Changes in neurofilament protein-immunoreactivity after kainic acid treatment of organotypic hippocampal slice cultures
Abstract
Neurofilament (NF) proteins are expressed in the majority of neurons in the central nervous system, and play a crucial role in the organization of neuronal shape and function. In the present study, we have used immunoblotting and immunocytochemical methods to study the light (NF-L), medium (NF-M ), and heavy (NF-H) molecular weight NF proteins in cultured organotypic hippocampal slices during the in vitro maturation and the changes after kainic acid (KA) treatment. In control cultures at 11 DIV throughout 25 DIV, CA3 pyramidal neurons and their proximal dendrites were heavily labeled with the antibodies against all three NF proteins. In CA1 pyramidal neurons, no staining was detected in any age group. A few weakly NF-L positive granule cells with fibers were detected in each age group, whereas NF-M and NF-H positive granule cells first appeared in the older cultures. The application of KA (5 μM) to the cultures for 48 hr, induced a pronounced cell death in the CA3 cell layers, and also moderately damaged granule cells. After the treatment, the immunoblot signal of NF-L and NF-M markedly decreased, whereas that of NF-H almost completely disappeared. The amount of NF-L positive fibers, however, dramatically increased in the molecular and hilar regions of the dentate gyrus in both age groups. Our results show the cellular heterogeneity in the distribution of NF protein triplet in cultured organotypic hippocampal slices. Kainic acid treatment induced changes, which mimicked those observed in the hippocampal region of epileptic animals. J. Neurosci. Res. 66:620–629, 2001. © 2001 Wiley-Liss, Inc.
Neurofilaments (NFs) are intermediate filament proteins that constitute a major component of the neuronal cytoskeleton (Schlaepfer, 1987; Nixon and Sihag, 1991). The three different NF proteins, low molecular weight, NF-L (68 kDa), medium molecular weight, NF-M (160 kDa), and high molecular weight, NF-H (200 kDa) are separate gene products and they are immunocytochemically distinct (Czosnek et al., 1980). NF proteins are formed by a heteropolymerization of NF-L with NF-M or NF-H (Lee and Cleveland, 1996). These proteins are considered to be a neuronal marker, which is absent in the glia and it is mainly localized in axons, but also in the soma and dendrites of neurons (Trojanowski et al., 1986; Van der Zee et al., 1997). Most populations of neurons are suggested to express NF proteins (Vickers and Costa, 1992), although their spatial distribution may vary in subclasses of neurons in different regions of the CNS (Trojanowski et al., 1986; Lee et al., 1987).
Alterations in the distribution, expression, and phosphorylation of NFs have been detected in various brain regions of adult animals in experimental conditions, e.g., after transient ischemia (Nakamura et al., 1992; Kaku et al., 1993) and kainic acid (KA)-induced seizures (Wang et al., 1994; Yang et al., 1995). Although NF proteins are of importance in the developing brain, their significance in pathological stages, e.g., in seizures, is poorly known in the immature brain, in particular in the hippocampus, a region of great importance in the pathophysiology of epilepsy (McNamara, 1994). A relatively simple and pharmacologically accessible method to study the role of NF proteins, is to use organotypic hippocampal slice cultures as an in vitro model of epilepsy. In these cultures, the synaptic organization and intrinsic hippocampal fiber pathways are developed corresponding to their in vivo counterparts (Frotscher and Gähwiler, 1988; Caeser and Aertsen, 1991; Gähwiler et al., 1997). Furthermore, the application of KA induces synaptic reorganization and functional changes (Routbort et al., 1999), which largely correspond to those detected in the hippocampus of epileptic animals in experimental conditions (Nadler et al., 1980; Sutula et al., 1989).
The focus of this immunocytochemical and immunoblotting study was on two main aspects. First, to establish the cellular distribution of NF-L, NF-M, and NF-H proteins in cultured organotypic hippocampal slices and, to detect eventual changes in their amount and staining pattern during the in vitro maturation. Second, whether or not the NF staining pattern and the amount of NF proteins are changed by treating the cultures with KA. This treatment was used as in vitro model of epilepsy. We used commercially available monoclonal antibodies that recognize phosphorylation- independent epitopes of NF-L, NF-M and NF-H proteins. To our knowledge this is the first study to show the amounts and cellular distribution of NF proteins and changes induced in them by KA treatment in organotypic hippocampal cultures during the in vitro maturation.
MATERIALS AND METHODS
Organotypic Hippocampal Slice Cultures
Hippocampal slice cultures were prepared using the method originally described by Stoppini et al. (1991). Hippocampi from postnatal 6–7 (P6-P7) Wistar rats were dissected and placed immediately in cold Gey's balanced salt solution (GIBCO BRL, Paisley, UK) supplemented with glucose (6.5 mg/ml). Four hundred micrometer slices were cut perpendicular to the septotemporal axis of the hippocampus using a McIllwain tissue chopper. Slices were carefully separated, trimmed and placed on semi-permeable membrane inserts (Millipore Corporation, Bedford, MA) (four slices per insert) in a 6-well plate containing 1.2 ml of culture medium (50% of minimum essential medium, 25% Hanks's balanced salt solution, 25% heat-inactivated horse serum, 25 mM HEPES, supplemented with 0.5 ml GlutaMaxII [GIBCO] and 6.5 mg/ml glucose, pH adjusted to 7.2). Slices from the middle third of the hippocampus were used for culturing. Culture medium was changed twice a week. No antibiotics or antimitotic drugs were used. Kainic acid (KA, 5 μM Sigma Chemical Company, St. Louis, MO) was diluted in culture medium, and applied for cultures at 7 and 14 days in vitro (DIV). After 48 hr (9 and 16 DIV respectively), inserts were transferred to new multi-well dishes containing fresh medium and cultured further for 2 days before collecting the slices for immunoblotting, and using them for immunocytochemistry and thionin staining.
Immunostaining
After a culture period ranging from 11–25 DIV, the cultures were processed for immunostaining. Culture medium was sucked off, the slices were briefly washed three times with ice cold 0.1 M phosphate buffer (PB, pH 7.4) and thereafter 4% ice-cold paraformaldehyde (PFA) in 0.1 M PB was added and left for 2 hr at +4°C. After rinsing with ice-cold PB, the slices were carefully mechanically detached from inserts and transferred into a net-well system (Costar Corning, Acton, MA), in which free-floating slices were immunostained. To prevent any structural damage of slices during medium changes and washes, net-wells with four slices each were removed from one multi-well to another (multi-wells contained 2.5 ml of medium in question) without touching the slices. Gentle rotation was used at all steps to ensure even distribution of reagents. First, to block the endogenous peroxidase activity, net-wells with floating slices were transferred into freshly prepared PB containing methanol (30%) and H2O2 (3%) and left for 30 min at room temperature (RT). After rinsing thoroughly with PB, slices were incubated in a blocking solution (BS) containing 4% bovine serum albumin (BSA), 4% donkey serum (DS), 0.1% Triton-X-100 for 1.5 hr at RT and thereafter with primary antibody diluted in BS at 4°C overnight. The dilution of 1:750 of the monoclonal primary antibodies NR4 (anti-NF68), NN18 (anti-NF160), and N52 (anti-NF200) (all from Sigma) was used. After rinsing briefly twice in PB and three times (each 10 min) in PB containing 0.1% Triton X-100 (PBX) at RT, slices were incubated with the secondary antibody [biotin-SP-conjugated donkey anti-mouse IgG (H+L), Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA), dilution 1:750–1:1,000] in PB containing 2% DS, 2% BSA, 0.1% Triton-X-100 for 1 hr at RT, followed by incubation with avidin-biotin-peroxidase complex (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA). After rinsing, 3,3′-diaminobenzidine (DAB) (Sigma) was used as a chromogen to detect immunoreactivity. Slices were rinsed and transferred to gelatin-coated glass slides and left to dry overnight at RT. After rehydration, slices were quickly dehydrated in graded ethanol, cleared in xylene and mounted in Permount. In each experiment, three to four slices in which the primary antibody was omitted but were otherwise treated as indicated above, served as negative controls. Preparations were examined in a Leica DM R microscope (Heerbrugg, Switzerland) under bright-field optics. The immunoreactivity in the CA3a/b, CA3c, CA1 pyramidal neurons, in the dentate granule cell layer (including both the supra and infrapyramidal cell layers), and in the molecular layers was scored using the following scheme: −, negative; ±, weakly positive; +, positive; ++, strongly positive; +++, very strongly positive. The CA3c region was defined as the CA3 cell layer located between the blades of the dentate granule cell layer, and the CA3a/b region as the CA3 cell layer excluding the CA3c region. Digital camera DC100 (Leica Inc., Deerfield, IL) was used to capture images that were further processed using Image J (version 1.20s), and Adobe Photoshop software (version 5.5, Adobe Systems, Inc., Mountain View, CA).
Thionin Staining
For thionin staining, the cultures on membrane inserts were briefly rinsed with PB (pH 7.4) and fixed for 15 min in 4% PFA in 0.1 M PB (pH 7.3) at RT. After rinsing with PB, slices were carefully mechanically detached from inserts, transferred to gelatin-coated glass slides and left to dry at RT from 2 hr up to overnight. After quick rehydration, slices were stained for 2 min in 0.5% thionin solution, dehydrated in graded ethanol, cleared in xylene and mounted in Permount. The stained slices were examined with a Leica DM R microscope separately by two observers under bright-field optics. The number of neurons in CA3a/b, CA3c, CA1, and granule cells of the dentate gyrus were scored using the following scheme: 0 = no stained neurons in the cell layer; 1 = some stained neurons; 2 = very sparse number of stained neurons; 3 = many stained neurons but with slightly disturbed cell layer integrity; 4 = many stained neurons with good cell layer integrity (regarded as normal). Cells with a large nuclear size were classified as neurons. Digital camera DC100 (Leica) was use to capture pictures, which were further prepared using Adobe Photoshop software.
SDS-PAGE Immunoblotting
For the immunoblotting studies, control (11, 18, and 25 DIV) and KA-treated (11 and 18 DIV) slices from two different culture batches (n = 25–33 slices in both batches and in each age and treatment group) were collected in ice cold homogenization buffer containing 50 mM TRIS-HCl (pH 7.4), 1% SDS, 2 mM EDTA, 1 mM PMSF, and 0.7 mM dithiothreitol. Slices were homogenized using Ultra-Turrax T25 homogenizer (Janke and Kunkel, Staufen, Germany), homogenates immediately boiled for 4 min and then centrifuged at 12,000 rpm for 30 min at 4°C. Supernatants were collected, frozen and stored at −80°C until used. Protein concentration of the samples was measured using Lowry based Bio-Rad DC Protein assay (Bio-Rad, Richmond, CA). Six micrograms of protein were applied to each line for SDS-PAGE and separated by electrophoresis with a 7.5% acrylamide minigel using Mini-protean II (Bio-Rad) and transferred to a polyvinylidene fluoride (PVDF) Immopilon-P (Millipore) membrane using semi-dry system (Transblot SD, Bio-Rad). Membranes were incubated overnight at 4°C with the primary antibodies NR4 (anti-NF68, 1:3,500), NN18 (anti-NF160, 1:4,000), N52 (anti-NF200, 1:3,000) (all from Sigma) and then with the HRP-conjugated secondary antibody (1:4,000) (Sigma) for 1 hr at RT and the signal was obtained using chemiluminescence ECL system (Amersham, Buckinghamshire, UK) and Hyperfilm ECL (Amersham). The optical signals were quantified with Image J 1.20s (NIH, USA) and the results are given as arbitrary units (a.u.) per mg of protein. The immunoblotting studies were repeated four times in each batch of slices.
Statistical Analysis
In the control groups, the statistical significance of differences in the immunoblots between the experimental groups was analyzed with the nonparametric ANOVA Kruskal-Wallis Test. The unpaired Student's t-test with Welch correction was used to analyze the differences between the controls and the KA-treated cultures of the same age, and between the two age groups in the KA-treated cultures. The overall group differences of the score numbers after thionin staining were assessed by one-way analysis of variance (ANOVA). If a significant effect was found, Tukey-Kramer Multiple Comparison Test was used to define the statistical significance of differences between the experimental groups and between the two age groups in KA-treated slices. GraphPad InStat (version 2.0) software (GraphPad Software, San Diego, CA) was used in all statistical analysis. The level of significance was set at P < 0.05.
RESULTS
NF-L, NF-M, and NF-H Immunoreactivity in Hippocampal Slices
Table I gives the distribution of NF-immunoreactivity at 11, 18, and 25 DIV in various hippocampal regions in slices cultured under normal conditions. In general, the changes in the immunoreactivity during the in vitro maturation of the slices were not pronounced. In all in vitro age groups, both the cell body and the proximal dendrites of the CA3a/b and CA3c pyramidal neurons were very strongly immunoreactive to NF-H and also strongly to NF-M and NF-L. DAB reaction product in the cell bodies was restricted to the rim of cytoplasm surrounding the unstained nucleus (Fig. 1A–C). Neither NF-H, NF-M, nor NF-L immunoreactivity was observed in the CA1 pyramidal neurons at any age (as an example see NF-H staining in Fig. 1A). A few weakly NF-L positive granule cells were detected in each age group, whereas some NF-H and NF-M positive granule cells only in the older cultures (Fig. 2A–C). In the molecular layers of dentate gyrus, the mean staining intensity with NF-H, NF-M and NF-L was weak through 11 to 25 DIV, and the amount of positively stained fibers remained stable between the different age groups.
| Region | 11 DIV | 18 DIV | 25 DIV |
|---|---|---|---|
| NF-L Immunoreactivity | |||
| Pyramidal neurons | |||
| CA1 | − | − | − |
| CA3a/b | ++ | ++ | +++ |
| CA3c | ++ | ++ | ++ |
| Dentate gyrus | |||
| Granule cells | ± | + | + |
| Molecular layers | + | + | + |
| NF-M Immunoreactivity | |||
| Pyramidal neurons: | |||
| CA1 | − | − | − |
| CA3a/b | +++ | +++ | ++ |
| CA3c | ++ | ++ | ++ |
| Dentate gyrus | |||
| Granule cells | − | ± | + |
| Molecular layers | + | ++ | + |
| NF-H Immunoreactivity | |||
| Pyramidal neurons | |||
| CA1 | − | − | − |
| CA3a/b | +++ | +++ | +++ |
| CA3c | +++ | +++ | +++ |
| Dentate gyrus | |||
| Granule cells | − | − | + |
| Molecular layers | + | + | + |
- * Scoring is a mean of 9–17 slices from 3–4 different culture batches. −, negative; ± weakly positive; +, positive; ++, strongly positive; +++, very strongly positive. DIV, days in vitro.
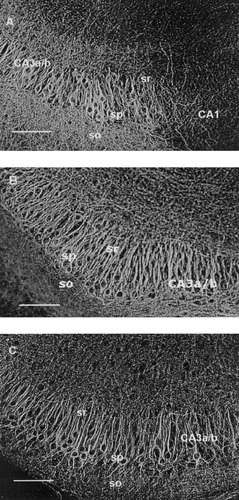
Representative inverted images of NF-H, NF-M and NF-L immunostaining in the pyramidal cell layers in hippocampal slices cultured in normal medium as described in the Materials and Methods. A: NF-H immunoreactivity in a hippocampal slice of 26 DIV. Note that no NF-H immunoreactivity is detected in the CA1 region. B: NF-M immunoreactivity in a hippocampal slice of 11 DIV. C: NF-L immunoreactivity in a hippocampal slice of 11 DIV. Abbreviations: so, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum. Scale bars = 100 μm.
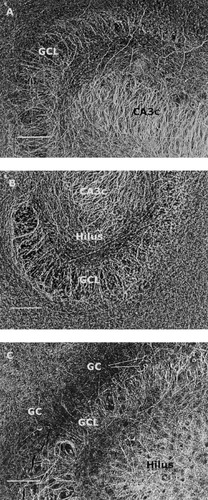
Representative inverted images of NF-H, NF-M and NF-L immunoreactivity in the dentate gyrus, hilar and CA3c regions in hippocampal slices cultured in normal culture medium. A: NF-H immunostaining in a slice of 11DIV. B: NF-M immunostaining in a slice of 18 DIV, and C: NF-L immunostaining in a slice of 18DIV. Because no (A,B) or very few granule cells (C) were stained, the accurate delineation of the granule cell layer is not possible. The immunostained fibers are mainly directed toward the molecular layers suggesting that these are dendrites. Note the sharp borders at the end zone of fiber outgrowth in the molecular layers (in particular in B), and the abundant staining in the CA3c pyramidal cell region in particular in A and B. Abbreviations: GC, granule cells; GCL, granule cell layer. Scale bars = 100 μm.
Kainic Acid Treatment Caused Neuronal Death and Changes in the NF Proteins
The vulnerability of hippocampal slices to KA during the in vitro maturation was first assessed by thionin staining in two in vitro age groups (11 and 18 DIV) 2 days after KA (5 μM hr) treatment. The most vulnerable regions at both in vitro ages were CA3a/b and CA3c, in which the number of pyramidal neurons dramatically decreased. Also, the number of dentate gyrus granule cells was markedly decreased in the treated cultures (Table II) (Fig. 3A,B). Despite a pronounced cell death in the KA-treated cultures, the main features of the cellular heterogeneous distribution and the immunoreactivity of NFs remained largely unchanged (Table III) when compared to control cultures. Surprisingly, in the KA-treated cultures both the intensity of NF-L staining and the amount of NF-L- positive fibers were strongly increased in the molecular layers of the dentate gyrus and in the hilar region (Fig. 4A). This staining pattern was also present at 18 DIV. NF-L staining also slightly increased in CA3a/b in both age groups, whereas NF-M and NF-H staining decreased in CA3 regions. Also, the NF-M immunoreactivity and the amount of NF-M positive fibers increased in the dentate gyrus molecular layers and in the hilar region (Fig. 4B), whereas no such changes were detected in the intensity of NF-H staining or in the amount of NF-H positive fibers (Fig. 4C). The pyramidal neurons of the CA1 region remained NF-H, NF-M, and NF-L negative at both age groups.
| Hippocampal region | Control cultures | Kainic acid treated | ||
|---|---|---|---|---|
| 11 DIV (n = 26) | 18 DIV (n = 36) | 11 DIV (n = 28) | 18 DIV (n = 31) | |
| CA3a/b | 4 | 4 | 2* | 2* |
| CA3c | 4 | 4 | 2* | 1a* |
| CA1 | 4 | 4 | 3 | 3 |
| Granule cells | 4 | 4 | 3 | 2a* |
- † Slice cultures were treated with 5 μM kainic acid for 48 hr at 7 and 14 DIV and thereafter cultured for 2 further days prior to staining with thionin blue. The cell death was scored as described in the Method section. Results are given as means. Number of slices is given in parentheses and represent slices from 4–6 different culture batches. DIV, days in vitro.
- a Significance of differences between the groups in KA-treated cultures.
- * P < 0.05 from the corresponding control.
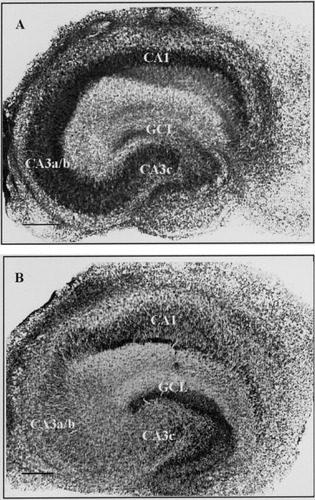
Thionin staining in representative hippocampal slices cultured for 11 days in normal medium (A) and in the presence of KA (5 μM, 48 hr) (B) as described in detail in the Materials and Methods. In the treated culture, the staining in the CA3a/b region is almost completely abolished indicating neuronal cell death. Note the widening of the pyramidal cell layers, which is a common phenomenon in cultured slices. Note that because the focusing of the image is done on the CA3 regions, the upper blade of the dentate gyrus in A is not properly visualized. Abbreviation: GCL, granule cell layer. Scale bars = 300 μm.
| Region | 11 DIV | 18 DIV |
|---|---|---|
| NF-L Immunoreactivity | ||
| Pyramidal neurons | ||
| CA1 | − | − |
| CA3a/b | +++ | +++ |
| CA3c | ++ | ++ |
| Dentate gyrus | ||
| Granule cells | ± | ++ |
| Molecular layers | +++ | ++ |
| NF-M Immunoreactivity | ||
| Pyramidal neurons | ||
| CA1 | − | − |
| CA3a/b | ++ | ++ |
| CA3c | + | ++ |
| Dentate gyrus | ||
| Granule cells | − | − |
| Molecular layers | ++ | ++ |
| NF-H Immunoreactivity | ||
| Pyramidal neurons | ||
| CA1 | − | − |
| CA3a/b | ++ | ++ |
| CA3c | ++ | ++ |
| Dentate gyrus | ||
| Granule cells | − | − |
| Molecular layers | + | + |
- *Scoring is a mean of 7–10 slices from 2–3 different culture batches. −, negative; ±, weakly positive; +, positive; ++, strongly positive; +++, very strongly positive. DIV, days in vitro.
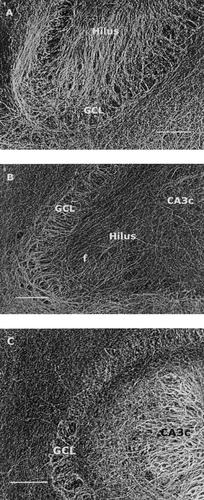
Inverted images of NF-L, NF-M, and NF-H immunostaining in hippocampal slices cultured in the presence of KA (5 μm, 48 hr) as described in the Methods. A: NF-L staining in one representative slice of 11 DIV. Note the massive increase in immunopositive fibers in the hilar region and molecular layers. A similar abundant increase in the amount of fibers was never seen in the respective areas in control cultures. B: NF-M immunostaining in one representative slice of 11 DIV and NF-H immunostaining in a slice of 18 DIV (C). Note that in all cases the outgrowth of fibers is restricted to certain molecular layers. The inner, middle and outer molecular layers cannot be reliably delineated. f, fibers; GCL, granule cell layer. Scale bars = 100 μm.
Immunoblotting
Figure 5A shows one representative immunoblot of NF-L, NF-M, and NF-H proteins in cultured slices at 11, 18, and 25 DIV and the changes after KA treatment. In the control cultures at 11 DIV throughout 25 DIV, the signal of NF-M was more intense than those of NF-L and NF-H (Fig. 5A), although it significantly (P < 0.01) decreased during the in vitro maturation of slices, whereas the signals of NF-L and NF-H remained unchanged (Fig. 5B–D). The treatment with KA significantly (P < 0.0001) decreased the signal of all three NF proteins in both age groups, the reduction being significantly (P < 0.003) more pronounced in the older than in the younger slices. The signal of NF-H protein was most dramatically decreased after KA treatment. The reduction was 75% at 11 DIV, and 83% at 18 DIV, whereas the reduction of NF-M signal was only 27% and 44% at 11 DIV and 18 DIV, respectively. The corresponding values for NF-L were 48% and 68%, respectively.
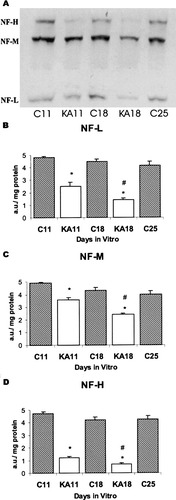
A: A representative immunoblot of NF-H, NF-M, and NF-L proteins in the control cultures at 11 (C11), 18 (C18) and 25 (C25) DIV, and at 11 (KA11) and 18 (KA18) DIV in KA treated cultures. Changes in the signals of NF-L (B), NF-M (C), and NF-H (D) proteins in immunoblots during the in vitro maturation of slices, and changes in them after KA treatment. The slices were processed for immunoblotting as described in the Materials and Methods section. The results are given as arbitrary units/mg protein. *Denotes the significance of differences from the corresponding control, and # denotes the significance of differences between 11 and 18 DIV in KA treated slices. Data are given as means ± SEM (bars) in each group (n = 8). a.u, arbitrary unit; C, control; KA, kainic acid.
DISCUSSION
Distribution and Amount of the Three Neurofilament Proteins in Cultured Organotypic Hippocampal Slices During the In Vitro Maturation
Our study showed a cell type selective, rather than ubiquitous distribution of the NF protein triplet in organotypic hippocampal slice cultures. This regionally heterogeneous distribution of NFs in the central nervous system, including the hippocampus, has previously been detected in adult rats, guinea pigs, and rabbits (Vickers and Costa, 1992; Yang et al., 1995; Van der Zee et al., 1997). Furthermore, we could not detect any pronounced developmental changes in the NF protein immunoreactivity as analyzed by immunoblotting and immunocytochemistry during the in vitro maturation of hippocampal tissue, which is comparable to the rat pups of P16–P32 of age, at least in the number of total postnatal days. The pyramidal neurons of CA3 regions together with their apical dendrites were heavily stained with all three NF-antibodies throughout the in vitro age groups. Consistent with these results, in P5 rat pups no SMI 311 immunoreactivity (antibody, that recognizes the non-phosphorylated epitopes on NF-M and NF-H subunits) has been detected in any hippocampal region at P5, but by P10 the staining pattern has generally resembled that of an adult rat and remained so at P15 (Shetty and Turner, 1995). In the study by Shetty and Turner (1995), the pyramidal neurons of CA3 and CA4 (corresponding to our CA3a/b and CA3c regions, respectively) and their apical dendrites have heavily been labeled, whereas the CA1 pyramidal neurons have been completely negative. Although this finding is in line with our present finding, some earlier studies have shown weak immunoreactivity of NF proteins in the CA1 neurons in adult guinea pigs, gerbils, and rabbits (Nakamura et al., 1992, Vickers and Costa, 1992, Van der Zee et al., 1997).
In contrast to the intense NF protein staining of neurons in CA3 and an absence of staining in CA1 pyramidal neurons, the other hippocampal regions showed a less clear-cut staining pattern. Strangely enough, only some weakly NF-L stained granule cells were detected throughout all age groups, but NF-M and NF-H positive granule cells only at older cultures. This is partly in keeping with the study of Shetty and Turner (1995) in which no SMI 311 immunoreactivity was observed in granule cells of P5-P15, 1-month-old or even in 4-month-old rats. The reason for the absence of NF-H staining in young cells could be due to the fact that granule cells are still proliferating at 11-25 DIV (Frotscher et al., 1995) and NF-H protein is suggested to appear later than NF-L during the cell maturation (Dahl and Bignami, 1986; Carden et al., 1987; Foster et al., 1987). There could also be species-related differences in the expression of NF proteins in various types of neurons. In 1-month-old rabbit, nearly the entire granule cell layer and pyramidal neurons have intensively been stained with antibody against NF-L protein (enzymatically dephosporylated pig NF-L) (Van der Zee et al., 1997). Although the comparison with earlier studies is difficult because several antibodies for the three NFs, which specifically recognize phosphorylated, non-phosphorylated, and phosphorylation-independent epitopes have been used, our present findings together with earlier studies suggest that in the developing hippocampus, the adult-like cellular heterogeneity of NF protein expression is already established at the early postnatal period. More importantly, our present findings strongly suggest that this in vivo cellular heterogeneity is expressed and preserved in vitro in hippocampal neurons in organotypic cultures. The reasons for the cellular heterogeneity of NF protein expression, at least as detected with immunocytochemical methods, in the hippocampus is not currently known.
Changes in the NF-L, MF-M, and NF-H Proteins in Kainic Acid Treated Organotypic Hippocampal Slices
In our cultured slices, a robust increase in NF-L and a moderate increase in NF-M immunoreactive fibers after KA treatment was detected in the restricted hippocampal areas, in the molecular layers and in the hilar region of the dentate gyrus in both in vitro age groups. The molecular layers normally contain the dendrites of granule cells (Caeser and Aertsen, 1991), but in epileptic brain they also contain the aberrantly sprouted axons of granule cells, the mossy fibers (Tauck and Nadler, 1985; Isokawa et al., 1993; Okazaki et al., 1999). In adult rats, an increase in NF-L immunoreactivity has been detected in the mossy fiber layer, in the distal apical dendritic segments of CA3, and in the dentate supragranular layer 4 weeks after KA injection (Wang et al., 1994). In the present study the changes, however, appeared at a much earlier phase. In contrast to relatively small changes in the pattern of cellular immunostaining after KA treatment, significant decreases were observed in the immunoblots of all three NFs. It is noteworthy, that the KA treatment differently affected the three NFs. The signal of NF-H was most dramatically decreased, followed by NF-L and NF-M, more so in the older slices. Consistent with these results, in adult rats a pronounced decrease in phosporylation-related immunoreactivity of NF-H has occurred as early as 1 day after KA treatment (Wang et al., 1994). Also a marked decrease in NF-H staining of mossy fibers has been detected 7 days after KA treatment (Yang et al., 1995). To our knowledge there are no studies on NF proteins in any experimental animal model of epilepsy in the immature brain. Because the intrinsic properties of hippocampus are well preserved in cultured slices (Dailey et al., 1994; Frotscher et al., 1995) we suggest that changes in the immunostaining of NF proteins after KA treatment in cultured slices are comparable to those observed in vivo. This reactivity is a part of a cascade leading to neuronal degeneration and is followed by plastic changes in the neurons of the immature hippocampus.
In addition to the variations in the amounts of the NF proteins in pathological stages, phosporylation/dephosphorylation is thought to be one early sign in situations such as ischemia, seizures and traumatic brain injury (Kaku et al., 1993; Wang et al., 1994; Lee and Cleveland, 1996), and changes in dendrites preceding those in axons (Posmantur et al., 2000). In adult rats a shift of NF-H from the phosphorylated to non-phosphorylated form has been detected in many brain areas, including the hippocampus, as early as 1 day after KA injection in adult rats (Wang et al., 1994), and within days after seizures induced by chronic electroconvulsive treatment (Vaidya et al., 2000). Because we used antibodies that recognize phosporylation-independent epitopes of the NF proteins, no conclusions can be drawn about the effect of KA on the phosphorylation/dephosphorylation of NF proteins in our slices.
Usefulness of Organotypic Hippocampal Slice Cultures As An In Vitro Model System of Reorganization and Plasticity in the Developing Hippocampus
The preparation of hippocampal slice cultures results in a total deprivation of afferent connections from the entorhinal cortex via the perforant pathway to the dentate gyrus. Loss of afferent excitatory connections to dentate gyrus induces sprouting of mossy fibers in adult and developing rats in vivo (Laurberg and Zimmer, 1981; Frotscher and Zimmer, 1983) and to some extent also in cultured hippocampal slices (Caeser and Aertsen, 1991; Routbort et al., 1999). The extensive mossy fiber sprouting and epileptiformic activity in the dentate gyrus, however, have been associated with epilepsy in both adult animal models of epilepsy (Cronin et al., 1992; Okazaki et al., 1999) and in cultured hippocampal slices (Routbort et al., 1999). In addition to mossy fiber sprouting, neuronal death in CA3 and CA1 regions has been detected in animal models of epilepsy in both adult (Sperk, 1994) and immature animals (Sankar et al., 1998, Thompson et al., 1998) as well as in cultured hippocampal slices upon KA treatment (Rimvall et al., 1987; Vornov and Coyle, 1991; Routbort et al., 1999). In keeping with our present findings, KA treatment has resulted in a pronounced loss of CA3 pyramidal neurons in the in vitro stages and conditions comparable to ours (Rimvall et al., 1987; Bruce et al., 1995).
Based on our findings we suggest that KA-induced changes in the immunoreactivity of NF proteins reflects not only the dynamic properties of these proteins but also the ongoing reorganization of neuronal cytoskeleton in cultured hippocampal slices. This finally leads to a robust mossy fiber sprouting, which is suggested to be intrinsic to the hippocampus and preserved in culture conditions (Routbort et al., 1999). Thus, KA treatment of hippocampal slices can be used as an in vitro model to study the significance of NF proteins in seizure-induced reorganization of the hippocampus in the developing brain under well-controlled experimental conditions.
Acknowledgements
IEH thanks professor James McNamara, the Epilepsy Research Unit, Duke University, NC, USA for a fruitful stay at the Research Unit, and Mark J. Routbort, MD, PhD, Duke University, for teaching the method of hippocampal slice cultures. We thank Sanna Soini, MSc and Kalevi Leppä, MD for technical assistance in processing the images. The financial support of the Arvo and Lea Ylppö Foundation and the Finnish Academy of Sciences to I.E.H, the Finnish Academy of Sciences to Esa R. Korpi for supplying the technical equipment, Sigrid Juselius Foundation to I.E.H. and H.B.L, and NIH grant (NS17771) to James McNamara are gratefully acknowledged.




