Cytomegalovirus Infection in Seropositive Kidney Transplant Recipients With Diverse Immunological Risks Under Preemptive Strategy
ABSTRACT
Cytomegalovirus (CMV) infection remains the most prevalent viral infection in kidney transplant recipients. Despite effective preventive strategies, the use of desensitization therapies and potent immunosuppressive agents in patients with high immunological risks underscores the continued importance of CMV as a major concern. This study aims to determine the incidence and outcomes of CMV infection in patients with diverse immunological risks under preemptive stategies. We analyzed 614 CMV-seropositive kidney transplant recipients managed under preemptive strategies. Of them, 231 patients (37.6%) underwent immunologically incompatible transplantation, including 75 ABO- and 156 HLA-incompatible transplants. During the median follow-up of 60 months, 354 patients (57.7%) experienced CMV infection. Multivariable analysis identified older recipient age, deceased donor, rituximab, and anti-thymocyte globulin as independent risk factors for CMV infection, while the use of mammalian target of rapamycin inhibitor was protective. Multivariable Cox regression analysis confirmed that CMV infection was independently associated with increased risks of death-censored graft loss (adjusted hazard ratio [aHR], 2.05; 95% confidence interval [CI], 1.17–3.59) and all-cause mortality (aHR, 3.40; 95% CI, 1.60–7.23). CMV infection adversely affects graft and patient outcomes in seropositive recipients managed under preemptive strategies. These findings emphasize the need for optimized CMV prevention strategies in recipients with high immunological risks, hereby intensified preemptive strategies or immunological risk-adapted antiviral prophylaxis are key options.
1 Introduction
Cytomegalovirus (CMV) infection remains the most prevalent viral complication following kidney transplantation (KT) [1]. Beyond the direct impact of the infection itself, CMV infection can lead to secondary complications, such as allograft rejection and other immune-mediated injuries [2]. These challenges significantly complicate posttransplant management, resulting in poorer graft survival and increased patient morbidity [3, 4]. Consequently, effective CMV prevention has become critical for improving transplant outcomes.
The most significant risk factor for CMV infection is the donor/recipient serostatus, in conjunction with the net state of immunosuppression [5]. International guidelines offer prevention strategies tailored to the serostatus profiles, and both prophylaxis and preemptive approaches are considered effective for seropositive recipients [6]. However, their efficacy and clinical impact in patients with diverse immunological risks are not well understood. This gap is particularly relevant in high risk patients—those undergoing ABO and/or HLA incompatible transplants—who require desensitization therapies and intensive immunosuppression.
Furthermore, while universal prophylaxis is commonly adopted in many Western countries, in South Korea, universal prophylaxis for CMV is not covered by national healthcare insurance for seropositive recipients unless infection is confirmed [7]. As a result, the preemptive strategy is used as the standard approach, even in high-risk patients undergoing desensitization and receiving potent immunosuppression. However, there is a lack of evidence regarding the incidence and clinical outcomes of CMV infection in high-risk populations managed with this strategy.
Given these gaps, there is an urgent need to expand our understanding of CMV prevention in relation to varying immunological risks. Therefore, this study addresses the incidence and impact of CMV infection in seropositive KT recipients managed under preemptive strategies, with a specific focus on individuals at high immunological risk.
2 Methods
2.1 Study Population
We screened consecutive adults who underwent KT between January 2010 and December 2020 at Severance Hospital, Seoul, Republic of Korea. Patients who received multiorgan transplants were excluded, as were those who were CMV-seronegative, lacked CMV serostatus data, or had insufficient CMV surveillance. We also excluded patients who experienced graft loss or death within 1 month posttransplant. After applying these criteria, 614 KT recipients were included in this study (Figure 1).
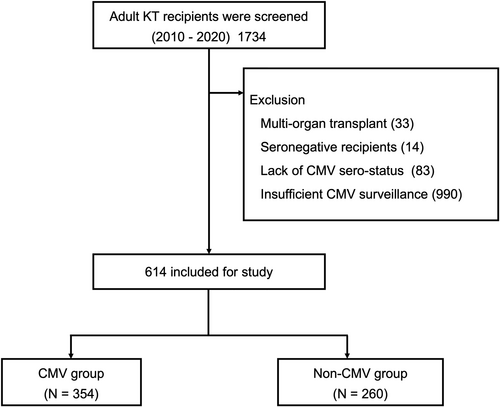
All study procedures were conducted in accordance with the Declaration of Helsinki and were approved by the Institutional Review Board of Severance Hospital (4-2023-1123). Informed consent was waived by the Institutional Review Board of Severance Hospital because of the retrospective nature of the study.
2.2 CMV Monitoring and Management
For CMV surveillance, we employed quantitative nucleic acid testing (QNAT) to measure CMV-DNA in plasma samples at predetermined intervals posttransplantation: 1, 3, 6, 9, and 12 months. Additional CMV-QNAT was performed at the physician's discretion for example, in cases of clinical symptoms suggestive of CMV infection, such as fever or neutropenia. CMV infection was defined as plasma CMV loads exceeding a lower limits of quantification (LLOQ) [8]. In this study, the LLOQ for plasma CMV DNA using QNAT was 500 copies/mL (equivalent to 195 IU/mL) before June 2020, and was updated to 34.5 IU/mL from June 2020 onward.
For treatment for CMV, oral valganciclovir or intravenous ganciclovir was administed. Plasma CMV-DNA loads were followed initially weekly and then bi-weekly until undetectable. Concurrently with antiviral treatment, we reduced the dosing of mycophenolate mofetil (MMF) by 50% based on the plasma CMV-DNA loads and the neutrophil counts, conducted every 1–2 weeks.
2.3 Immunosuppression
Immunosuppression and desensitization therapies were performed as described previously [9, 10]. Basiliximab or anti-thymocyte globulin (ATG) was selected for induction based on each patient's immunological risk. Maintenance immunosuppression consisted of a calcineurin inhibitor (CNI, tacrolimus or cyclosporine), prednisolone, and MMF. The initial dose of methylprednisolone (500–1000 mg) was gradually reduced and replaced with oral prednisolone (5–10 mg/day) during the first 3 weeks after transplantation. MMF was initiated at 1.0–1.5 g/day and then adjusted to minimize adverse effects such as neutropenia or gastrointestinal disturbances. mTOR inhibitors were selectively used in patients enrolled in clinical trials or with a cancer history [11].
Patients undergoing ABO- and/or HLA-incompatible KT received desensitization therapy with rituximab, plasmapheresis, with or without intravenous immunoglobulin (IVIG) [9]. In the present study, HLA-incompatible KT was defined as KT in patients with detectable donor-specific antibody of any strength (cytotoxic crossmatch, flowcytometric crossmatch, or single-antigen assay). Rituximab was administered as a single dose within 1 month before KT. A 375 mg/m2 dose was given to those undergoing HLA-incompatible KT or ABO-incompatible KT with anti-A/B IgG titers ≥ 1:256, while a fixed dose of 200 mg was given to ABO-incompatible KT recipients with titers ≤ 1:128. The number of plasmapheresis sessions depended on baseline antibody titers, crossmatch results, response to plasmapheresis, and real-time antibody measurements during desensitization. Patients receiving HLA-incompatible KT or ABO-incompatible KT with titers ≥ 1:256 also received 100 mg/kg IVIG after each plasmapheresis. Patients with panel-reactive antibodies > 50% received single dose of rituximab before KT.
2.4 Study Endpoints
The primary study endpoint was the occurrence of CMV infection. The secondary endpoints included death-censored graft survival and patient survival. Death-censored graft loss was defined as a return to long-term dialysis or re-transplantation. Death-censored graft survival was calculated from the date of transplantation to the date of graft loss or December 31, 2022 (the end of the follow-up period). In cases of death with a functioning graft, graft survival was censored at the time of death.
2.5 Statistical Analysis
Data were expressed as frequency, mean with standard deviation or median with interquartile range (IQR), depending on the data type. Categorical variables were compared using the chi-square or Fisher's exact test, as appropriate. Continuous variables were compared using Student's t-test for parametric data or the Mann–Whitney test for nonparametric data. CMV risk factor was evaluated with multivariable logistic analysis. CMV-free survival, death-censored graft survival, and patient survival were analyzed using Kaplan–Meier curves and the log-rank test. Cox proportional hazard regression models were used to evaluate the associations between the CMV infection and time to events (death-censored graft loss and patient death). Three models were created: model 1, unadjusted; model 2, adjusted for age, sex, and deceased donor; and model 3, adjusted for the model 2 factors plus diabetes mellitus, duration of dialysis, ABO-incompatible KT and HLA-incompatible KT. Statistical analyses were performed using SAS statistical software (version 9.4; SAS Inc., Cary, NC, USA) and R (version 3.4.3; R Foundation for Statistical Computing, Vienna, Austria). All tests were two-tailed, and p-values less than 0.05 were considered statistically significant.
3 Results
3.1 Baseline Characteristics
The baseline characteristics of the study population are summarized in Table 1. Patients who developed CMV infection had higher HLA mismatch, longer dialysis duration, and older donor age compared with those who did not. The proportion of living donors was significantly higher in the non-CMV group than in the CMV group (71.9% vs. 54.8%; p < 0.001). Recipient age, recipient/donor sex, body mass index, diabetes mellitus, and re-transplantation rates did not differ significantly between the two groups.
| Variables | Non-CMV group (n = 260) | CMV group (n = 354) | p value |
|---|---|---|---|
| Recipient age, year | 49.1 ± 11.3 | 50.5 ± 11.5 | 0.127 |
| Sex, female | 119 (45.8) | 171 (48.3) | 0.534 |
| BMI, kg/m2 | 22.7 ± 3.4 | 22.9 ± 3.3 | 0.453 |
| Diabetes mellitus | 75 (28.8) | 106 (29.9) | 0.768 |
| Re-transplant | 22 (8.5) | 32 (9.0) | 0.803 |
| HLA mismatch | 3.1 ± 1.6 | 3.4 ± 1.6 | 0.027 |
| Duration of dialysis, month | 11.5 [1.0, 81.8] | 36.0 [2.0, 101.3] | 0.004 |
| Living donor | 187 (71.9) | 194 (54.8) | < 0.001 |
| Donor age, year | 44.3 ± 12.5 | 47.7 ± 13.2 | 0.001 |
| Donor sex, female | 124 (47.7) | 162 (45.8) | 0.636 |
- Note: Data are presented as number (percentage), mean ± standard deviation, or median [interquartile range].
- Abbreviations: BMI, body mass index; HLA, human leukocyte antigen.
3.2 Immunologic Risk and Immunosuppression
Of the total 614 patients, 231 (37.6%) underwent immunologically incompatible KT: 75 ABO-incompatible and 156 HLA-incompatible (Table 2). Among these, 28 patients had both ABO- and HLA-incompatible KT. The proportion of ABO-incompatible cases was significantly lower in the CMV group than in the non-CMV group (10.2% vs. 15.0%; p < 0.001), while HLA-incompatible KT was significantly more common in the CMV group (34.5% vs. 13.1%; p < 0.001).
| Variables | Non-CMV group (n = 260) | CMV group (n = 354) | p value |
|---|---|---|---|
| Pretransplant immunological risk | |||
| ABO-incompatible KT | 39 (15.0) | 36 (10.2) | < 0.001 |
| HLA-incompatible KT | 34 (13.1) | 122 (34.5) | < 0.001 |
| CDC XM (+) | 5 (1.9) | 26 (7.3) | |
| Flow cytometry XM (+) | 12 (4.6) | 49 (13.8) | |
| Donor-specific antibody (+) | 17 (6.5) | 47 (13.3) | |
| Induction agents | < 0.001 | ||
| No induction | 0 (0.0) | 2 (0.6) | |
| IL-2 inhibitor | 185 (71.2) | 104 (29.4) | |
| Anti-thymoglobulin | 75 (28.8) | 248 (70.0) | |
| Low cumulative dose (≤ 6.0 mg/kg) | 59/75 (78.7) | 127/248 (51.2) | |
| High cumulative dose (> 6.0 mg/kg) | 16/75 (21.3) | 121/248 (48.8) | |
| Maintenance immunosuppressants | 0.125 | ||
| Tacrolimus | 237 (91.2) | 334 (94.4) | |
| Cyclosporine | 23 (8.8) | 20 (5.6) | |
| MMF | 203 (78.1) | 315 (89.0) | < 0.001 |
| mTOR inhibitor | 36 (13.8) | 12 (3.4) | < 0.001 |
- Note: Data are presented as number (percentage), mean ± standard deviation, or median [interquartile range].
- Abbreviations: CDC, complement-dependent cytotoxicity; HLA, human leukocyte antigen; IL, interleukin; MMF, mycofenolate mofetil; mTOR, mammalian target of rapamycin; XM, crossmatch.
Induction agent use also differed significantly (p < 0.001). In the non-CMV group, 71.2% received basiliximab, versus 29.4% in the CMV group. Conversely, 70.0% of CMV-infected patients received ATG, versus 28.8% in the non-CMV group. Regarding ATG dosage, 48.8% (121/248) of patients with CMV infection received > 6.0 mg/kg, whereas 78.7% (59/75) in the non-CMV group received ≤ 6.0 mg/kg. In terms of maintenance immunosuppressants, more than 90% of patients in both groups used tacrolimus. In the CMV group, MMF was used significantly more frequently (89.0% vs. 78.1%; p < 0.001), whereas mTOR inhibitors were used significantly less frequently (3.4% vs. 13.8%; p < 0.001) compared to the non-CMV group.
3.3 CMV Infection
The median follow-up for the entire cohort was 60 months (IQR, 39–101). During this period, 354 patients (57.7%) experienced CMV infection, with a median time to onset of 29 days (IQR, 16–65) (Supporting Information S1: Figure S1). The median peak CMV-DNA loads was 3763.5 IU/mL (IQR, 937.0–28 567.5). Among those with CMV infection, 7.3% (26/354) developed CMV syndrome, and three patients (0.8%) had tissue-invasive disease (one enterocolitis, one pneumonitis, one nephritis).
3.4 Risk Factors for CMV Infection
Multivariable logistic regression identified older recipient age (adjusted odds ratio [aOR], 1.015; 95% CI, 1.000–1.029; p = 0.044), use of rituximab (aOR, 1.717; 95% CI, 1.113–2.649; p = 0.015), and deceased donor transplantation (aOR, 1.683; 95% CI, 1.081–2.619; p = 0.021) as significant risk factors for CMV infection (Table 3). ATG was also a risk factor regardless of cumulative dose. A high ATG dose (> 6.0 mg/kg) had an aOR of 10.595 (95% CI, 5.804–19.340; p < 0.001), while a lower dose (≤ 6.0 mg/kg) had an aOR of 2.712 (95% CI, 1.776–4.144; p < 0.001). Conversely, mTOR inhibitor use was associated with a lower risk of CMV infection (aOR, 0.277; 95% CI, 0.126–0.612; p = 0.001).
| Multivariablea | ||
|---|---|---|
| Variables | aOR (95% CI) | p value |
| Recipient age | 1.015 (1.000, 1.029) | 0.044 |
| Use of Rituximab | 1.717 (1.113, 2.649) | 0.015 |
| Use of Anti-thymoglobulin | ||
| No use | Reference | |
| Low cumulative dose (≤ 6.0 mg/kg) | 2.712 (1.776, 4.144) | < 0.001 |
| High cumulative dose (> 6.0 mg/kg) | 10.595 (5.804, 19.340) | < 0.001 |
| Deceased donor | 1.683 (1.081, 2.619) | 0.021 |
| Use of mTOR inhibitor | 0.277 (0.126, 0.612) | 0.001 |
- Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; mTOR, mammalian target of rapamycin.
- a Model was established using the backward conditional method, entering covariates with p < 0.2 on univariable analysis.
3.5 Graft and Patient Outcomes
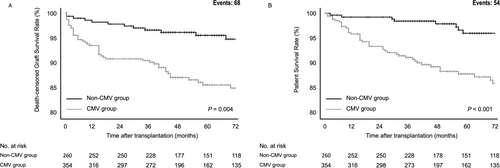
CMV-infected patients had significantly lower death-censored graft survival than those without CMV (p = 0.004). The death-censored graft survival rates in the CMV group were 90.8%, 87.1%, and 84.9% at 2, 4, and 6 years, respectively, compared with 97.7%, 96.0%, and 94.7% in the non-CMV group (Figure 2A). Patient survival was also significantly lower in the CMV group (p < 0.001). Specifically, the patient survival rates were 93.4%, 89.3%, and 86.6% at 2, 4, and 6 years, respectively, versus 99.2%, 97.9%, and 96.0% in the non-CMV group (Figure 2B).
The most common cause of death-censored graft loss in both groups was acute rejection. In the CMV group, acute rejection accounted for 47.0% of death-censored graft losses, compared to 26.3% in the non-CMV group. Chronic rejection and BK virus nephropathy were the next most common causes (Table 4).
| Causes of DCGL | Non-CMV group (n = 19) | CMV group (n = 49) |
|---|---|---|
| Rejection | 8 (42.1) | 31 (63.2) |
| Acute rejection | 5 (26.3) | 23 (46.9) |
| TCMR | — | 5 |
| ABMR | 3 | 11 |
| TCMR + ABMR | 2 | 7 |
| Chronic rejection | 3 (15.8) | 8 (16.3) |
| TCMR | — | 4 |
| ABMR | 3 | 4 |
| Glomerulonephritis | 1 (5.3) | — |
| Graft infection | 4 (21.1) | 6 (12.2) |
| BKV nephritis | 4 | 5 |
| Invasive aspergillosis | 0 | 1 |
| Graft thrombosis | — | 1 (2.0) |
| Noncompliance | — | 1 (2.0) |
| Primary nonfunction | 1 (5.3) | 3 (6.1) |
| Others | 3 (15.8) | 7 (14.3) |
- Note: Data are presented as number (percentage).
- Abbreviations: ABMR, antibody-mediated rejection; BKV, BK virus; DCGL, death-censored graft loss; TCMR, T cell-mediated rejection.
In the multivariable Cox proportional hazards model, CMV infection was significantly associated with death-censored graft loss (aHR, 2.050; 95% CI, 1.171–3.591; p = 0.012, Model 3) and all-cause mortality (aHR, 3.402; 95% CI, 1.600–7.234; p = 0.001, Model 3) across all three models (Table 5).
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| DCGL | ||||||
| CMV infection | 2.119 (1.247, 3.600) | 0.005 | 1.933 (1.125, 3.319) | 0.017 | 2.050 (1.171, 3.591) | 0.012 |
| Patient death | ||||||
| CMV infection | 4.255 (2.078, 8.713) | < 0.001 | 3.774 (1.818, 7.835) | < 0.001 | 3.402 (1.600, 7.234) | 0.001 |
- Note: Model 1, unadjusted model; model 2, adjusted for age, sex, donor age and deceased donor; model 3, adjusted for model 2 factors plus diabetes mellitus, duration of dialysis, ABO-incompatible KT and HLA-incompatible KT.
- Abbreviations: CI, confidence interval; DCGL, death-censored graft loss; HR, hazard ratio.
4 Discussion
This study highlights the considerable impact of immunological risk factors on CMV infection rates among seropositive KT recipients managed with a preemptive strategy. Specifically, we observed that ATG and rituximab use—particularly in high-risk settings such as ABO- or HLA-incompatible KT—significantly increases the risk of CMV infection. Furthermore, multivariable analysis confirmed that CMV infection correlates with reduced death-censored graft survival and patient survival. These findings emphasize the importance of integrating immunological risk factors into CMV prevention strategies for KT recipients and suggest that personalized approaches may enhance patient outcomes.
Current international guidelines classify seropositive KT recipients as having “intermediate risk,” recommending either 3-month prophylaxis or a preemptive approach [6]. However, our findings show that actual CMV risk can vary substantially within this category, driven by immunological risk factors including induction agents and desensitization treatments. Seropositive recipients treated with ATG or rituximab, especially those with ABO or HLA incompatibilities, appear to be at higher risk. This indicates that universal management strategies may be insufficient and that stratifying CMV risk based on immunological factors is critical. Such stratification may guide future studies that compare prophylaxis and preemptive strategies specifically tailored to high-risk, seropositive recipients.
ATG, frequently used to prevent acute rejection in high immunological-risk recipients [12-14], is strongly associated with CMV infection when administered at higher doses [4, 15-17]. A previous clinical trial comparing ATG doses of 4.5 mg/kg and 6.0 mg/kg demonstrated higher CMV infection rates in the latter group, regardless of prophylaxis [15]. Another study found that a single 3 mg/kg dose of ATG reduced CMV infection risk in high-risk KT recipients without prophylaxis [16]. In line with these findings, our study shows that ATG doses > 6.0 mg/kg increase CMV infection risk by approximately 10.6-fold compared to no ATG use, while doses ≤ 6.0 mg/kg raise the risk by 2.7-fold. These data suggest that universal prophylaxis, rather than a preemptive strategy, may be warranted in seropositive patients receiving ATG.
Rituximab, a chimeric monoclonal antibody targeting CD20 on B cells, plays a crucial role in desensitization for KT [18-20]. While some studies report on rituximab's impact on CMV infection in hematological malignancies [21-23], there is limited research on its effect in KT recipients [9, 24]. This is because it is difficult to isolate the contribution of a specific agent like rituximab, given the use of various other desensitization therapies and potent induction agents in these patients. Our prior research did not detect a significant difference in CMV infection based on rituximab dose, but that study featured less-active CMV monitoring and a smaller sample size [9]. To the best of our knowledge, the current study is the first to definitively link rituximab use to higher CMV infection risk in KT recipients.
In KT recipients, mTOR inhibitors can substitute for MMF alongside tacrolimus [25]. Notably, mTOR inhibitors exhibit some antiviral effects [26], potentially due to the role of the mTOR pathway in CMV replication, as well as enhanced CD4+ and CD8 + T cells against CMV [27, 28]. Several multicenter prospective and randomized controlled trials have confirmed that mTOR inhibitors reduce CMV incidence, regardless of recipient serostatus or preventive strategies [29-31]. Our study corroborates these findings, indicating that mTOR inhibitor use is protective against CMV infection.
Previous research has often focused on short-term CMV incidence under prophylaxis or preemptive strategies [32-34], but less is known about long-term outcomes in the current era. Recent data from the United Network for Organ Sharing show that CMV mismatch remains an independent risk factor for graft loss and mortality [35]. Our results align with these observations, demonstrating that CMV infection under preemptive strategies impairs long-term graft and patient survival. In our study, acute rejection was a major cause of death-censored graft loss, consistent with the known but not fully elucidated link between CMV infection and rejection [2]. Plausible explanations include CMV-induced changes in immune cell activity that promote rejection, such as upregulating adhesion molecules, enhancing allograft infiltration and inflammation, modulating MHC expression, and inducing inflammatory NK and T cell responses.
We conducted this study in South Korea, where universal prophylaxis for CMV is not covered by national healthcare insurance for seropositive recipients until infection is confirmed. Consequently, we relied solely on a preemptive strategy even in high-risk patients. While universal prophylaxis is commonly used in many Western countries, it remains challenging in others—such as Brazil, Japan, and South Korea—due to insurance limitations [7, 36, 37]. Few studies have examined CMV occurrence and outcomes in a preemptive setting that also accounts for known risk factors like desensitization and potent immunosuppression. Our findings may guide the development of improved preventive strategies for seropositive patients in various healthcare settings worldwide.
This study has several limitations. First, it was a single-center retrospective study. However, single-center designs can benefit from uniform CMV monitoring, consistent immunosuppressive regimens, standardized patient management, and more complete outcome data. Second, we could not compare preemptive and prophylaxis approaches in seropositive recipients due to the lack of insurance coverage for prophylaxis. Finally, the LLOQ for CMV QNAT was revised once during the study period, which may have introduced minor variability in the timing of CMV detection and initiation of preemptive therapy.
In conclusion, CMV infection adversely affects graft and patient outcomes in seropositive KT recipients managed with a preemptive strategy. These findings emphasize the need for optimized CMV prevention strategies in recipients with high immunological risks, hereby intensified preemptive strategies or immunological risk-adapted antiviral prophylaxis are key options.
Author Contributions
Research idea and study design: Mun Chae Choi and Juhan Lee. Data acquisition: Mun Chae Choi, Minyu Kang, Hwa-hee Koh, Seung Hyuk Yim, Hyun Jeong Kim, and Juhan Lee. Data analysis/interpretation: Mun Chae Choi, Minyu Kang, Hwa-hee Koh, Seung Hyuk Yim, and Juhan Lee. Statistical analysis: Mun Chae Choi and Juhan Lee. Supervision and mentorship: Su Jin Jung, Hyung Woo Kim, Jaeseok Yang, Beom Seok Kim, Kyu Ha Huh, Myoung Soo Kim, and Juhan Lee. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual's own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Acknowledgments
The authors would like to express their sincere gratitude to Professor Hans H. Hirsch (University Hospital Basel, Switzerland) for his invaluable insights and suggestions during the development of this manuscript. This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2022-KH129774).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




