Lactate Dehydrogenase/Albumin to Urea Ratio: A Novel Prognostic Indicator for Adverse Outcomes in Patients With Severe Fever With Thrombocytopenia Syndrome
Ruihua Zhang, Yameng Mu, Mengyuan Zhang, Zhihai Chen, and Yaxian Kong contributed equally to this study.
ABSTRACT
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging infectious disease with significant mortality risks. This study evaluated the predictive value of the lactate dehydrogenase/albumin to urea ratio (LAU) for lethal outcomes in SFTS patients. Clinical data from 348 patients (295 survivors, 53 non-survivors) admitted to Yantai Infectious Disease Hospital (2022-2023) were analyzed. Multivariate analysis identified older age, SFTS-associated encephalopathy, SFTSV RNA, decreased albumin and serum calcium, increased lactate dehydrogenase, and elevated LAU as independent mortality risk factors. LAU demonstrated strong predictive accuracy (AUC: 0.79; 95% CI: 0.72-0.85; sensitivity 69%, specificity 75%), with an optimal cutoff of 144.685 U·mmol/g·L. Survival analysis linked high LAU to poor prognosis, and longitudinal trends showed decreasing LAU with recovery in survivors but rising levels in non-survivors. These findings highlight LAU as a novel, practical biomarker for early risk stratification and prognosis improvement in SFTS.
1 Introduction
Severe fever with thrombocytopenia syndrome (SFTS) was first discovered as a viral infectious disease in China in 2009 [1]. It was subsequently reported in South Korea in 2011 and in Japan in 2012 [2, 3]. Clinical manifestations are diverse, and mild cases may include fever, fatigue, gastrointestinal symptoms, thrombocytopenia, and leukopenia. Severe cases may manifest as bleeding, neurological damage, myocardial damage, multiple organ dysfunction, or even death. Haemaphysalis longicornis and Rhipicephalus microplus are possible carriers of severe fever with thrombocytopenia syndrome virus (SFTSV), which can transmit pathogens through tick bites [4-6]. In recent years, there have been reports of animal-to-human transmission [7] and human-to-human transmission [8, 9]. Since its incidence and mortality are gradually rising, and there is a lack of effective treatment methods, it has attracted increasing attention worldwide [10].
During clinical treatment, we have found that patients with SFTS, even if their symptoms are normal or mild, sometimes experience rapid deterioration of their condition, with a mortality rate of up to 30% [11]. Therefore, it is crucial to closely monitor fast, practical, and reliable indicators. There have been reports that various biomarkers can indicate adverse outcomes in patients with SFTS, such as neutrophil-to-lymphocyte ratio (NLR) [12], platelet-to-lymphocyte ratio (PLR) [13], and platelet-to-albumin ratio (PAR) [14]. However, dysregulation of the immune response caused by infection can lead to abnormal hematological parameters, making the diagnostic performance of these markers less stable. Studies have also proposed scoring models that include multiple clinical parameters, but their clinical applications are too complex [15]. Therefore, the search for simpler and more reliable composite biomarkers needs to be performed.
SFTSV infection can cause multiorgan damage, and lactate dehydrogenase (LDH), blood urea nitrogen (BUN), and serum albumin (ALB) are relevant indicators of multiorgan damage [16-18]. In clinical practice, these indicators change with the progression of the disease, which also proves this point. Previous studies have indicated the predictive effect of the composite biomarker BUN/ALB ratio (BAR) on SFTS [19]. However, this composite indicator lacks the indicators of myocardial injury indicators, which was a crucial clinical characteristic for patients with SFTS. Therefore, we attempted to include myocardial injury biomarkers to construct a more reasonable composite indicator. Previous studies have found that patients with a high level of LDH/albumin to urea (LAU) have an increased risk of death from coronavirus disease 2019 (COVID-19). LAU has been proposed as a potential predictor of COVID-19-induced fatal clinical complications in hospitalized patients [20]. To the best of our knowledge, there is no research focusing on the role of LAU in predicting the prognosis of SFTS, which prompts us to consider whether LAU can serve as a reliable biomarker for adverse outcomes in hospitalized patients with SFTS.
This study aimed to explore and evaluate the diagnostic value of LAU in SFTS patients by analyzing the influencing factors associated with poor prognosis of SFTS, with the aim of early identification of high-risk patients, timely diagnosis and treatment, and improving prognosis.
2 Materials and Methods
2.1 Research Object and Data Collection
This retrospective study included 348 patients with SFTS who were hospitalized at the Yantai Infectious Disease Hospital between January 2022 and December 2023. The inclusion criteria were as follows: (1) age ≥ 18 years old; (2) separate SFTSV or positive SFTSV nucleic acid testing from patient samples, or antibody titer increased four-fold in the double sample [21]. The exclusion criteria: (1) Incomplete clinical data (including demographic data, clinical symptoms, and laboratory data); (2) presence of any of the following diseases: autoimmune disease, chronic blood disease, chronic liver disease, end-stage renal failure, or tumor disease; (3) accompanying other acute infections in the past month: COVID-19, EB virus, and so on (screened through self-reporting plus confirmatory testing).
This study was conducted in line with the principles of the Helsinki Declaration. It was approved by the Local Ethics Committee of the Leading Center of Beijing Ditan Hospital, Capital Medical University (No. DTEC-KY2022-022-03). The patients and their families were informed of the study and signed relevant informed consent forms.
All patients received the treatment strategy recommended by the SFTS management plan and were divided into death and survival groups based on their survival outcomes. The observational endpoint of this study was death or survival. Death is defined as death caused by any reason, while survivors are those whose body temperature gradually returns to normal and whose symptoms alleviate to the point where they can be discharged. Follow up patients who have discontinued treatment or been discharged due to personal reasons until 28 days after onset of symptoms. Collect clinical data of patients within 24 h after admission through the electronic medical record system of Yantai Infectious Disease Hospital, including demographic (gender, age, underlying diseases, and outcome), clinical symptoms (fever, fatigue, anorexia, nausea, abdominal pain, diabetes, cough, bleeding, lymph node enlargement, and SFTS associated encephalopathy [SFTSAE]), and laboratory data (SFTSV RNA, white blood cell [WBC], neutrophil [NEU], lymphocyte [LYM], monocyte [MON], platelet [PLT], alanine aminotransaminase [ALT], aspartate aminotransferase [AST], ALB, kalium [K], calcium [Ca], BUN, creatinine [CREA], creatine kinase [CK], LDH, activated partial thromboplastin time [APTT]), all data were entered into an Excel file and cross-checked by two trained researchers. Among them, LAU = LDH * BUN/ALB; NLR = NEU/lymphocyte counts; PLR = PLT/lymphocyte counts; PAR = PLT/ALB; BAR = BUN/ALB.
Definition: Bleeding was defined as the presence of any of the following conditions: bleeding from the mouth or gums, skin bruising or patches, black stools, or bloody stools. SFTSAE was defined as the presence of any of the following conditions: dizziness, headache, confusion, coma, convulsions, muscle tremors, or neurological signs.
SFTSV RNA detection: Viral RNA was extracted from blood samples of all SFTS patients using a virus RNA kit (Daan Gene Company, Guangzhou, China). The viral load of SFTSV RNA was determined using a certified real-time fluorescent quantitative PCR kit with a sensitivity of 10 TCID50/mL and linear range of 1.0 × 10²−1.0 × 10⁶ TCID50/mL, following the manufacturer's instructions.
2.2 Statistical Analysis
The statistical analyses were conducted using the following software: SPSS 27.0 (SPSS Inc., Chicago, IL, USA), R Statistical Software (version 4.4.0; R Core Team 2022), GraphPad Prism 9.5, and Zstats v1.0 (www.zstats.net).
Normally distributed meteorological data were expressed as mean ± standard deviation (x ± s), and an independent sample t-test was used for comparison between groups. The measurement data of skewed distribution are expressed as the median (P25-P75), and the Mann–Whitney U test was used for comparison between groups. Categorical variables were expressed as ratios and categorical variables were compared between groups using the chi-square test or Fisher's exact probability test. All tests were two-tailed, and statistical significance was set at p < 0.05.
Univariate logistic regression was used to identify potential prognostic factors, and variables with p-values less than 0.05 were subsequently included in the multivariate logistic regression analysis. Using receiver operating characteristic (ROC) curve analysis, we determined the predictive ability of each indicator for mortality. The probability critical point for the optimal combination of sensitivity and specificity was determined using Youden's index. Kaplan–Meier survival analysis was used to evaluate the cumulative survival rates of groups with lower and higher LAU thresholds, and a logarithmic rank test was used to confirm the validity of the differences. Spearman's correlation analysis was used to explore the relationship between LAU and various clinical parameters. If the r value was greater than 0.2 and the p-value was less than 0.05, the correlation was considered significant (r > 0.2, p < 0.05).
3 Results
3.1 Demographic and General Characteristics
A total of 358 patients with SFTS diagnosed at Yantai Infectious Disease Hospital from January 2022 to December 2023 were screened using the inclusion and exclusion criteria. Finally, 348 patients were included in the study, with 53 in the death group and a mortality rate of 15.23%. A flowchart of the study is shown in Figure S1. The median age of all patients was 67, and the median age of the death group was higher than that of the survival group. Among all patients, there were 152 males (43.68%), and there was no significant gender difference between the survival and death groups. Among the previously existing basic diseases of patients with SFTS, the most common were hypertension (n = 89, 25.57%), diabetes (n = 65, 18.68%), cerebrovascular disease (n = 19, 5.46%), and coronary heart disease (n = 19, 5.46%). However, there was no significant difference between the survival and death groups of patients with SFTS with the above basic diseases (Table 1).
| All (n = 348) | Survival (n = 295) | Death (n = 53) | p | |
|---|---|---|---|---|
| Age (year) | 67.00 (59.00, 73.00) | 67.00 (58.00, 73.00) | 71.00 (62.00, 76.00) | 0.011 |
| Gender, n (%) | 0.391 | |||
| Male | 152 (43.68) | 126 (42.71) | 26 (49.06) | |
| Female | 196 (56.32) | 169 (57.29) | 27 (50.94) | |
| Underlying diseases, n (%) | ||||
| Diabetes, n (%) | 65 (18.68) | 56 (18.98) | 9 (16.98) | 0.731 |
| Hypertension, n (%) | 89 (25.57) | 74 (25.08) | 15 (28.30) | 0.621 |
| Coronary heart disease, n (%) | 19 (5.46) | 17 (5.76) | 2 (3.77) | 0.796 |
| Cerebrovascular disease, n (%) | 19 (5.46) | 16 (5.42) | 3 (5.66) | 1.000 |
| Clinical symptoms, n (%) | ||||
| Fever, n (%) | 334 (95.98) | 283 (95.93) | 51 (96.23) | 1.000 |
| Fatigue, n (%) | 317 (91.09) | 269 (91.19) | 48 (90.57) | 1.000 |
| Anorexia, n (%) | 325 (93.39) | 275 (93.22) | 50 (94.34) | 0.999 |
| Nausea, n (%) | 159 (45.69) | 140 (47.46) | 19 (35.85) | 0.118 |
| Abdominal pain, n (%) | 44 (12.64) | 39 (13.22) | 5 (9.43) | 0.445 |
| Diarrhea, n (%) | 119 (34.20) | 99 (33.56) | 20 (37.74) | 0.555 |
| Cough, n (%) | 82 (23.56) | 71 (24.07) | 11 (20.75) | 0.601 |
| Bleeding, n (%) | 49 (14.08) | 32 (10.85) | 17 (32.08) | < 0.001 |
| Lymph node enlargement, n (%) | 108 (31.03) | 90 (30.51) | 18 (33.96) | 0.617 |
| SFTAE, n (%) | 179 (51.44) | 136 (46.10) | 43 (81.13) | < 0.001 |
- Abbreviations: SFTS, severe fever with thrombocytopenia syndrome; SFTSAE, SFTS associated encephalopathy.
In terms of clinical manifestations, the common clinical manifestations of SFTS patients include fever in 334 cases (95.98%), anorexia in 325 cases (93.39%), fatigue in 317 cases (91.09%), SFTSAE in 179 cases (51.44%), nausea in 159 cases (45.69%), diarrhea in 119 cases (34.20%), lymph node enlargement in 108 cases (31.03%), cough in 82 cases (23.56%), bleeding in 49 cases (14.08%), and abdominal pain in 44 cases (12.64%). The number of patients with bleeding (32.08% vs. 10.85%, p < 0.001) and SFTSAE (81.13% vs. 46.10%, p < 0.001) was significantly higher in the death group than in the survival group (Table 1).
The laboratory test results of all patients upon admission are shown in Table 2. The laboratory indicators that showed significant differences between the survival group and the death group of SFTS patients are as follows: SFTSV RNA (p < 0.001), MON count (p = 0.010), PLT (p < 0.001), ALT (p = 0.016), AST (p < 0.001), K+ (p = 0.007), Ca2+ (p < 0.001), BUN (p < 0.001), CREA (p < 0.001), CK (p < 0.001), LDH (p < 0.001), APTT (p < 0.001), and LAU (p < 0.001).
| All (n = 348) | Survival (n = 295) | Death (n = 53) | p | |
|---|---|---|---|---|
| SFTSV RNAa (log10 TCID50/mL) | 3.28 (2.55, 4.07) | 3.05 (2.38, 3.82) | 4.62 (4.01, 5.62) | < 0.001 |
| WBCa (×109/L) | 2.12 (1.48, 3.46) | 2.15 (1.48, 3.50) | 2.07 (1.37, 2.89) | 0.352 |
| NEUa (×109/L) | 1.31 (0.84, 2.17) | 1.29 (0.83, 2.17) | 1.34 (0.96, 2.20) | 0.495 |
| LYMa (×109/L) | 0.51 (0.32, 0.81) | 0.54 (0.32, 0.84) | 0.43 (0.27, 0.62) | 0.051 |
| MONa (×109/L) | 0.15 (0.08, 0.33) | 0.15 (0.08, 0.39) | 0.10 (0.06, 0.20) | 0.010 |
| PLTa (×109/L) | 67.00 (51.00, 87.00) | 69.00 (53.50, 88.00) | 58.00 (41.00, 77.00) | < 0.001 |
| ALTa (U/L) | 71.60 (43.08, 130.82) | 66.80 (39.55, 116.60) | 87.90 (54.70, 178.20) | 0.016 |
| ASTa (U/L) | 147.65 (84.77, 295.45) | 130.90 (76.05, 255.40) | 292.90 (171.00, 681.90) | < 0.001 |
| ALBa (g/L) | 31.90 (29.00, 34.73) | 31.90 (29.10, 34.90) | 31.30 (28.30, 34.30) | 0.105 |
| K+a (mmol/L) | 3.61 (3.35, 3.97) | 3.59 (3.31, 3.95) | 3.79 (3.43, 4.39) | 0.007 |
| Ca2+a (mmol/L) | 2.03 (1.94, 2.12) | 2.03 (1.96, 2.14) | 1.96 (1.86, 2.05) | < 0.001 |
| BUNa (mmol/L) | 5.50 (4.08, 7.80) | 5.29 (3.90, 7.39) | 8.50 (5.60, 12.93) | < 0.001 |
| CREAa (umol/L) | 66.30 (54.27, 85.40) | 65.20 (53.00, 83.05) | 83.60 (61.40, 132.10) | < 0.001 |
| CKa (U/L) | 431.00 (197.75, 1047.50) | 386.00 (179.50, 942.50) | 879.00 (388.00, 2009.00) | < 0.001 |
| LDHa (U/L) | 595.50 (408.25, 929.50) | 540.00 (378.00, 842.50) | 999.00 (619.00, 1838.00) | < 0.001 |
| APTTa (s) | 48.90 (43.18, 57.02) | 48.00 (42.30, 53.55) | 62.10 (53.00, 77.00) | < 0.001 |
| LAUa (U mmol/g L) | 106.13 (59.31, 198.69) | 86.90 (55.34, 170.85) | 233.80 (145.34, 653.61) | < 0.001 |
- Abbreviations: ALB, albumin; ALT, alanine aminotransaminase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Ca, calcium; CK, creatine kinase; CREA, creatinine; HGB, hemoglobin; K, kalium; LAU, lactate dehydrogenase/albumin to urea ratio; LDH, lactate dehydrogenase; LYM, lymphocyte; MON, monocyte; NEU, neutrophil; PLT, platelet; SFTS, severe fever with thrombocytopenia syndrome; SFTSV, SFTS virus; WBC, white blood cell.
- a By means of the nonparametric test, expressed as median (P25–P75), M is the median, P25 is the lower quartile, P75 is the upper quartile.
3.2 Risk Factors for Death
Based on the clinical characteristics and laboratory parameters of patients, univariate logistic regression analysis showed that bleeding, SFTSAE, age, SFTSV RNA, PLT, AST, ALB, K+, Ca2+, BUN, CREA, CK, LDH, APTT, and LAU were associated with adverse mortality outcomes. Subsequently, these factors were included in a multiple logistic regression analysis, and the results showed that older age (odds ratio [OR]:1.05; 95% confidence interval [CI]: 1.01–1.10; p = 0.037), SFTSAE (OR: 2.60; 95% CI: 1.04–6.50; p = 0.04), high SFTSV RNA (OR: 5.53; 95% CI: 3.06–10.00; p < 0.001), decreased ALB (OR: 1.13; 95% CI: 1.01–1.25; p = 0.027), decreased serum Ca2+ (OR: 0.02; 95% CI: 0.00–0.24; p = 0.002), increased LDH (OR: 0.99; 95% CI: 0.99–0.99; p = 0.044), increased LAU (OR: 1.01; 95% CI: 1.01–1.01; p = 0.014) were independent risk factors for mortality in SFTS patients (Table 3).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | p | OR (95% CI) | p | OR (95% CI) |
| Age | 0.012 | 1.04 (1.01–1.07) | 0.037 | 1.05 (1.01–1.10) |
| Gender | 0.392 | 0.77 (0.43–1.39) | ||
| Diabetes | 0.731 | 0.87 (0.40–1.89) | ||
| Hypertension | 0.621 | 1.18 (0.61–2.27) | ||
| Coronary heart disease | 0.560 | 0.64 (0.14–2.86) | ||
| Cerebrovascular disease | 0.944 | 1.05 (0.29–3.72) | ||
| Fever | 0.920 | 1.08 (0.23–4.98) | ||
| Fatigue | 0.884 | 0.93 (0.34–2.54) | ||
| Anorexia | 0.763 | 1.21 (0.35–4.23) | ||
| Nausea | 0.62 | (0.34–1.13) | ||
| Abdominal pain | 0.68 | (0.26–1.82) | ||
| Diarrhea | 1.20 | (0.65–2.20) | ||
| Cough | 0.83 | (0.40–1.69) | ||
| Bleeding | < 0.001 | 3.88 (1.96–7.69) | ||
| Lymph node enlargement | 0.617 | 1.17 (0.63–2.18) | ||
| SFTAE | < 0.001 | 5.03 (2.43–10.38) | 0.040 | 2.60 (1.04–6.50) |
| SFTSV RNA | < 0.001 | 4.37 (2.95–6.48) | < 0.001 | 5.53 (3.06–10.00) |
| WBC | 0.991 | 1.00 (0.89–1.13) | ||
| LYM | 0.946 | 1.00 (0.91–1.11) | ||
| MON | 0.69 | (0.28–1.69) | ||
| PLT | 0.001 | 0.98 (0.97–0.99) | ||
| ALT | 0.095 | 1.00 (1.00–1.00) | ||
| AST | < 0.001 | 1.01 (1.01–1.01) | ||
| ALB | 0.018 | 0.92 (0.86–0.99) | 0.027 | 1.13 (1.01–1.25) |
| K+ | < 0.001 | 2.65 (1.57–4.47) | ||
| Ca2+ | < 0.001 | 0.02 (0.00–0.17) | 0.002 | 0.02 (0.00–0.24) |
| BUN | < 0.001 | 1.14 (1.08–1.21) | ||
| CREA | < 0.001 | 1.01 (1.01–1.01) | ||
| CK | 0.006 | 1.01 (1.01–1.01) | ||
| LDH | < 0.001 | 1.01 (1.01–1.01) | 0.044 | 0.99 (0.99–0.99) |
| APTT | < 0.001 | 1.06 (1.04–1.08) | ||
| LAU | < 0.001 | 1.01 (1.01–1.01) | 0.014 | 1.01 (1.01–1.01) |
- Abbreviations: 95% CI, 95% confidence interval; ALB, albumin; ALT, alanine aminotransaminase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Ca, calcium; CK, creatine kinase; CREA, creatinine; K, kalium; LAU, lactate dehydrogenase/albumin to urea ratio; LDH, lactate dehydrogenase; LYM, lymphocyte; MON, monocyte; OR, odds ratio; PLT, platelet; SFTS, severe fever with thrombocytopenia syndrome; SFTSV, SFTS virus; WBC, white blood cell.
3.3 Diagnostic Performance of LAU for Mortality Risk in SFTS Patients
To determine the diagnostic performance of LAU for fatal outcomes in patients with SFTS, the predictive efficiency of LAU was compared with that of several independent risk factors mentioned above. As shown in Figure 1A, the area under the curve (AUC) of SFTSV RNA is 0.87 (95% CI: 0.82–0.92; p < 0.0001), the LAU is 0.79 (95% CI: 0.72–0.85; p < 0.0001), the LDH is 0.75 (95% CI: 0.68–0.83; p < 0.0001), serum Ca2+ is 0.68 (95% CI: 0.60–0.76; p < 0.0001), age is 0.61 (95% CI: 0.53–0.69; p = 0.0108), and the ALB is 0.57 (95% CI: 0.49–0.65; p = 0.1049) (Table 4).
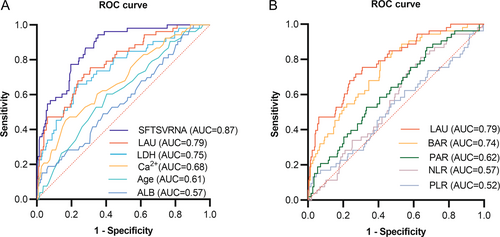
| Biomarkers | AUC | 95% CI | p value | Cut-off value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| Age | 0.61 | 0.53–0.69 | 0.0108 | 68.5 | 60 | 60 |
| SFTSV RNA | 0.87 | 0.82–0.92 | < 0.0001 | 3.474 | 65 | 94 |
| ALB | 0.57 | 0.49–0.65 | 0.1049 | 26.1 | 6 | 81 |
| Ca2+ | 0.68 | 0.60–0.76 | < 0.0001 | 1.925 | 17 | 55 |
| LDH | 0.75 | 0.68–0.83 | < 0.0001 | 891 | 78 | 64 |
| LAU | 0.79 | 0.72–0.85 | < 0.0001 | 144.685 | 69 | 75 |
| PAR | 0.62 | 0.54–0.70 | 0.0047 | 1.887 | 33 | 47 |
| PLR | 0.52 | 0.43–0.61 | 0.5873 | 32.224 | 3 | 87 |
| BAR | 0.74 | 0.67–0.81 | < 0.0001 | 5.29 | 59 | 77 |
| NLR | 0.57 | 0.49–0.64 | 0.1272 | 2.13 | 42 | 75 |
- Abbreviations: 95% CI, 95% confidence interval; ALB, albumin; AUC, area under the curve; BAR, blood urea nitrogen-to-albumin ratio; Ca, calcium; LAU, lactate dehydrogenase/albumin to urea ratio; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; PAR, platelet-to-albumin ratio; PLR, platelet-to-lymphocyte ratio; SFTSV, SFTS virus.
In addition, we compared LAU with several other classic metrics, such as NLR, PAR, PLR, and BAR. As shown in Figure 1B, the LAU is 0.79 (95% CI: 0.72–0.85; p < 0.0001), the BAR is 0.74 (95% CI: 0.67–0.81; p < 0.0001), the PAR is 0.62 (95% CI: 0.54–0.70; p = 0.0047), the NLR is 0.57 (95% CI: 0.49–0.64; p = 0.1272), and the PLR is 0.52 (95% CI: 0.43–0.61; p = 0.5873) (Table 4).
The above results indicate that, in addition to SFTSV RNA, LAU better predicts the poor prognosis of patients with SFTS upon admission than other indicators and has a more reliable predictive performance. The optimal critical value for LAU determined by the maximum Youden index was 144.685 U mmol/g L, with a sensitivity of 69% and specificity of 75% (Table 4).
3.4 Correlation Between LAU and Laboratory Parameters
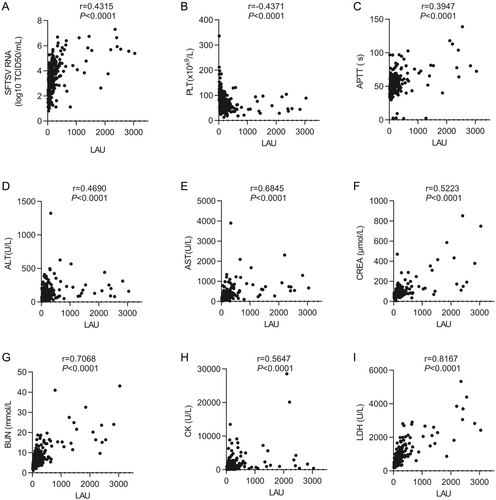
There were significant differences in viral load, cardiac function (CK and LDH), liver function (ALT and AST), renal function (BUN and CREA), and coagulation function (PLT and APTT) between the death and survival groups upon admission (Table 2). Therefore, the correlation between the LAU level and these indicators was evaluated (Figure 2). The results showed that LAU was highly correlated with LDH (r = 0.8167, p < 0.0001), moderately correlated with AST (r = 0.6845, p < 0.0001), CREA (r = 0.5223, p < 0.0001), BUN (r = 0.7068, p < 0.0001), CK (r = 0.5647, p < 0.0001), weakly correlated with ALT (r = 0.4690, p < 0.0001), APTT (r = 0.3947, p < 0.0001), and SFTSV RNA (r = 0.4315, p < 0.0001), but negatively correlated with PLT (r = −0.4371, p < 0.0001). These results indicate that the LAU level is consistent with the level of clinical functional indicators.
3.5 The Impact of Varying Levels of LAU on the Clinical Characteristics of Patients With SFTS
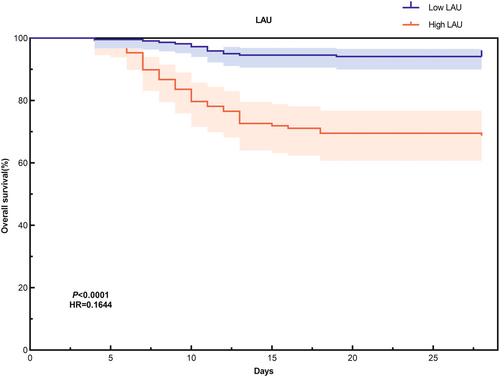
The results of multiple logistic regression analysis indicate that LAU serves as an independent risk factor for mortality in patients with SFTS. Based on the critical value of 144.685 U mmol/g L, all patients were categorized into low LAU and high LAU groups. Compared to the low LAU group, the high LAU group exhibited older age (p = 0.014), higher admission viral load (p < 0.001), and elevated levels of ALT (p < 0.001), AST (p < 0.001), K+ (p < 0.001), BUN (p < 0.001), CREA (p < 0.001), CK (p < 0.001), LDH (p < 0.001), and APTT (p < 0.001). Conversely, the high LAU group displayed lower levels of PLT (p < 0.001), ALB (p < 0.001), and Ca2+ (p < 0.001) (Table S2). Furthermore, the mortality rate (p < 0.001), history of diabetes (p = 0.007), and the incidence of SFTAE (p < 0.001) were notably higher in the high LAU group (Table S1). No significant differences were observed in other indicators between the two groups. To further elucidate the diagnostic value of LAU for predicting poor prognosis in SFTS patients 28 days post-onset, we conducted a Kaplan–Meier survival curve analysis. The findings revealed that SFTS patients with low LAU levels had significantly longer survival times compared to those with high LAU levels (p < 0.001, log-rank test) (Figure 3). This further underscores the association between elevated LAU levels and adverse outcomes in SFTS patients.
3.6 Dynamic Changes in LAU Levels in Survival and Death Groups of Patients
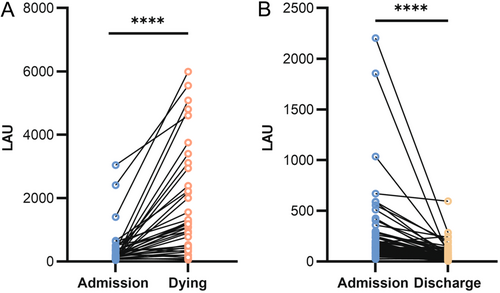
To further investigate the relationship between LAU levels and the prognosis of patients with SFTS, we tracked the dynamic changes in LAU levels during the initial and final blood tests of some survival and death groups throughout their hospitalization. The results showed that in the death group (n = 37), the LAU levels of patients increased with disease progression (Figure 4A). In contrast, in the survivor group (n = 69), as the patient's condition improved and SFTS virus nucleic acid testing was negative, the LAU level significantly decreased (Figure 4B).
4 Discussion
SFTS, a newly emerging infectious disease, progresses rapidly and has a high mortality rate. The early identification of high-risk patients is particularly important. This study retrospectively analyzed 348 patients with SFTS admitted to Yantai Infectious Disease Hospital. Logistic regression analysis of clinical data from the survival and death groups revealed that older age, SFTSAE, SFTSV RNA, decreased ALB and serum Ca2+, increased LDH, and increased LAU were independent risk factors for mortality in SFTS patients. Most importantly, this study is the first to evaluate the reliable predictive performance of LAU for a poor prognosis in patients with SFTS.
SFTSV infection primarily affects the live [17, 22], kidneys [23], and heart [24], with severe cases potentially leading to multiple organ failure or fatality [25, 26]. Although the pathogenesis of SFTSV remains incompletely understood, it is believed to involve direct viral infection and immune-mediated damage through cytokine storms [27]. The liver serves as a principal target organ for SFTSV infection. Studies have demonstrated that the virus can infect liver macrophages [28, 29] and epithelial cells [30], replicating within these cells and causing direct cellular damage. Furthermore, SFTSV infection significantly stimulates the release of pro-inflammatory cytokines (such as interleukin-6, interleukin-8, and tumor necrosis factor-α) and chemokines (such as C-C Motif Chemokine Ligand 5 and interferon γ inducible protein-10), which exacerbate liver inflammation and contribute to immunopathological damage [30]. Zhang's research also showed that the presence of liver injury at admission was an independent risk factor of fatal outcome [17]. ALB is a protein synthesized by the liver that can reflect the nutritional status and plasma osmotic pressure of the body. It has previously been used as a prognostic biomarker for various infections such as sepsis [31]. A meta-analysis has shown that ALB is associated with the risk of mortality in patients with SFTS [32]. This finding aligns with both our research results and clinical observations, as liver dysfunction and increased vascular permeability induced by SFTSV infection can lead to a reduction in ALB levels. Additionally, renal dysfunction may contribute to increased protein filtration or decreased reabsorption, further impacting ALB concentration. Although the specific mechanism underlying SFTSV-related kidney injury remains unclear, the virus has been detected in the kidney tissue of patients [28], suggesting a potential direct invasion of kidney cells by the virus. Furthermore, SFTSV infection can trigger cytokine storms, resulting in microcirculatory disorders, increased vascular permeability, and even the induction of thrombosis and disseminated intravascular coagulation [15, 33]. Additionally, elevated cytokine levels can directly damage renal endothelial cells and tubular epithelial cells, thereby exacerbating renal dysfunction [34, 35]. BUN is the end product of protein metabolism in the body and can reflect function of the kidneys [36]. Previous studies have reported that elevated BUN levels are associated with death from various diseases, such as lung disease, pancreatitis, sepsis, and COVID-19 [37, 38]. A study conducted in 2018 utilized immunohistochemistry and immunofluorescence techniques to detect viral nucleoprotein antigens in the heart tissue of patients with SFTS. This study confirmed that the virus can directly invade myocardial cells or cardiac microvascular endothelial cells, resulting in cardiac damage [26]. Furthermore, severe SFTSV infections can provoke excessive immune responses, initiating cytokine storms that may lead to myocarditis, microcirculatory disorders, and even multiple organ dysfunction [33]. LDH is widely present in human tissues, and any cellular damage caused by infection, organ damage, or systemic inflammation can lead to an increase in the serum LDH concentration [39]. Therefore, serum LDH levels often increase with infection severity and reflect the degree of infection-induced cell damage [40]. Liu's study demonstrated LDH is an independent risk factor for cytokine storm in patients with SFTS [15]. Our research also indicates that an elevated LDH level is an independent risk factor for mortality in patients with SFTS. However, when used alone, serum LDH and ALB levels may be affected by many medical conditions. For example, elevated LDH levels are caused by anemia, and severe hypoalbuminemia is caused by malnutrition. Therefore, the combined application of these three indicators could better reflect the disease status of patients with SFTS.
As is well known, LDH, BUN, and ALB are commonly used serum indicators in clinical practice, which have the characteristics of short detection time, convenient acquisition, and repeatable measurement. As a novel composite biomarker, LAU encompasses the three aforementioned indicators and has previously been a potential predictor of fatal clinical complications in hospitalized COVID-19 patients. Shokr's study found that patients with a high LAU ratio have an increased risk of death due to COVID-19 infection [20]. Despite the significant differences in virological characteristics, target cells, and replication mechanisms between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and SFTSV, the LAU ratio serves as a composite sensitive indicator reflecting systemic damage and metabolic disorders in the host, making it applicable to various severe infections. In addition, clinical Bunyavirus nucleic acid testing requires certain medical conditions, which makes it difficult for remote areas to promptly undergo virological testing. However, LAU testing is simple and cost-effective and can be extended to rural hospitals. At the same time, the results are easy to interpret, do not require complex calculations, and can quickly assess the risk of death. This helps doctors focus more effectively on patients with a high risk of fatal outcomes.
This study demonstrated that LAU had a more reliable predictive performance for adverse outcomes in patients with SFTS. First, ROC curve analysis indicates that In addition to SFTSV RNA, LAU is a better predictor of poor prognosis for SFTS patients upon admission than other indicators. In terms of laboratory parameters, we also evaluated the correlation between LAU levels and viral load, cardiac function (CK and LDH), liver function (ALT and AST), renal function (BUN and CREA), and coagulation function (PLT and APTT) indicators and found that LAU levels were consistent with clinical functional indicators. Because some SFTS patients with significantly increased LAU still survived, we used the optimal cutoff value for LAU to divide the patients into low and high LAU groups. We systematically compared the clinical and laboratory parameters between the low LAU group and the high LAU group. Our findings indicate that the high LAU group was older, exhibited a higher viral load upon admission, had a significantly greater incidence of SFTAE, and experienced more severe damage to the heart, liver, and kidneys. According to Kaplan–Meier survival curve analysis, patients with low LAU levels had significantly longer survival times than those with high LAU levels. These results further validate the potential clinical application of this critical value in treatment decision-making. In addition, we also followed up the LAU levels of some survivors and deceased patients and found that the LAU levels of the deceased group increased with disease progression, while the LAU levels of the survival group patients significantly decreased with disease improvement. Therefore, the study has significant clinical implications. Doctors can reduce the risk of mortality in patients with SFTS by conducting LAU quantitative assessment for timely diagnosis and treatment.
However, this study had three limitations. First, this was a single-center retrospective study, and the efficacy and promotion of indicators may require further validation through multicenter research. Second, due to the lack of data, some meaningful test results, including CRP, D-dimer, blood amylase levels, and related cytokines, have not been studied and analyzed. Third, our hybrid screening (self-report plus confirmatory testing) may have missed undetected co-infections (such as COVID-19, EB virus), potentially introducing confounding. Future studies should implement standardized lab screening to exclude such coinfections and improve the robustness of research results. Finally, owing to the relatively small number of deaths found in this study cohort, there may be a statistical bias. Therefore, future multicenter and follow-up studies should involve larger queue sizes.
5 Conclusion
As a new infectious disease, the incidence rate of SFTS is increasing annually, and the mortality rate is still high. The study has found that older age, SFTSAE, high levels of SFTSV RNA, decreased ALB and serum Ca2+, increased LDH, and elevated levels of LAU were independent risk factors for mortality in SFTS patients. Most importantly, our study provides the first evidence that LAU can serve as an early warning biomarker for adverse outcomes in patients with SFTS.
Author Contributions
Ruihua Zhang: conceptualization, methodology, formal analysis, writing – original draft, writing – review and editing, project administration. Yameng Mu: conceptualization, investigation, data curation, writing – original draft, writing – review and editing, supervision. Mengyuan Zhang: conceptualization, formal analysis, investigation, visualization, writing – original draft, writing – review and editing. Hongxiao Wu: methodology, formal analysis, visualization. Yanli Xu: resources, data curation. Chenxi Zhao: resources, validation. Zishuai Liu: investigation, visualization. Ling Lin: resources, data curation. Wei Zhang: resources, formal analysis. Yaxian Kong: conceptualization, supervision, project administration. Zhihai Chen: conceptualization, supervision, project administration, funding acquisition. All authors contributed to the manuscript revision.
Acknowledgments
We would like to express our gratitude to all the healthcare workers who contributed to this study. Thanks for the data support provided by the Department of Infectious Diseases at Yantai Qishan Hospital. The study was supported by the National Key R&D Program of China (Grant No. 2022YFF1203201), Beijing Research Center for Respiratory Infectious Diseases Project (BJRID2024-009).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




