDH), interleukin (IL)-4, and interferon (IFN)-γ primers were purchased from Clontech. Unique primers for CCR6 and CCR8 were designed from sequences provided by National Center for Biotechnology Information (NCBI) and were determined to be specific and not to contain similar sequences or cross react with other known murine CC, CXC, C, or CX3C chemokine receptors. CCR primer sets were synthesized by Gibco. PCR conditions for G3PDH, IL-4, and IFN-γ were as follows: 94°C for 3 min, followed by 40 cycles of 45 sec at 94°C, 45 sec at 62°C, and 1 min at 72°C, with a final extension at 72°C for 3 min. PCR conditions for CCR1–4 and CCR6–8 were as follows: 94°C for 3 min, followed by 40 cycles of 30 sec at 94°C, 30 sec at 62°C, and 2 min at 72°C, with a final extension at 72°C for 3 min. PCR conditions for the CCR5 primer set were 94°C for 3 min, followed by 40 cycles of 30 sec at 94°C, 30 sec at 55°C, and 1 min at 72°C, with a final extension at 72°C for 3 min. Primer sequences used in this study were: CCR1 (546 base pairs) sense 5′-AGCCTACCCCACAACTACAGAA-3′, antisense 5′-CTTGTAGGGGAAATGAGGGCTA-3′ (Tanabe et al., 1997); CCR2 (253 base pairs) sense 5′-GGTCATGATCCCTATGTGG-3′, antisense 5′-CTGGGCACCTGATTTAAAGG-3′ (Boring et al., 1997); CCR3 (383 base pairs) sense 5′-TGGGCAACATGATGGTTGTG-3′, antisense 5′-GCTGTCTTGAGACTCATGGA-3′ (Ruth et al., 1998; Oliveira and Lukacs, 2001); CCR4 (1,090 base pairs) sense 5′-CCAAAGATGAATGCCACAGAG-3′, antisense 5′-CCTTACAAAGCGTCACGGAAG-3′ (Tanabe et al., 1997); CCR5 (362 base pairs) sense 5′-GCTGAAGAGCGTGACTGATA-3′, antisense 5′-GAGGACTGCATGTATAATGA-3′ (Oliveira and Lukacs, 2001); CCR6 (650 base pairs) sense 5′-GGGCAACATTATGGTGGTGATGAC-3′; antisense 5′-ACCGCAGTCACGAGGAGGACCATG-3′; CCR7 (344 base pairs) sense 5′-ACAGCGGCCTCCAGAAGAACAGCGG-3′; antisense 5′-TGACGTCATAGGCAATGTTGAGCTG-3′ (Yanagihara et al., 1998); CCR8 (424 base pairs) sense 5′-CGATGGAGCCCAACGTCACG-3′; antisense 5′-GGCCGTCCTCACCTTGATGGC-3′; and G3PDH (452 base pairs) sense 5′-ACCACAGTCCATGCCATCAC-3′, antisense 5′-TCCACCACCCTGTTGCTGTA-3′. Positive and negative controls were included in each RT-PCR. Positive control for IL-4 and IFN-γ was purchased from Clonetech. Positive control RNA used for CCR RT-PCR was from 16 hr anti-CD3-stimulated SJL splenocytes. A negative control without RT added was included for each target analyzed. Negative control RNA used was an RT– sample from 16 hr anti-CD3-stimulated SJL splenocytes.
RESULTS
Chemokine Receptor mRNA Expression by Activated CD4+ T Cells
An essential step during tissue-specific inflammation is the chemokine-induced recruitment of inflammatory cells (Oppenheim et al., 1991; Springer, 1994). Chemokine expression during the initiation and progression of EAE has previously been demonstrated for several different mouse strains (Glabinski et al., 1995, 1998; Godiska et al., 1995; Karpus and Kennedy, 1997; Karpus and Ransohoff, 1998; Juedes et al., 2000), including SJL mice, following adoptive transfer of encephalitogenic T cells (Karpus et al., 1995; Karpus and Kennedy, 1997). In an effort to understand better the role of CC chemokine receptor expression during the pathogenesis of EAE, we examined peripheral CD4+ T cells from normal, unprimed mice, draining lymph node CD4+ T cells isolated from PLP139–151 antigen-primed mice, and draining lymph node CD4+ T cells from PLP139–151 antigen-primed mice after in vitro PLP139–151 antigen-specific activation. Specifically, we examined mRNA expression for CC chemokine receptors by RT-PCR. The results shown in Figure 1 demonstrate CCR1, -2, -3, -5, -6, -7, and -8 mRNA expression by normal lymph node-derived CD4+ T cells. CD4+ lymph node T cells from PLP139–151-primed mice also expressed CCR1, -2, -3, -5, -6, -7, and -8 mRNA. Although the RT-PCR technique used in these experiments is not quantitative, it is clear from Figure 1 that antigen reactivation of PLP139–151-primed CD4+ T cells results in up-regulation of CCR4 mRNA expression. It also appears that CCR1 mRNA expression may be up-regulated as well.
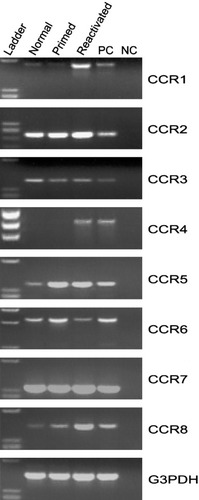
CC chemokine receptor mRNA expression by normal, antigen-primed, and antigen-primed and reactivated T cells. Data show CCR1–8 mRNA expression from normal inguinal and periaortic lymph node, draining lymph node, and in vitro-activated draining lymph node CD4+ T cells. Positive control (PC) and negative control (NC) are included for each primer set. The data shown are representative of three independent experiments.
Chemokine Receptor Protein Expression by CD4+ T Cells Following T Cell Activation
We next analyzed CD4+ T cell-specific CCR1–4 protein expression following T cell activation. The rationale for choosing to examine CCR1–4 protein expression included limited polyclonal antibody availability as well as the apparent up-regulation of CCR1 and CCR4 mRNA following T cell activation (Fig. 1). CCR1–4 protein expression was assessed by flow cytometry on lymph node-derived CD4+ T cells from normal, unprimed mice. The results shown in Figure 2A indicate a basal level of CCR2 and CCR3 expression, with minimal CCR1 and CCR4 expression by normal, resting CD4+ lymph node cells. We next examined ex vivo CCR expression by CD4+ T cells from PLP139–151 antigen-primed mice. The results shown in Figure 2B indicate that, after primary PLP139–151-specific T cell stimulation, CCR2 and -3 are expressed, with minimal CCR1 and -4 expression, similarly to normal CD4+ T cells (Fig. 2A). We examined CCR expression on CD4+ T cells from PLP139–151-primed mice following secondary, in vitro restimulation with PLP139–151. The results shown in Figure 2C demonstrate substantial CCR1–4 expression. These latter results are potentially important; it is this reactivated CD4+ T cell population that is adoptively transferred to induce passive EAE. Although polyclonal anti-CCR5 antibody is available, the staining procedure never yielded reproducible results by flow cytometry (data not shown). These data indicate differential chemokine receptor expression by T cells after activation that may contribute to their accumulation in the target organ during the pathogenesis of disease.
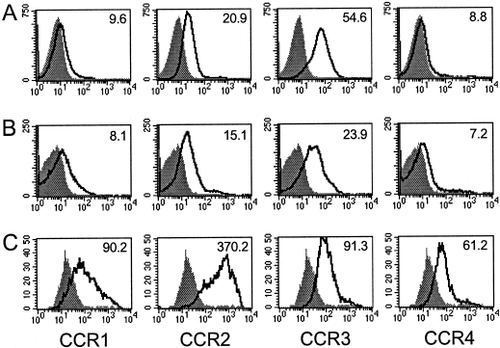
CC chemokine receptor protein expression on normal, antigen-primed, and antigen-primed and reactivated cells. A: CCR1–4 protein expression on inguinal and periaortic lymph node-derived CD4+ T cells from normal mice. B: CCR1–4 protein expression on CD4+ T cells from antigen-primed mice. C: CCR1–4 protein expression on CD4+ T cells from antigen-primed mice subsequently in vitro antigen reactivated. Cells were washed and stained with antibodies for CCR1–4 and analyzed by flow cytometry for protein surface expression. The x-axis represents the mean fluorescence intensity of each antibody, and the y-axis represents relative cell counts. The bolface line represents mean fluorescence intensity for each CCR, and the shaded area represents background isotype control staining. The numbers in the individual histograms refer to the increase in the geometric mean fluorescent intensity (ΔGMFI) as a measurement for increased specific staining. The data shown are representative of three independent experiments.
Selective CCR1 Expression by CNS-Derived CD4+ T Cells During Acute EAE
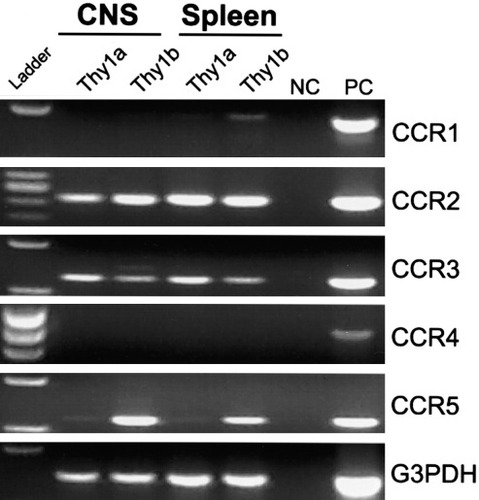
Because CCR1 and CCR4 were substantially up-regulated by CD4+ T cells following PLP139–151 in vitro reactivation, we asked whether these receptors were also expressed by encephalitogenic CD4+ T cells in the CNS during acute clinical EAE. To identify encephalitogenic CD4+ T cells in the CNS of SJL mice with EAE, we utilized the previously described SJL.Thy1a congenic mouse system that would allow us to track antigen-activated CD4+Thy1a+ T cells using an adoptive EAE regimen in a normal Thy1b+ host SJL mouse (Skundric et al., 1993). SJL.Thy1a congenic mice were primed with PLP139–151 in CFA, draining lymph node cells were isolated and restimulated in vitro with PLP139–151, and viable CD4+Thy1a+ T cell blasts were transferred intravenously to normal SJL recipients. Although these cells were not antigen-specific clones, they were highly enriched for antigen-specific, IFN-γ-producing CD4+ T cells as measured by flow cytometric intracellular cytokine expression (data not shown). This experimental design allowed us to sort CD4+Thy1a+ donor-derived disease-inducing T cells separately from CD4+Thy1b+ host-derived T cells isolated from various tissue compartments. We examined CCR mRNA expression by flow cytometric sorted CD4+ T cells isolated from both the CNS and the spleen at the peak of acute EAE, day 14 postadoptive transfer. Specifically, CCR1–5 expression by RT-PCR was examined. At the peak of acute EAE, encephalitogenic CD4+Thy1a+ donor T cells isolated from the CNS expressed mRNA for CCR1–5 (Fig. 3). Encephalitogenic CD4+Thy1a+ cells isolated from the spleens of the same animals expressed CCR2–5, but not CCR1 (Fig. 3). At the peak of acute EAE, CNS-infiltrating host-derived CD4+Thy1b+ cells expressed CCR1–5 mRNA (Fig. 3). CD4+Thy1b+ host-derived cells isolated from the spleen expressed CCR2–4, but not CCR1 or -5 mRNA (Fig. 3). CCR1 was the only receptor mRNA detected in cells infiltrating the CNS that was not expressed by peripheral splenic donor or host CD4+ T cells (Fig. 3). CCR1 is one of the receptors for the ligand CCL3 (Murphy et al., 2000) and has been implicated as an important chemokine receptor controlling the selective recruitment of cells into inflammatory lesions during the development of EAE (Rottman et al., 2000; Liang et al., 2000). Our results demonstrate differential chemokine receptor expression by CNS-infiltrating T cells and, more significantly, that there is selective expression of a subset of CCL3 receptors on CNS-accumulating donor and host CD4+ T cells during the pathogenesis of disease.
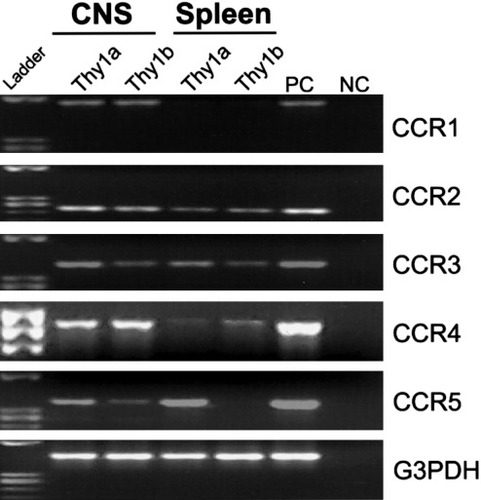
Differential CCR mRNA expression by CNS-infiltrating CD4+ cells. EAE was induced by the adoptive transfer of SJL.Thy1a PLP139–151-specific T cell blasts to naive SJL (Thy1b) mice. Animals were allowed to develop acute disease symptoms. CD4+Thy1a+ and CD4+Thy1b+ cells were sorted by flow cytometry from the CNS infiltrate and spleen at peak acute clinical disease, day 14 postadoptive transfer. CCR1–5 mRNA expression was examined by RT-PCR. Positive control (PC) and negative control (NC) are included for each primer set. The data shown are representative of two independent experiments.
Chemokine Receptor Protein Expression on CD4+ T Cells During Clinical EAE
Given the differential regulation of CCR expression in T cell antigen activation (Figs. 1-3), we next analyzed the protein expression on CNS-infiltrating mononuclear cells during clinical disease. To examine this, disease was induced by the adoptive transfer of SJL.Thy1a PLP139–151-specific T cell blasts to normal SJL (Thy1b) mice as previously described. Two days posttransfer, representative mice were analyzed for the CCR1–4 protein expression on adoptively transferred encephalitogenic CD4+Thy1a+ cells isolated from the peripheral blood and spleen (data not shown). Circulating CD4+Thy1a+ T cells from the peripheral blood had subpopulations of cells with high levels of CCR1–3 protein expression, whereas CD4+Thy1a+ cells accumulating in the spleen 2 days posttransfer did not exhibit increased CCR expression (data not shown). It is important to note that disease-initiating CD4+Thy1a+ cells can be detected in the CNS 2 days posttransfer, but insufficient numbers were present to perform CCR1–4 flow cytometric analysis (data not shown). We next examined CCR1–4 expression on CNS-infiltrating CD4+Thy1a+ encephalitogenic T cells at the peak of acute EAE, day 14 postadoptive transfer, and the results shown in Figure 4A indicate that the disease-inducing PLP139–151-specific CD4+Thy1a+ T cells predominantly expressed CCR1. CCR2–4 staining was not above background. CCR1–4 protein expression on CD4+Thy1a+ encephalitogenic donor cells isolated from the spleens of recipient mice (Thy1b) during acute disease exhibited no substantial staining for chemokine receptors above background (Fig. 4B). At this same time during clinical disease, CCR1–4 protein expression on CNS-infiltrating CD4+Thy1b+ host-derived T cells was examined. Subpopulations of CD4+Thy1b+ cells in the CNS were positive for CCR1–4 (Fig. 4C). Cells isolated from the spleens of the same mice did not have significant CCR1, -3, or -4 expression but did have CCR2 expression (Fig. 4D). These results demonstrate differential CCR1 expression on CNS-infiltrating T cells isolated during the clinical disease. CCR1 was not expressed on CD4 T cells isolated from the spleen of the same mice.
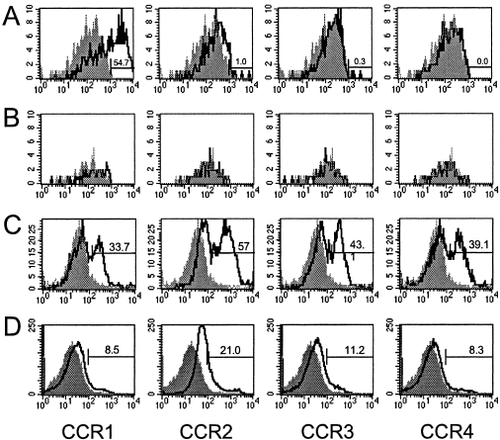
CCR1 protein expression by encephalitogenic, PLP139–151 antigen-specific CD4+Thy1a+ cells isolated from acute EAE mice. Disease was induced by the adoptive transfer of SJL.Thy1a PLP139–151-specific T cell blasts to normal SJL (Thy1b) mice. Animals were allowed to develop peak acute clinical disease, day 14 postadoptive transfer, when CNS-infiltrating CD4+Thy1a+ cells were analyzed for CCR expression by flow cytometry. A: CCR1–4 protein expression on encephalitogenic donor CD4+Thy1a+ cells isolated from the CNS of Thy1b recipient animals. B: CCR1–4 protein expression on encephalitogenic donor CD4+Thy1a+ cells isolated from the spleens of Thy1b recipient animals. C: CCR1–4 protein expression on host CD4+Thy1b+ cells isolated from the CNS of Thy1b recipient animals. D: CCR1–4 protein expression on host CD4+Thy1b+ cells isolated from the spleens of Thy1b recipient animals. The x-axis represents the mean fluorescence intensity of each antibody, and the y-axis represents relative cell counts. The boldface line represents mean fluorescence intensity for each CCR, and the shaded area indicates background isotype control antibody staining. The data shown are representative of two independent experiments.
Chemokine Receptor Expression on CD4+ T Cells During Relapsing EAE
We next examined CCR mRNA expression on CNS-infiltrating T cells to determine whether there was differential CCR expression during relapsing EAE. At the peak of relapsing EAE, day 35 postadoptive transfer, CD4+ T cells were isolated from both the CNS and the spleen and analyzed for CCR mRNA expression. Specifically, CCR1–5 expression was examined by RT-PCR. At the peak of relapsing EAE, encephalitogenic CD4+Thy1a+ donor T cells isolated from the CNS expressed mRNA for CCR2, -3, and -5 (Fig. 5). Encephalitogenic CD4+Thy1a+ cells isolated from the spleens of the same animals expressed the same subset of receptors, CCR2, -3, and -5 (Fig. 5). At the peak of relapsing EAE, CNS-infiltrating host-derived CD4+Thy1b+ cells expressed CCR2, -3, and -5 mRNA (Fig. 5). CD4+Thy1b+ host-derived cells isolated from the spleen expressed CCR1, -2, -3, and -5 mRNA (Fig. 5). These results indicate that there were no significant differences in CCR2–5 expression from donor or host-derived cells from either the CNS or the spleen. These results demonstrate the lack of CCR1 expression, suggesting a differential expression pattern compared to acute EAE.
CCR mRNA expression by CNS-infiltrating CD4+ cells during relapsing EAE. EAE was induced by the adoptive transfer of SJL.Thy1a PLP139–151-specific T cell blasts to naive SJL (Thy1b) mice. Animals were allowed to develop relapsing disease symptoms. CD4+Thy1a+ and CD4+Thy1b+ cells were sorted by flow cytometry from the CNS infiltrate and spleen at peak relapsing clinical disease, day 35 postadoptive transfer. CCR1–5, mRNA expression was examined by RT-PCR. Positive control (PC) and negative control (NC) are included for each primer set. The data shown are representative of two independent experiments.
CNS-Infiltrating Cells Produce Proinflammatory Cytokines
Additional experiments were performed to correlate Th1 effector function with CCR expression and subsequent chemokine-mediated infiltration. We would expect, given the findings that EAE is a CD4+ Th1-mediated demyelinating disease of the CNS, the infiltrating cells to produce a Th1-type cytokine profile. We asked whether sorted CNS-infiltrating CD4+ cells expressed mRNA for the proinflammatory cytokine IFN-γ, or the antiinflammatory cytokine IL-4 directly ex vivo, without antigen restimulation. Encephalitogenic donor CD4+Thy1a+ and host CD4+Thy1b+ cells were isolated from the CNS and spleen during peak clinical EAE, day 14 postadoptive transfer, and examined for IL-4 and IFN-γ cytokine expression by RT-PCR. CD4+Thy1a+ and CD4+Thy1b+ cells from the CNS expressed IFN-γ mRNA but not IL-4 (Fig. 6). In contrast, CD4+Thy1a+ and CD4+Thy1b+ cells sorted from the spleen expressed IL-4 but not IFN-γ mRNA (Fig. 6). These results demonstrate effector cytokine production by cells accumulating in the CNS that also exhibit differential CCR1 expression during clinical disease.
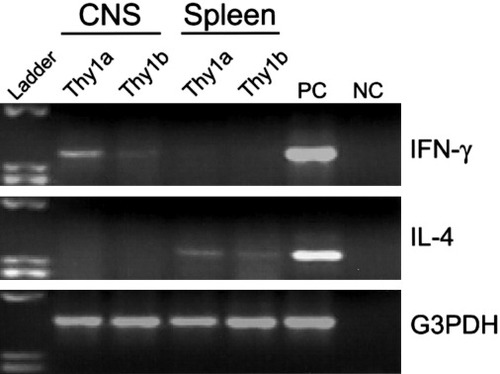
Infiltrating CD4+ cells express IFN-γ but not IL-4 mRNA in the CNS during acute EAE. IL-4, IFN-γ, and G3PDH mRNA expression by RT-PCR was examined on CD4+Thy1a+ and CD4+Thy1b+ cells sorted from the CNS and spleen during the peak of acute disease, day 14 postadoptive transfer. CD4+Thy1a+ and Thy1b+ cells from the CNS expressed IFN-γ mRNA but not IL-4. In contrast, CD4+Thy1a+ and Thy1b+ cells sorted from the spleen expressed IL-4 but not IFN-γ mRNA. Positive control (PC) and negative control (NC) are included for each primer set. The data shown are representative of two independent experiments.
Anti-CCL3 Treatment Decreases Encephalitogenic T Cell Accumulation in the CNS
Flow cytometric analysis was performed on infiltrating cells isolated from the CNS and spleen during the peak of acute EAE from mice treated with anti-CCL3 or control normal rabbit serum (NRS). EAE was induced by adoptive transfer of CD4+Thy1a+ PLP139–151-specific blasts into normal SJL Thy1b+ animals. At the peak of acute EAE, day 10 postadoptive transfer, cells were isolated from the CNS and spleen and stained for surface expression of CD4 and either Thy1a or Thy1b. Results shown in Figure 7A demonstrate a 22-fold reduction in the percentage of CD4+Thy1a+ encephalitogenic T cells accumulating in the target tissue of recipients that received anti-CCL3 treatment. There was a 13-fold reduction in the percentage of host-derived CD4+Thy1b+ T cells accumulating in the CNS following anti-CCL3 treatment (Fig. 7A). There were no significant differences in the donor or host CD4+ T cell percentages in the spleens of these same animals (Fig. 7B). Similarly to our previous findings, anti-CCL3 treatment did not alter the antigen-specific T cell-proliferative or cytokine responses (data not shown). Collectively, the results from these experiments demonstrate differential CCR1 expression by CNS-derived encephalitogenic CD4+ T cells and that in vivo inhibition of CCL3, a ligand for CCR1, reduces the accumulation of encephalitogenic CD4+ T cells in the CNS during acute EAE.
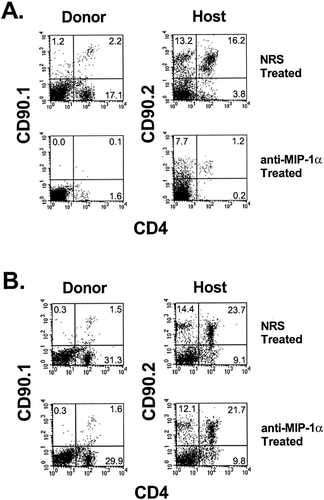
Anti-CCL3 antibody treatment decreases the accumulation of CD4+ T cells in the CNS. Flow cytometric analysis was performed on infiltrating cells isolated from the CNS and spleen during the peak of acute EAE from mice treated with anti-MIP-1α antibody or normal rabbit serum (NRS). EAE was induced by adoptive transfer of Thy1a+ PLP139–151-specific blasts into normal SJL (Thy1b) animals. Nine animals per group were treated intraperitoneally at day 0 and day 2 postadoptive transfer with 0.5 ml anti-CCL3 or NRS as control antibody treatment. After 10 days, cells were isolated from the CNS and spleen from both groups. Cells were stained for surface expression of CD4 and either Thy1a or Thy1b. A: Analysis of CNS-infiltrating T cells. B: Analysis of splenic T cells. The percentage of doubly positive cells is indicated as a percentage of total cellular infiltrate determined by forward- and side-scatter cell characteristics. The data shown are representative of two independent experiments.
DISCUSSION
EAE is a CD4+ Th1-mediated autoimmune disease model for MS and is characterized by CNS-infiltrating monocytes and lymphocytes, resulting in a relapsing, remitting paralytic disease in the appropriate mouse strains. Regulation of disease pathogenesis includes migration of activated T cells from peripheral lymphoid tissues into the CNS. In the present study we demonstrate selective CC chemokine receptor expression by CNS-infiltrating, encephalitogenic CD4+ T cells. Specifically, CCR1 mRNA expression is restricted to CNS- and not splenic-infiltrating CD4+Thy1a+ encephalitogenic and CD4+Thy1b+ T cells (Fig. 3). We also have provided the first evidence for CCR1 ligand (CCL3) expression directly regulating the accumulation of encephalitogenic CD4+ T cells in the CNS (Fig. 7). These results have led us to conclude that the combination of CCR1 expression by encephalitogenic T cells and CNS CCL3 expression are key contributors to acute disease pathogenesis by regulating disease-inducing T cell accumulation.
Several recent studies using genetically deficient mice have shown that CCR1 (Rottman et al., 2000) and CCR2 (Fife et al., 2000) expression is important for the development of acute EAE. In the CCR1 knockout mice, there was approximately a 50% decrease in clinical disease severity; however, the mechanism behind this attenuation was not explored. Insofar as both T cells and monocytes have been shown to express CCR1 (Gao et al., 1997), it is possible that CCR1 expression by either lymphocytes or monocytes or perhaps both is required for EAE development. In the CCR2 knockout mice, there was almost a total absence of disease because of a failure of monocytes to traffic to the CNS (Fife et al., 2000). These two examples are in contrast to EAE induction in CCR5 knockout mice, in which the same level of disease severity was seen as in wild-type control animals (Tran et al., 2000). The current data support a role for CCR1 in the development of EAE by suggesting that T cell accumulation occurs, at least in part, through the activation of CCR1 by appropriate ligands. The fact that anti-CCL3 treatment inhibits both encephalitogenic T cell accumulation and clinical disease in SJL mice indicates that CCR1 and/or CCR5 play an important role in the pathogenesis of disease. Because CCR1, and not CCR5, is expressed only by CNS-infiltrating cells (Fig. 3) and CCR5-deficient mice show EAE severity equal to that in controls (Tran et al., 2000), it is reasonable to postulate that CCL3 in our model functions primarily through CCR1 and not CCR5. Indeed, a low-molecular-weight antagonist of CCR1 has shown efficacy in the inhibition of clinical EAE (Hesselgesser et al., 1998; Liang et al., 2000).
In light of these observations, we propose the following model of EAE pathogenesis. Antigen-specific T cell activation induces clonal expansion and proinflammatory cytokine expression, which potentially serves to up-regulate CCR1 expression (Bonecchi et al., 1999; Colantonio et al., 1999). After Th1 activation and differentiation, a process understood in experimental models but not in MS, these lymphocytes enter the blood. Once in the blood, activated cells interact via adhesion molecules with vascular endothelial cells that make up the blood vessels. The initial interactions between activated T cells and cerebral vascular endothelial cells are not well understood; however, encephalitogenic T cells have been shown to secrete lymphotoxin (LT) and tumor necrosis factor-α (TNF-α), which in turn may activate endothelial cells to increase expression of ICAM-1 and VCAM-1 (Powell et al., 1990; Rosenman et al., 1995; Lou et al., 1996). Activated T cells express VLA-4 (CD49d/CD29) and LFA-1 (CD11a/CD18), which bind to VCAM-1 (CD106) and ICAM-1 (CD54) on endothelium (Yednock et al., 1992; Baron et al., 1993). The interactions of these integrins and adhesion molecules is important for the development of EAE, because disease pathogenesis can be inhibited by using blocking antibodies to the integrins VLA-4 (Yednock et al., 1992) and LFA-1 (Kobayashi et al., 1995) as well as the adhesion molecule ICAM-1 (Morrissey et al., 1996). In addition to T cell infiltration, CNS monocyte infiltration is a critical parameter in the pathogenesis of demyelinating disease, as demonstrated by depletion studies. Inability to express CCR2 results in a failure of monocytes to accumulate in the CNS, thereby significantly attenuating clinical and histological demyelinating disease.
It is not well understood whether chemokines are necessary for the initial transendothelial migration and infiltration of lymphocytes and monocytes into the CNS. However, there is ample evidence that a component of the tissue-specific autoimmune inflammatory disease pathogenesis includes chemotactic-induced recruitment of leukocytes into the specific tissue. Many reports have previously demonstrated descriptive and functional relationships between production of chemokines in the CNS and development of acute and/or relapsing EAE in a variety of mouse strains (Hulkower et al., 1993; Ransohoff et al., 1993; Godiska et al., 1995; Karpus et al., 1995; Glabinski et al., 1997, 1999; Kennedy et al., 1998; Juedes et al., 2000; Matejuk et al., 2000; Rajan et al., 2000). The current results shown in Figure 7 demonstrate a 22-fold reduction in the percentage of CD4+Thy1a+ encephalitogenic T cells accumulating in the target tissue when recipients were treated with anti-CCL3, a CCR1 ligand. These results do not directly demonstrate a role for chemokines and chemokine receptors in the initial migration of T cells into the CNS; however, they support the idea that chemokines and their specific receptors contribute to the accumulation of pathogenic T cells in the CNS, thereby regulating disease progression.
A question arises of whether chemokines and their receptors are similarly associated with MS pathogenesis. Several reports examining either chemokine expression in the cerebrospinal fluid or the parenchymal tissue have demonstrated the presence of chemokines, including CCL3 (Miyagishi et al., 1995), in the CNS (Hvas et al., 1997; McManus et al., 1998; Simpson et al., 1998; Van et al., 1999; Sorensen et al., 1999; Balashov et al., 1999). Likewise, increases in chemokine receptor expression have also been noted in the cerebrospinal fluid and CNS of MS patients (Sorensen et al., 1999; Balashov et al., 1999; Simpson et al., 2000a,b). The expression of chemokines in the CNS during episodes of clinical MS and the corresponding increase of specific chemokine receptor-positive cells raise the possibility of receptor-specific treatment modalities (Ransohoff and Bacon, 2000). The efficacy of such treatment strategies would depend on identification of restricted receptor expression, such as the differential CCR1 expression we have shown in the present study.
Acknowledgements
This study was supported by NIH grants T32 AI07476 (B.T.F.), R01 NS34510 (W.J.K.), and R01 AI35935 (W.J.K.).




