Reduced phospholipase C-β activity and isoform expression in the cerebellum of TS65DN mouse: A model of down syndrome
Abstract
Agonist- and guanine-nucleotide-stimulated phospholipase C-β (PLC) activity was characterized in crude plasma membrane preparations from cerebral cortex, hippocampus and cerebellum of Ts65Dn mice, a model for Down syndrome, and their control littermates. The levels of expression of PLC-β(1–4) isoforms and G-protein αq/11 subunits were also quantified by Western blot analysis to establish their contribution to the patterns of PLC functioning. PLC activity regulated by G-proteins and muscarinic and 5-HT2 receptors presented a regional distribution in both control and Ts65Dn mice. In both groups of mice, the intensity of PLC responses to maximal activation by calcium followed the sequence cerebellum > cortex > hippocampus. Both basal and maximal PLC activities, however, were significantly lower in cerebellar membranes of Ts65Dn than in control mice. This difference was mostly revealed in crude plasma membranes prepared from cerebellum at the level of G-protein-dependent-PLC activity because the concentration-response curve to GTPγS showed a reduction of the maximal effect in Ts65Dn mice, with no change in sensitivity (EC50). Western blot analysis showed a heterogeneous distribution of PLC-β(1–4) isoforms in both groups of mice. The levels of PLC-β4 isoform, however, were significantly lower in the cerebellum of Ts65Dn than in control mice. We conclude that the cerebellum of Ts65Dn mice has severe deficiencies in PLC activity stimulated by guanine nucleotides, which are specifically related to a lower level of expression of the PLC-β4 isoform, a fact that may account for the neurological phenotype observed in this murine model of Down syndrome. J. Neurosci. Res. 66:540–550, 2001. © 2001 Wiley-Liss, Inc.
Trisomy for human chromosome 21 (HSA 21), or Down syndrome (DS), is the most common genetic cause of mental retardation. Some DS phenotypes, including mental retardation, occur in all affected individuals, whereas other characteristics show variable penetrance. Mice provide a powerful experimental system for studies of mammalian aneuploidy. Ts65Dn mice are trisomic for a region of mouse chromosome 16 homologous to HSA 21 (Davisson et al., 1993), and are the most complete animal model for DS currently available (Dierssen et al., 1999). Ts65Dn mice present many DS-like features, including significant learning deficits in a variety of behavioral tests (Reeves et al., 1995; Escorihuela et al., 1995, 1998; Cousons-Read and Crnic, 1996; Demas et al., 1996, 1998; Holtzman et al., 1996), motor dysfunction (Costa et al., 1999), decrease in the density of the granule cell layer of the dentate gyrus (Insausti et al., 1998) and in the number of asymmetric excitatory synapses in the temporal cortex (Kurt et al., 2000), and reduction of cerebellar volume due to a reduction of both the internal granular layer and the molecular layer (Baxter et al., 2000).
Few neurochemical studies have been carried out in this model. Although no gross abnormalities were found in the main neuroamine systems of the brain (Megías et al., 1997), we have demonstrated a consistent depression in the function of adenylyl cyclase, one of the most relevant intraneuronal signaling pathways, in the cerebral cortex and hippocampus of Ts65Dn mice, documented by the reduced response to both β-adrenergic and forskolin stimulation (Dierssen et al., 1996, 1997). Considering the implications that the disturbed functioning of the main pathways leading to protein phosphorylation may have on behavior and learning, we have extended our studies to analyze the function of a phosphoinositide-specific phospholipase C (PLC), the other major effector by which neurotransmitter receptors trigger the formation of second messengers in mammalian brain. In fact, previous work provides new impetus for studying phosphoinositide metabolism in DS, and highlights the usefulness of the Ts65Dn mouse brain in uncovering the functional activity of this second messenger system. It has been consistently demonstrated, both in individuals with DS and in Ts65Dn mice, the presence of increased levels of myo-inositol in several brain regions including frontal cortex, basal ganglia and cerebellum (Shetty et al., 1995, 2000; Berry et al., 1999; Huang et al., 2000), probably related with the increased uptake and expression of the Na+/myo-inositol cotransporter in cells of individuals with DS (Fruen and Lester, 1990, 1991; Galdzicki et al., 1998). Despite the large changes in DS brain myo-inositol levels, the total phospholipid content and specifically the levels of phosphatidylinositol (PI) were significantly decreased, suggesting a general defect in brain lipid metabolism in DS subjects (Murphy et al., 2000).
The PLC system has been revealed to comprise a family of multiple isoenzymes by protein purification and molecular cloning. The 10 mammalian PLC isoenzymes (excluding alternatively spliced forms) identified to date can be divided into three structural types, β, γ, and δ (Rhee and Bae, 1997). Although, all PLC isoenzymes are activated by Ca2+ in vitro, the structural differences are responsible for the fact that different PLC isoenzymes are linked to membrane receptors through distinct mechanisms (Rhee and Bae, 1997). The PLC-β (β1–β4) isoforms have been described to be regulated by αq/11 and β, γ subunits of heterotrimeric G proteins (Smrcka et al., 1991; Camps et al., 1992; Smrcka and Sternweis, 1993; Hepler et al., 1993). The receptors activating G-protein αq family members in mammalian systems do not discriminate between αq- and α11-subunits (Wange et al., 1991; Offermanns et al., 1994). Similarly, there appears to be little difference between the ability of both G-protein α-subunits to regulate PLC-β isoforms (Hepler at al., 1993; Jiang et al., 1994; Lee et al., 1994).
The main purpose of the present experiments was to assess the functioning of the PLC signaling pathway in different areas of the brain of Ts65Dn mice. As a first approach, we have explored the changes induced on phosphatidylinositol 4,5-bisphosphate (PIP2) breakdown by the stimulation of neurotransmitter receptors- and G-protein-coupling to PLC-β with the muscarinic agonist carbachol and 5-HT2 agonist 5-methyltryptamine, and GTPγS (a non hydrolyzable analog of GTP) respectively, in cortical, hippocampal and cerebellar membranes of Ts65Dn mice and their control littermates. In addition, we have also quantified the protein levels of several elements of the PLC transmembrane signaling complex by using Western blot techniques. This has allowed us to compare the levels of αq/11 subunits and PLC-β1–4 isoforms and their functional coupling in crude (“high speed”) plasma membranes obtained from the same mouse brain sample. This study represents the first analysis of the activity of the PLC signaling pathway in the Ts65Dn mouse brain. A preliminary report was previously presented (Sallés et al., 1998).
MATERIALS AND METHODS
Materials
Atropine, carbachol (Cch), ketanserin, 5-methyltryptamine (5-MT), phosphatidylinositol 4,5-bisphosphate (PIP2), and ATP were obtained from Sigma Chemical Company, (St. Louis, MO). Guanosine 5′-0-[3-thiotriphosphate] (GTPγS) from Boehringer-Mannheim Biochemicals, (Indianapolis, IN), and [3H]-PIP2 (specific activity 6 Ci/mmol) came from New England Nuclear (Dupont, Boston, MA). Rabbit polyclonal anti-PLC-β(1–4) isoforms and anti-αq/11 subunits antibodies were purchased from Santa Cruz Biotechnology, (Santa Cruz, CA). Mouse monoclonal anti-actin antibody was purchased from Sigma. Horseradish peroxidase-conjugated anti-rabbit IgG and anti-mouse IgG, Enhanced chemiluminescence (ECL) Plus reagent and Rainbow (protein size marker) were obtained from Amersham Pharmacia Biotechnology, (Piscataway, NJ). All other chemicals were obtained from Sigma and Bio-Rad (Richmond, CA).
Animals
Groups of 16 male Ts65Dn mice and their control littermates were used in all experiments. Animals were housed four to a cage in a temperature-controlled room with a 12:12 hr light:dark cycle, and had free access to food and water. All experiments were carried out according to the European Community Council Directive of 24 November 1986 (86/609/EEC). After decapitation, the 42–52-day-old mouse brains were removed and placed on ice. The cerebral cortex, the hippocampus, and the cerebellum were dissected free. The three brain regions were frozen on dry ice and stored at −75°C until use.
Preparation of Crude Plasma Membranes
Frozen pooled samples (cortices, hippocampi or cerebella) from two brains were thawed in ice-cold 20 mM Tris-HCl buffer, pH 7.0, containing 1 mM EGTA (Tris/EGTA buffer) before homogenization, then homogenized in 20 vol of the same hypotonic buffer using a glass homogenizer with a Teflon pestle (twenty strokes with a motor-driven pestle at maximum setting). A crude plasma membrane preparation was isolated as described previously for rat cerebral cortex (Claro et al., 1989; Sallés et al., 1993). We normally aliquot the membranes in microcentrifuge tubes and keep the pellets at −75°C until use. Protein was measured using the Bio-Rad dye reagent.
Phospholipase C Assay
Membrane pellets were resuspended at a concentration of 2.5 mg protein/mL in a cold buffer consisting of 25 mM Tris-maleate, 5 mM ATP, 15 mM MgCl2, and 25 mM LiCl, pH 6.8 (adjusted with KOH). Reactions (100 μL total volume) were initiated by adding 40 μL of membranes (100 μg protein) to tubes with 25 mM Tris-maleate, pH 6.8, containing [3H]-PIP2 (final concentration 30 μM), 1 mM sodium deoxycholate, 3 mM EGTA and CaCl2 necessary to yield different free calcium concentrations (calculated according to Harafugi and Ogawa, 1980), and GTPγS, and agonist when appropriate. Tubes were incubated at 37°C for 15 min. The reactions were stopped with 1.2 mL chloroform/methanol (1:2, v/v), then 0.5 mL each of chloroform and 0.25 M HCl were added to create two phases, and were thoroughly vortexed. After centrifugation, a 1-mL aliquots of the upper aqueous phases containing [3H]-inositol phosphates were mixed with 4 mL of Optiphase Hi-Safe® for scintillation counting.
Immunodetection of G-Protein αq/11 Subunits and the PLCβ (1–4) Isoforms
Crude plasma membrane proteins were solubilized in sample buffer (10% glycerol, 5% 2-mercaptoethanol, 2% sodium dodecylsulfate [SDS] and 62.5 mM Tris-HCl, pH 6.8), and equal protein amounts (6 μg of membrane protein) were resolved by electrophoresis in 10% SDS-polyacrylamide gels and transferred by electroblotting on to polyvinylidene difluoride membranes (Amersham). Blots were blocked in 5% nonfat dry milk/phosphate-buffered saline containing 0.5% BSA and 0.2% Tween (PBS-T) for 1 hr, and were incubated overnight with either PLC-β(1–4) antiserum (1:5,000) or αq/11 antiserum (1:1,000). Blots were washed and incubated with secondary antibody diluted to 1:4,000 in blocking buffer for 2 hr at room temperature. Immunoreactive bands were visualized with the ECL system according to the manufacturer instructions (Amersham). Each gel contained a prestained broad-range protein ladder to measure molecular masses of individual bands. In some experiments, blots were subsequently incubated in stripping solution [62.5 mM Tris (pH 6.7), 2% (wt/vol) SDS, and 100 mM β-mercaptoethanol] for 30 min at 50°C, washed twice with PBS-T, and reprobed with anti-actin (1:2,000) as described above, to test whether nonspecific effects (i.e., consistency of protein loading) could occur in our experimental conditions.
The amount of protein loaded for each protein was determined by pilot experiments to lie within the linear range of detection (i.e., see Fig. 4). These aliquots (1–8 μg of membrane protein) were obtained from the same samples, electrophoresed on identical gels in duplicate, and immunoblotted at the same time under identical conditions.
Data Analysis and Presentation
All PLC assays were carried out in triplicate and replicated in at least three independent experiments. The data are usually presented as mean ± SE for the indicated number of experiments. Significance of mean differences were analyzed by unpaired two-tailed Student's t-tests or analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparison tests using the INSTAT program (GraphPAD). Statistical significance was set at the 95% confidence level.
PLC-β(1–4)- and αq/11-specific bands were quantitated using a Bio-Rad GS-700 imaging scanning densitometer in the transmittance mode, with analysis of subsequent signals using the Molecular Analyst for Windows program. Differences in PLC-β isoforms and G-protein αq/11 subunits immunoreactivities between crude membrane preparations prepared from the control and Ts65Dn brain sample, which were also run-yoked on the same blot, were analyzed with unpaired two-tailed Student's t-test. The minimal level of significance was taken as P < 0.05.
RESULTS
Regulation of PLC-β Activity by GTPγS and Agonists in Mouse Brain Cortical Membranes
In the first set of experiments, we measured basal breakdown of [3H]-PIP2 and its stimulation by KCl (20 mM) and agonists in the absence or presence of GTPγS. Based on previous concentration-response studies carried out in rat and human brain cortical crude membranes, a concentration of 3 μ GTPγS plus 1 mM Cch or plus 300 μM 5-MT was selected to activate PLC-β activity. ANOVA (one-way) showed significant differences between groups in either control [F(6,27) = 432.99, P < 0.0001] or Ts65Dn cortical membranes [F(6,27) = 535.59, P < 0.0001]. Post-hoc Tukey-Kramer multiple comparisons test revealed that basal activity of PLC was not stimulated either by KCl (control: q =1.78, NS; Ts65Dn: q = 1.30, NS) or the agonists Cch (control: q = 0.86, NS; Ts65Dn: q = 1.20, NS) and 5-MT (control: q = 1.89, NS; Ts65Dn: q = 1.38, NS). GTPγS clearly stimulated basal [3H]-PIP2 breakdown in either control (q = 29.20, P < 0.001) and Ts65Dn (q = 31.60, P < 0.001) cortical membranes. Further stimulation was induced by the addition of Cch (control: q = 17.39, P < 0.001; Ts65Dn: q = 19.74, P < 0.001) and 5-MT (control: q = 11.56, P < 0.001; Ts65Dn: q = 12.27, P < 0.001), indicating that the response to the agonists was strictly dependent on the presence of GTPγS (Fig. 1). To assess the ligand specificities of the responses to Cch and 5-MT, the responses were examined in the presence of the 5-HT2 antagonist ketanserin (10 μM) and the muscarinic antagonist atropine (10 μM). ANOVA showed a significant overall antagonists effects in either control [F(11,47) = 311.90, P < 0.0001) or Ts65Dn [F(11,47) = 519.97, P < 0.0001]. As shown in Figure 1, the response to 5-MT (300 μM) was specifically blocked by ketanserin (control: q = 11.73, P < 0.001; Ts65Dn: q = 15.76, P < 0.001) but not by atropine (control: q = 2.23, NS; Ts65Dn: q = 0.59, NS), whereas that of Cch (1 mM) was only blocked by atropine (control: q = 18.46, P < 0.001; Ts65Dn: q = 21.73, P < 0.001).
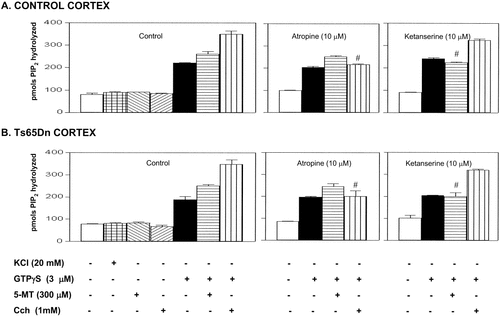
Selective inhibition of the GTPγS-dependent Cch or 5-MT activation of PLC-β by atropine and ketanserin in control (A) and Ts65Dn (B) cortical brain membranes. Membranes were incubated as indicated in Materials and Methods and no more additions for basal conditions (empty bars), or in the presence of 3 μM GTPγS (solid bars), and 3 μM GTPγS plus 300 μM 5-MT (horizontal bars), and 3 μM GTPγS plus 1 mM Cch (vertical bars). Values shown are means of four independent experiments done in triplicate and are expressed as pmol of hydrolyzed [3H]-PIP2 over 15 min. Note the lack of PLC responsiveness to 20 mM KCl (mesh bars), and agonists in the absence of GTPγS (300 μM 5-MT, left diagonal bars; or 1 mM Cch, right diagonal bars). #P < 0.001 vs. corresponding control cortical membranes (Tukey-Kramer multiple comparison tests after significant ANOVA).
Regulation of PLC Activity by GTPγS, Agonists, and Calcium in Several Brain Areas
Stimulation of [3H]-PIP2 breakdown was measured in crude membranes prepared from cerebellum, cerebral cortex and hippocampus in control and Ts65Dn mice. The regional distribution of PLC activity in both groups of mice, with respect to basal, GTPγS-, and agonists- (GTPγS-dependent) stimulated activities are shown in Figure 2A. Basal PLC activity showed a marked regional distribution in either control (ANOVA [F(2,11) = 54.9] P < 0.0001) or Ts65Dn brain (ANOVA [F(2,11) = 24.1] P < 0.001): cerebellum > cortex > hippocampus. The activation of G-proteins with 3 μM GTPγS stimulated [3H]-PIP2 hydrolysis with varying efficacies in either control [F(2,11) = 76.61, P < 0.0001] or Ts65Dn brain [F(2,11)=67, P < 0.0001]. This stimulation was higher in cerebellar than in cerebral or hippocampal membranes, both in control (q = 13.42, P < 0.001) and Ts65Dn mice (q = 10.34, P < 0.001); however, PLC activities under GTPγS conditions were significantly higher (29.4 ± 1%, n = 4, P < 0.001, unpaired Student's t-test) in control than in Ts65Dn cerebellar membranes. The response to GTPγS was further increased by the addition of maximal concentrations of Cch (1 mM) or 5-MT (300 μ M) in either control and Ts65Dn brain membranes, with the exception of cerebellum where no obvious Cch responses were obtained (Fig. 2A). These observations were confirmed by the statistical analysis. In cortical and hippocampal membranes of control brain, Cch stimulated [3H]-PIP2 hydrolysis by 53 ± 4.5% and 95 ± 4%, respectively, over the GTPγS-stimulated values, with the stimulation values being statistically different between them (P < 0.001, unpaired Student's t-test). In cortical and hippocampal membranes of Ts65Dn brain, Cch stimulated [3H]-PIP2 hydrolysis by 55 ± 5% and 100 ± 6%, respectively, over the GTPγS-stimulated values, with the stimulation values being statistically different between them (P < 0.001, unpaired Student's t-test). There were not, however, differences between control and TS65Dn mice. 5-MT stimulated [3H]-PIP2 hydrolysis over the GTPγS response by 35.5 ± 1.8% and 28.5 ± 2.1%, 42.8 ± 2.3% and 39.2 ± 1.8%, and, 12.4 ± 0.6% and 12.1 ± 0.7%, in cortical, hippocampal and cerebellar membranes of control and Ts65Dn brain, respectively, There were not, however, differences between control and Ts65Dn mice. Therefore, although the net PLC response to 5-MT in Ts65Dn cerebellar membranes was not as great as in control cerebellar membranes (Fig. 2A), the data show that there was no further decrease of GTPγS-dependent PLC activation when both GTPγS and the agonist were simultaneously present. In other words, the responses of 5-MT over the GTPγS effects were not significantly different (control: 12.4 ± 0.6%; Ts65Dn: 12.1 ± 0.7%, NS, unpaired Student's t-test).
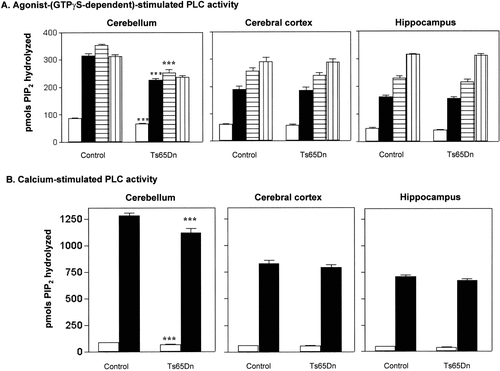
Effect of PLC stimuli on the production of [3H]-inositol phosphates in cortical, hippocampal and cerebellar membranes of control and Ts65Dn mice. A: Profile of agonist stimulation of [3H]-PIP2 hydrolysis in mouse brain membranes. Membranes were incubated as indicated in Materials and Methods and when present, 3 μM GTPγS and 300 μM 5-MT or 1 mM Cch as follows: basal (empty bars), GTPγS (solid bars), GTPγS plus 5-MT (horizontal bars), and GTPγS plus Cch (vertical bars). B: Calcium-stimulated [3H]-PIP2 breakdown in mouse brain membranes. Membranes were incubated with CaCl2 necessary to yield 50 nM (basal conditions, empty bars) and 10 μM free calcium concentration (solid bars). Values shown are the mean ± SE of four independent experiments done in triplicate and are presented as pmol of hydrolyzed [3H]-PIP2 over 15 min. One-way ANOVA showed significant regional differences both in control and Ts65Dn animals, and between groups of animals in the cerebellum. ***P < 0.001 vs. corresponding control cerebellar membranes group (unpaired two-tailed Student's t-test after significant ANOVA).
To investigate if the regional variations of basal PLC activity were due to alterations in the sensitivity to calcium or levels of PLC activities, the response of PLC to maximal concentration of calcium (10 μM) was examined. As shown in Figure 2B, calcium produced a significant increase in total PLC activity that, as in basal conditions, was higher in cerebellar than in cortical and hippocampal membranes, in either control (ANOVA [F(2,11) = 92.36]) P < 0.0001) or TS65Dn brain (ANOVA [F(2,11) = 66.62] P < 0.0001). Furthermore, there were significant differences in either basal or calcium-stimulated PLC activity between Ts65Dn and control cerebellar membranes, which was lower (basal: 23 ± 1%, n = 4, P < 0.001; calcium: 12 ± 0.3%, n = 4, P < 0.001; unpaired Student's t-test) in trisomic mice.
Concentration-Response Curves to GTPγS in Mouse Brain Cortical and Cerebellar Membranes
To examine G protein coupling to PLC-β in control and Ts65Dn cerebellar membranes, full concentration-response curves for GTPγS were carried out. Maximal stimulation over basal at 30 μM GTPγS, and half-maximal (apparent EC50 value for GTPγS) with approximately 0.2 μM GTPγS were attained in both experimental groups (Fig. 3), consistent with previous results in rat and human brain cortical membranes. Maximal stimulation showed a significant decrease (40%, n = 4, P < 0.05, unpaired Student's t-test) in the Emax values for GTPγS in Ts65Dn compared to control mice. When data were expressed as percentage of stimulation over the corresponding basal values, however, the maximal responses were not significantly different, suggesting an underlying defect downstream of the G-proteins.
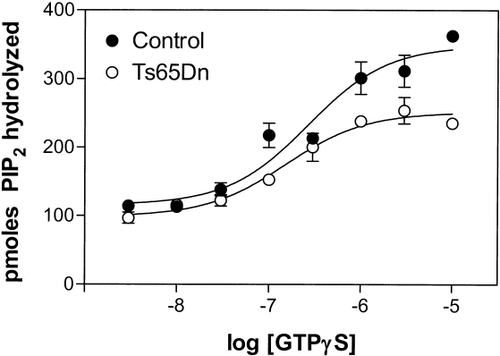
GTPγS concentration-dependence of PLC activity in mouse brain cerebellar membranes. Control (•) and Ts65Dn (○) cerebellar membranes were assayed for GTPγS. Symbols shown are mean ± SE of four independent experiments done in triplicate and are presented as pmol of [3H]-PIP2 hydrolyzed. The concentration-response curves were analyzed by nonlinear regression using the GraphPad Prism software. Values for Emax, and EC50 of the concentration-response curves are mean ± SEM of the values estimated by the fitting program: control cerebellar membranes, Emax = 349 ± 20 pmol, EC50 = 0.26 ± 0.05 μM; Ts65Dn cerebellar membrane, Emax = 252 ± 7 pmol, EC50 = 0.17 ± 0.04 μM. Values for pmol of [3H]-PIP2 converted to [3H]-inositol phosphates over 15 min in basal conditions averaged 116 ± 8 pmol in control cerebellar membranes, and 88 ± 6 pmol in Ts65Dn cerebellar membranes.
In cortical membranes, maximal stimulation by GTPγS was 310% and 320% over basal in control and Ts65Dn mice, respectively, the difference being not significant. Half-maximal stimulation was achieved at 0.20 and 0.17 μM GTPγS for control and trisomic mice, respectively.
Levels of αq/11 Subunits and PLC-β (1–4) Isoforms in Mouse Brain Membranes
To determine whether the decreased G protein-mediated functional responses in cerebellar membranes was associated with a specific decrease in the density of PLC-β (1–4) isoforms or αq/11 subunits, we measured the immunolabeling of these proteins in cortical, hippocampal and cerebellar membranes in both groups of animals. Figure 4 shows representative immunoblots of αq/11 subunits and PLC-β1 isoform immunoreactivities in crude membranes from Ts65Dn and control mouse brain cortex. Two distinct bands migrating at expected molecular masses (≈165 and ≈40 kDa) are shown. Analysis of the standard curves revealed a linear relationship between the amount of protein (1–8 μg) in each lane and the relative optical density of the αq/11 subunits band and of PLC-β1 isoform band (see legend Fig. 4A). Furthermore, the slopes of the straight lines obtained either for PLC-β1 or αq/11 were not statistically different between control and Ts65Dn cortical membranes. The relative values of signals of PLC-β1 isoform and G-protein αq/11 subunits for several brain areas of Ts65Dn and control animals are presented in Figure 4B. An aliquot of pooled standard cortical membrane proteins from control animals was run on one lane of every gel to minimize the inter-assay variation, and the optical density units obtained from each sample were normalized against those from a pooled cortical standard. The protein level for the PLC-β1 isoform was reduced by ≈53% in cerebellar compared to cortical or hippocampal membranes, either of Ts65Dn (46.8 ± 1.3%, n = 3, P < 0.001) or control animals (47.4 ± 1.1%, n = 3, P < 0.001). For the αq/11 subunits, although increases were evident in αq/11 immunoreactivity in cortical and hippocampal compared to cerebellar membranes (Fig. 4B), the differences were not statistically significant. Again, there were no significant differences in the relative values of the corresponding band of the αq/11 subunits between Ts65Dn brains and control animals, irrespective of the brain region analyzed.
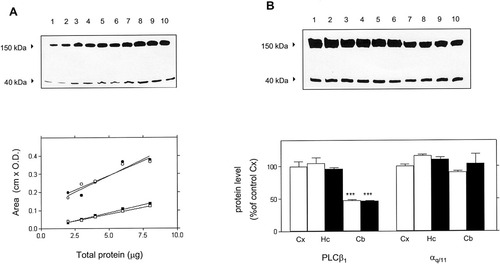
Quantitation of immunoblotting. A: Standard curves of PLC-β1 isoenzyme and αq/11 subunits immunoreactivities in crude membranes of control (lanes 1, 3, 5, 7, and 9) and Ts65Dn (lanes 2, 4, 6, 8, and 10) mouse cerebral cortex. Upper panel: Immunoblot of representative standard curves. Lower panel: Standard curves were generated by immunoblotting using incremental amounts of mouse cortical membranes ranging from 1–8 μg of protein. The correlation coefficients were obtained by linear regression analysis and were as follow: control animals: PLC-β1 isoform (r = 0.9127, ○), and G-protein αq/11 subunits (r = 0.9866, □). Ts65Dn animals: PLC-β1 isoform (r = 0.8955, •), and G-protein αq/11 subunits (r = 0.9941, ▪). The differences between the slopes for PLC-β1 in control and Ts65Dn animals were not statistically significant (F[1,6] = 0.18856; P = 0.6793). The differences between the slopes for αq/11 in control and Ts65Dn animals were not statistically significant (F[1,6] = 5.2057; P = 0.0626). B: Upper panel: Representative Western blots of PLC-β1 isoform and G-protein αq/11 subunits immunoreactivities in duplicate samples of cortical (lanes 1, 2), hippocampal (lanes 3–6) and cerebellar (lanes 7–10) membranes from controls (lanes 1–4, 7, and 8) and Ts65Dn (lanes 5, 6, 9, and 10) mice brain. Lower panel: Quantitation of immunoblotting. Differences on PLC-β1 and αq/11 was assessed in three separate immunoblot experiments carried out in duplicate, using 6 μg of protein from cortical, hippocampal and cerebellar membranes from control (open bars) and Ts65Dn (solid bars) brain samples. Data are expressed as the mean ± SEM percentage of the corresponding matched control cortical membranes. ***P < 0.001 vs. control cortical membranes (unpaired two-tailed Student's t-test).
To fully investigate the PLC-β isoform composition of cortical and cerebellar membranes, PLC-β2, PLC-β3 and PLC-β4 isoforms were analyzed. As expected, the PLC-βs examined were expressed in both brain regions. For the PLC-β2 isoform (data not shown), the resultant data of the major band (migrating at 145 kDa) did not allow to obtain accurate estimates due to the weak signal detected in the two regions studied. In any case, there were no significant differences in the relative values between Ts65Dn brains and controls.
The relative values of signals of PLC-β3 and PLC-β4 isoforms in cortical and cerebellar membranes for Ts65Dn mice and controls are presented in Figure 5. For the PLC-β3 isoform, the resultant data of the major band (migrating at 152 kDa) showed a significant increase of ≈140% in cerebellar compared to cortical membranes either of Ts65Dn (148 ± 7%, n = 6, P < 0.0001) or control animals (138 ± 5%, n = 6, P < 0.0001, unpaired Student's t-test), but no significant change was found in either cortical or cerebellar membranes of Ts65Dn brains when compared to controls (Fig. 5A). For the PLC-β4 isoform (=129 kDa), there were group differences as well as regional ones; mean levels of PLC-β4 were higher in cerebellar compared to cortical membranes either of Ts65Dn (113.6 ± 2.5%, n = 6, P < 0.0001) or control animals (155.1 ± 3.4%, n = 6, P < 0.0001), but in Ts65Dn cerebellar membranes the PLC-β4 levels were significantly reduced (30%, P < 0.0001, unpaired Student's t-test) when compared to control cerebellar membranes (Fig. 5B). To test whether nonspecific effects (i.e., consistency of protein loading) could occur in our experimental conditions, immunostaining for the cytoskeletal protein actin was subsequently carried out in all blots (Fig. 5). As shown in Figure 5, the immunoblots revealed no differences in actin immunoreactivities among control and Ts65Dn brain membranes.
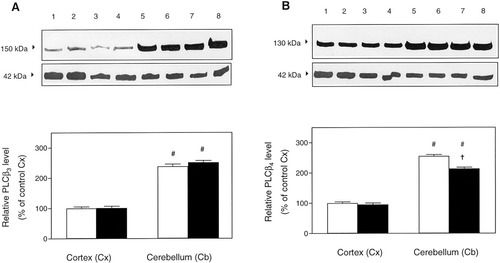
Fig. 5. Quantitation of immunoblotting. A: Upper panel: Representative Western blot of PLC-β3 isoform and actin immunoreactivities in duplicate samples of cortical (lanes 1–4) and cerebellar (lanes 5–8) membranes from controls (lanes 1, 2, 5, and 6) and Ts65Dn (lanes 3, 4, 7, and 8) brain samples. Lower panel: Differences on PLC-β3 was assessed in six separate immunoblot experiments carried out in duplicate, using 6 μg of protein from cortical and cerebellar membranes prepared from control (open bars) and Ts65Dn (solid bars) brain samples. B: Upper panel: Representative Western blot of PLC-β4 isoform and actin immunoreactivities in duplicate samples of cortical (lanes 1–4) and cerebellar (lanes 5–8) membranes from controls (lanes 1, 2, 5, and 6) and Ts65Dn (lanes 3, 4, 7, and 8) brain samples. Lower panel: Differences on PLC-β4 was assessed in six separate immunoblot experiments carried out in duplicate, using 6 μg of protein from cortical and cerebellar membranes prepared from control (open bars) and Ts65Dn (solid bars) brain samples. To ensure that the same amounts of proteins were loaded, the blots were stripped and reprobed with anti-actin antibody. Note that the PLC-β3 and PLC-β4 Western blots have been combined with actin immunodetection on the same membranes, to visualize directly consistency of protein loading. Data are expressed as the mean ± SEM percentage of the corresponding matched control cortical membranes. #P < 0.0001 vs. corresponding cortical membranes group; †P < 0.0001 vs. corresponding control brain sample (unpaired two-tailed Student's t-test).
DISCUSSION
Previous reports have demonstrated the feasibility of measuring the activity of receptor-coupled, G-protein-mediated phosphoinositide hydrolysis in crude (also noted as “high speed”) plasma membranes prepared from rat and human brain (Claro et al., 1989; Sallés et al., 1993; Jope et al., 1994). Following similar methodological procedures, which permit the investigation of direct coupling of G-proteins and muscarinic and serotonergic receptors to PLC-β, we have conducted a functional study of PLC activity in several brain areas of mice, and have compared these activities in normal and Ts65Dn mice, a model for Down syndrome. In addition, we have simultaneously studied the expression of four phospholipase C-β isoforms plus the G-protein αq/11 subunits in the same mouse brain areas. To the best of our knowledge, this is the first time that the expression of all known functional PLC-β isoforms and G-protein αq/11 subunits was investigated in parallel with PLC activity stimulated by calcium, guanine nucleotides and agonists in the mammalian brain.
Our functional studies have shown that: 1) basal and maximal calcium stimulation of [3H]-PIP2 hydrolysis in cerebellum were higher than in cerebral cortex and hippocampus of control and Ts65Dn mice; 2) cerebellar basal as well as stimulated PLC activities were lower in Ts65Dn mice than in their control littermates; and 3) concentration-response curves for GTPγS in cerebellum of Ts65Dn mice were depressed compared to control; the fact that no obvious differences were observed in the EC50 values of GTPγS between cerebellar and cortical in both Ts65Dn and control mice indicates the presence of a similar sensitivity to GTPγS in all mouse brain cerebral membranes. These findings are consistent with the possibility that concentrations of G-protein αq/11 subunits or PLC-β1/4 isoforms are rate limiting for inositol lipid hydrolysis in response to agonists in control and Ts65Dn cerebellar membranes. Interestingly, although under G-protein stimulating conditions the production of inositol phosphates was higher in control than in Ts65Dn cerebellar membranes, the percentage of stimulation over basal values were similar, findings that suggests that PLC-β1/4 isoforms levels rather than the availability of G-protein αq/11 subunits may limit the rate of phosphoinositide hydrolysis.
The disturbance in PLC signaling restricted to cerebellum of Ts65Dn mice contrasts to the previously reported on the adenylyl cyclase signal in cortex and hippocampus, but not in cerebellum, of the same model (Dierssen et al., 1996, 1997). These findings emphasize that several different brain regions need to be considered in analyzing the signaling pathways in the brain of DS individuals, as it appears that different pathways are altered in different brain regions.
The enhanced overall PLC activity in control cerebellum (as defined by calcium stimulated [3H]-PIP2 hydrolysis) could arise from increased levels of enzymatic activity, compared to those of Ts65Dn mice, that can be also responsible for the higher cerebellar response to GTPγS stimulation in control mice (as shown in Fig. 2A and Fig. 3). Remarkably, the reduction of cerebellar PLC activity in Ts65Dn mice was more evident under GTPγS- than under calcium-stimulating conditions. It likely reflects the presence and contribution of a more complex population of PLC isoenzymes to the basal PLC activity, when measured in crude plasma membranes. Taken together, these data suggest the presence of a decreased level of the PLC-β isoforms in the cerebellum of Ts65Dn mice, rather than the inability of GTPγS to bind to G-proteins.
To determine whether the observed impairments of G-protein modulation of PLC could be attributed to specific defect in the G-protein αq/11 subunits or in the PLC-β (1–4) isoforms, their protein levels were examined by Western blot analysis. No significant brain regional differences were documented in the levels of G-protein αq/11 subunits in either control or Ts65Dn mice. Protein levels of PLC-β2 were very weak in the mouse brain. The regional distribution patterns of expression for PLC-β3 and PLC-β4 differed markedly from that of PLC-β1 in both groups of mice. Expression for PLC-β1 in the cerebral cortex and hippocampus was much higher than in cerebellum, whereas expression for PLC-β3 and PLC-β4 were higher in cerebellum than in cerebral cortex. Accordingly, a near inversion of the ratios PLC-β1/PLC-β3 and PLC-β1/PLC-β4 was observed between cerebral cortex and cerebellum. Although these data represent relative, not absolute, measures of protein levels within these regions, they suggest nonetheless possibly significant differences in their contribution to the overall PLC-β transmembrane signaling capacities.
No significant differences in the PLC-β1, PLC-β2 and PLC-β3 isoforms were appreciated in cerebellum and cerebral cortex of Ts65Dn mice, compared to their controls. Protein levels of the PLC-β4 isoform, however, were found to be reduced significantly and exclusively in the cerebellum of TS65Dn mice (33%). These results indicate a region-specific change of the PLC-β4 isoform in the Ts65Dn brain that matches the functional changes observed in PLC activity. Because there was no significant variation in the levels of G-protein αq/11 subunits in control vs. Ts65Dn crude plasma membranes, it can be concluded that the decreased density of the PLC-β4 isoform would be sufficient to account for the changes observed in the GTPγS concentration-response curve that specifically affected the Emax values of the guanine nucleotide analog.
Several changes in cerebellum have been documented in both individuals with DS and Ts65Dn mice. A reduction in cerebellar volume is consistently present in DS (Crome et al., 1966; Raz et al., 1995; Aylward et al., 1997), where a reduction of the cerebellar granule cells (GC) density has been documented (Baxter et al., 2000). A similar reduction in cerebellar volume of Ts65Dn mice has been reported (Baxter et al., 2000), that can be explained by a reduction in the thickness of the internal granule cell layer and the molecular layer of the cerebellar cortex, as a consequence of the reduction in the total number of GC and Purkinje cells (PC) (76% and 89.5% of euploid animals, respectively). Interestingly, both present and previous studies indicate that PLC-β1 and PLC-β4 represent major neuronal PLC-β isoforms in various regions of the adult brain, whereas in the cerebellum, PLC-β3 and PLC-β4 are major isoforms with lower levels of PLC-β1 (Tanaka and Kondo, 1994; Watanabe et al., 1998). Specifically, the distribution of PLC-β3 mRNA is confined to the PC layer in the cerebellum, whereas PLC-β4 mRNA is expressed strongly in PC, moderately in GC and weakly in the molecular layer neurons (Watanabe et al., 1998). It is, therefore, possible that the decreased level of PLC-β4, but not PLC-β3, found in the cerebellum of Ts65Dn mice, might be consequence, at least in part, of the decreased number of GC for these mice.
DS individuals display hypotonia in infancy. Children and adults also display fine motor deficits that have attributed to problems in cognition or motor control mechanisms (Latash and Corcos, 1991). The reduction in the number of cerebellar neurons and in the function of the cerebellar transmission processes in the Ts65Dn mice can be reflected in tests of motor function. Although several reports did not document deficits in sensorimotor tests (Escorihuela et al., 1995; Baxter et al., 2000), sensitive fine and gross motor tests have shown mild to severe disturbances of equilibrium and motor coordination in Ts65Dn mice (Costa et al., 1999), that might be attributed to cerebellar dysfunction. Interestingly, a role in motor learning for mGluR1, G-protein αq/11 subunits, PLC-β4 isoform, and PKC-γ isoform present in cerebellum is now well supported by recent experiments in transgenic mice (see review by Daniel et al., 1998). Thus, mice deficient in mGluR1, Gαq/11, PLC-β4 or PKC-γ may show impaired motor coordination (Offermanns et al., 1994, 1997; Kano et al., 1995, 1997; Kim et al., 1997). The impairment of motor coordination, however, may not be strictly proportional to the concentration of each of these factors, as demonstrated in heterozygous αq/11 deficient mice that did not show defects in motor coordination even though their αq/11 levels were reduced (Offermanns et al., 1997).
In conclusion, our study shows region-specific cerebellar reduction in the overall PLC activity (basal and GTPγS-stimulated conditions) in the Ts65Dn mice, a model for DS. These changes are matched by specific changes in the protein levels of PLC-β4 isoform. These findings provide a new insight in the definition of the cerebellar phenotype appearing in DS individuals.
Acknowledgements
The authors would like to thank Dr. Oscar Goñi for helpful technical assistance in our initial Western blot experiments. This work was supported by grants SAF/98-0064-C02-02 and UPV-G15/98 to J.S. from the Spanish Ministry of Education and the University of the Basque Country, respectively, and by grants SAF99-0092-C02-02 and Foundation “Marcelino Botín” to J.F. I.R.A. received a fellowship from Euskal Herriko Unibertsitatea/University of the Basque Country, and M.A.L. from Foundation “Marqués de Valdecilla”.




