Viral Kinetics During Acute Chikungunya Virus Infection: Insights Into Potential Role of Monoclonal Antibodies in Viral Clearance and Prophylaxis Using Mathematical Modeling
The research project was performed at Institut Pasteur du Cambodge, Cambodia.
ABSTRACT
Chikungunya virus (CHIKV), an arthritogenic alphavirus, is a significant public health threat in endemic and newly affected regions. This study investigates viral kinetics, immune responses, and the potential of monoclonal antibody (mAb) therapies to mitigate viraemia and transmission during acute CHIKV infection, providing novel insights into early intervention strategies. Using data from 29 patients in Cambodia, serial sampling and viral load quantification revealed that the population-average peak viral load occurred ~1.87 days prior to symptom onset. Children demonstrated higher peak viral loads and faster replication rates compared to adults, although symptom severity and burden were similar across age groups. IgM antibodies appeared earlier in adults (median: 4.1 days) than in children (median: 5.1 days; p = 0.036). C-reactive protein (CRP) levels were transiently elevated in about 50% of patients but showed no correlation with disease severity. Mathematical modeling highlighted that prophylactic mAb therapies, when administered 3 days before symptoms onset, could substantially reduce viral load and potentially prevent detectable viraemia. While these findings underscore the potential of mAbs as an early therapeutic strategy, further studies are necessary to evaluate the robustness of these results and assess their practical implications to curb CHIKV outbreaks by minimizing viraemia and presymptomatic transmission.
1 Introduction
Chikungunya virus (CHIKV), an arthritogenic alphavirus, is primarily transmitted by Aedes mosquitoes, particularly Aedes aegypti and Aedes albopictus, in tropical and subtropical regions. Recent expansion of mosquito populations into temperate areas has raised concerns about the increasing risk of CHIKV outbreaks in new regions [1-3]. CHIKV is endemic in Africa and Asia and has more recently spread to other continents, including Europe and Latin America, where it has been detected in autochthonous populations and has established endemic transmission, respectively [4]. In Cambodia, CHIKV was first documented in the 1960s. After an apparent disappearance, the virus reemerged in 2011, with the Indian Ocean lineage carrying the A226V mutation [5]. CHIKV circulation ceased after 2013 but reemerged in 2020 before once again disappearing in 2023.
CHIKV outbreaks are typically unpredictable and begin with a sudden, explosive onset, affecting a large proportion of the population in a short time [6]. Attack rates in affected areas can range from 30% to 75%, underscoring the substantial public health burden of these outbreaks [7, 8].
Clinically, CHIKV infection manifests as an acute febrile illness characterized by a rapid onset of high fever, polyarthralgia, rash, and myalgia. While the acute symptoms usually resolve within 7–10 days, about 40%–60% of patients develop chronic arthralgia that can persist for months or even years, leading to long-term disability and reduced quality of life [9, 10]. These sequelae highlight the need for effective strategies to mitigate both the acute and chronic impacts of CHIKV infection.
Viraemia is typically highest within the first 48–72 h after symptom onset and usually lasts 5–7 days, although viral RNA has been detected for up to 12 days in some severe cases [11, 12]. The decline in viral load coincides with the appearance of neutralizing antibodies, which play a key role in viral clearance [13]. CHIKV infection has been linked to more severe disease manifestations, such as encephalopathy, cardiovascular, hepatitis, and multiorgan failure [14-16]. This underscores the importance of early diagnostic and therapeutic interventions to limit disease progression and severity.
In recent years, monoclonal antibodies (mAbs) targeting CHIKV have emerged as a promising therapeutic option. Several preclinical studies have demonstrated the efficacy of mAbs in neutralizing CHIKV in animal models, reducing viral load, and preventing the progression to severe disease [17-22]. These antibodies have shown potential in targeting specific epitopes on the CHIKV envelope proteins, thereby inhibiting viral entry into host cells and accelerating viral clearance [23]. Early-phase clinical trials have provided encouraging results, with mAbs demonstrating both safety and efficacy in reducing viraemia and mitigating joint inflammation in CHIKV-infected patients [24]. These findings position mAbs as a critical tool for both therapeutic intervention and outbreak control.
Despite these advancements, detailed studies on the within-subject viral kinetics during acute CHIKV infection remain limited. Current data often come from small numbers of patients or population-level estimates based on single-time-point samples, which may not fully capture the variability and complexity of viral kinetics in individual patients [25-29]. Understanding how viral load fluctuates over time in individual patients and its association with clinical outcomes is crucial for optimizing therapeutic strategies, including the timing of monoclonal antibody administration. To fill this gap, the present study provides a comprehensive analysis of serial viraemia measurements in a cohort of CHIKV patients during the acute phase of infection. These findings aim to refine viral kinetic models and inform future antiviral and immunotherapeutic strategies, with the ultimate goal of reducing viraemia, limiting transmission, and preventing severe and chronic disease outcomes.
2 Materials and Methods
2.1 Study Setting and Patient Enrollment
This study was conducted in the Cambodian provinces of Kampong Thom, Kandal, and Phnom Penh between 2021 and 2022 (Supporting Information S2: Figure S1). Patients presenting with acute CHIKV infection were enrolled from participating hospitals. The study received ethical approval from the Cambodian National Ethics Committee for Health Research (Approval Date: 26th October 2020, NECHR No. 262). All participants provided informed consent for the use of their blood samples and anonymized clinical data. For pediatric patients, parental consent was required.
Eligible participants included males and females aged 48 months and older who presented within 72 h of acute febrile illness (≥ 38.5°C) with sudden-onset joint pain and had CHIKV infection confirmed via RT-PCR. Written informed consent was required, and for patients under 18 years, consent was obtained from a parent or legal guardian, with inclusion contingent on the ability to collect an adequate blood sample and at least two follow-up sampling. Patients coinfected with dengue virus were excluded from the study, though prior dengue exposure did not disqualify participation.
2.2 Clinical and Laboratory Data Collection
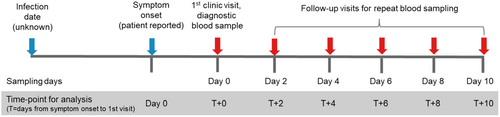
At the initial hospital presentation, demographic details, clinical symptoms, and the timing of symptom onset relative to clinic presentation were recorded (Supporting Information S2: Table S1). Blood samples were collected at the first visit (Day 0) and at regular 2-day intervals for 10 days (Figure 1). Follow-up blood sampling as well as clinical symptoms after Day 0 were collected either during hospitalization or, for patients who were discharged, at their households. No further follow-up after Day 10 was performed. Each blood sample was tested for viral load, anti-CHIKV IgM and IgG antibodies, and C-reactive protein (CRP). Additionally, the total symptom burden was evaluated by counting the number of 14 prespecified symptoms reported by each patient at each visit (Table 1).
| Characteristic | Overall | < 18 years old | Adults |
|---|---|---|---|
| N = 29 | N = 15a | N = 14 | |
| Age (median (range)) | 16 (3, 70) | 10 (3, 16) | 30 (19, 70) |
| Sex | |||
| Female | 14 (48%) | 5 (33%) | 9 (64%) |
| Male | 15 (52%) | 10 (67%) | 5 (36%) |
| Log10 viral load | 7.50 (3.04, 9.92) | 8.59 (3.60, 9.92) | 7.29 (3.04, 8.57) |
| Overall number of symptoms (median (range)) | 6 (2, 12) | 6 (2, 12) | 6 (2, 10) |
| Number of musculoskeletal symptoms (median (range)) | 2 (0, 4) | 2 (0, 3) | 2 (0, 4) |
| Fever | 26 (90%) | 14 (93%) | 12 (86%) |
| Headache | 20 (69%) | 10 (67%) | 10 (71%) |
| Skin rash | 23 (79%) | 13 (87%) | 10 (71%) |
| Arthralgia | 22 (76%) | 11 (73%) | 11 (79%) |
| Fatigue or tiredness | 22 (76%) | 12 (80%) | 10 (71%) |
| Myalgia | 14 (48%) | 6 (40%) | 8 (57%) |
| Itching | 9 (32%) | 4 (29%) | 5 (36%) |
| Weakness or asthenia | 9 (31%) | 5 (33%) | 4 (29%) |
| Stiffness | 6 (21%) | 3 (21%) | 3 (21%) |
| Nausea or vomiting | 6 (21%) | 5 (33%) | 1 (7%) |
| Shivering | 5 (17%) | 3 (20%) | 2 (14%) |
| Arthritis | 3 (10%) | 1 (7%) | 2 (14%) |
| Orbital pain | 3 (10%) | 1 (7%) | 2 (14%) |
| Diarrhea | 2 (7%) | 2 (13%) | 0 (0%) |
- a Frequency of symptoms obtained only from 13 subjects < 18 years due to young age of two subjects.
2.3 Laboratory Assays
All assays were conducted at the Virology Unit and Medical Biology Laboratory Unit of the Institut Pasteur du Cambodge (IPC) in Phnom Penh. Whole blood samples collected at local sites were centrifuged (1000–2000g for 10 min at 4°C) to separate serum, which was aliquoted into RNase- and DNase-free 1.5 mL polypropylene Eppendorf tubes. Serum samples were transported and stored at −80°C for subsequent testing.
Detection and quantification of CHIKV were performed on acute and subsequent serum samples. Viral RNA was extracted using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) per manufacturer's instructions. The extracted RNA was tested for CHIKV using real-time RT-PCR (RT-qPCR) adapted from Pastorino et al. [30] targeting the E1 gene with SuperScript III Platinum One-Step Quantitative RT-PCR Kit (Invitrogen, Carlsbad, CA, USA). The reactions were conducted in 25 μL volume containing 5 μL of RNA, 12.5 μL of 2× Thermoscript Reaction Mix Buffer, 1 μL of 50 mM MgSO4, 0.1 μM of the TaqMan probe, 0.2 μM of each primer, and 0.5 μL of the enzyme mix (Invitrogen). Real-time RT-PCR assays were carried out in the Bio-Rad CFX96 Real-Time Detection System (Bio-Rad, Hercules, CA, USA) using the following thermal cycling parameters: reverse transcription at 50°C for 15 min, inactivation at 95°C for 2 min followed by 45 cycles of fluorescence detection at 95°C for 15 s, and annealing and extension at 60°C for 1 min. Viral loads were expressed as RNA copy numbers based on a standard curve of known plasmid copy numbers. A cycle threshold (Ct) value > 40 indicated the absence of CHIKV.
CRP concentrations were measured using a commercially available EIA kit (Abcam ref. ab99995), with a lower limit of quantification of 5 mg/L. Anti-CHIKV IgM antibodies were quantified using an in-house MAC-ELISA method [31] with antigens derived from a Cambodian CHIKV strain isolated in 2012. Briefly 96-well microtiter plates were coated with goat anti-human IgM and 100 µL of serum samples and controls diluted 1∶100 in phosphate buffered saline + Tween 20 0.5% (PBS-T) + 5% nonfat dried milk (PBS-T-NDM) were added and incubated for 1 h at 37°C. After washing with PBS-T, CHIKV suckling mouse-brain derived antigen produced by sucrose acetone extraction was added to each well at a concentration of 16 hemagglutination units, and incubated at 4°C overnight. The plate was washed with PBS-T, monoclonal antibody to the antigens being detected was added, incubated at 37°C, washed and peroxidase conjugated anti-mouse Fab H + L was added. The final reaction was developed and read at 405 nm on an automated plate reader. A result was considered positive for CHIKV IgM antibodies when the optical density (OD) was higher than 0.1 (threshold determined by measuring the OD of CHIKV-negative human serum). IgG antibodies were measured using the CHIKJJ Detect IgG ELISA (InBios, Seattle, WA), with OD < 1.0 considered negative according to manufacturer's recommendation.
2.4 Statistical Analysis
Demographic and clinical characteristics of participants were summarized using absolute and relative frequencies for categorical variables and medians with ranges (minimum–maximum) for continuous variables. Comparisons between adults and children were performed using Pearson's χ2 test or Fisher's exact test for categorical variables and the Mann–Whitney U test for continuous variables.
2.4.1 Mathematical Modeling
Plasma viral load measurements were used to construct both individual and population-average viraemia-time curves. A nonlinear mixed-effects model was applied to fit the individual viral load data, using a Target-Cell Limited (TCL) mathematical model to describe CHIKV viral dynamics.
The TCL mathematical model, a widely used framework for modeling viral dynamics, is defined by the following equations, which describe the interactions between target cells, infected cells, and viral load. The detailed equations and methodology are presented in Supporting Information S1: Supporting Methods.
Key viral kinetics parameters were derived from the fitted model, including maximum viral load, time to reach maximum viral load (days), time of first detectable viral load (days), time to last detectable viral load (days) and duration of detectable viral load (days). These parameters were compared between adults and < 18 years old.
Immune biomarkers (IgM, IgG, and CRP) were modeled at the individual level using a nonlinear mixed-effects model for IgM and IgG, and a linear mixed-effects model for CRP. A logistic function was fitted for IgM and IgG, and 3-degrees polynomial function was used to model CRP concentrations over time.
Symptom trajectories were described at the population level. For each symptom, the proportion of patients presenting the symptom was calculated according to the time since symptoms onset. A local polynomial regression fitting (LOESS) was used to capture trends in symptom evolution across timepoints.
2.4.2 Antiviral Strategies Simulation
To explore the potential impact of antiviral interventions, we conducted simulations based on the modeled viraemia-time profile. These simulations assumed an antiviral agent, specifically an anti-CHIKV monoclonal antibody, that increases viral clearance by a factor of 5 and reduce target cell infection by varying degrees (90%–100%). The simulations assessed the efficacy of these interventions across different administration timings, ranging from prophylactic treatment (3 days before symptom onset) to postsymptomatic treatment (up to 7 days after symptom onset). Effectiveness was estimated compared to a baseline scenario with no antiviral on the following parameters: area under the viraemia curve (AUC), maximum viral load (log10 copies/mL), and duration of detectable viraemia.
3 Results
3.1 Patient Characteristics and Clinical Symptoms
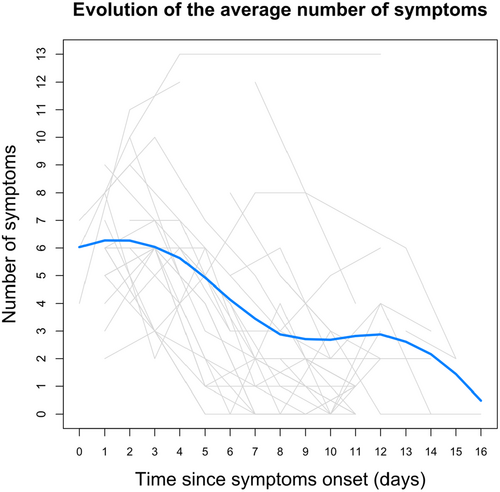
A total of 46 acute CHIKV infection cases were identified, and among them 29 patients, all with Khmer ethnicity, who met all the inclusion and exclusion criteria with at least two follow-up sampling time-points were enrolled in the study. The cohort's demographic characteristics and clinical characteristics, stratified by age group, are shown in Table 1. The most frequently reported symptoms were similar in adults and in children, with both groups experiencing fever, headache, and arthralgia. However, gastrointestinal symptoms (nausea, vomiting, or diarrhea) were more frequent in children. In general, fever resolved within the first 7 days; however, some individuals experienced elevated temperatures up to 10–12 days after symptom onset. The evolution of the total symptom burden and individual symptom frequencies was summarized (Figure 2 and Supporting Information S2: Figure S2).
3.2 Viral Load Kinetics
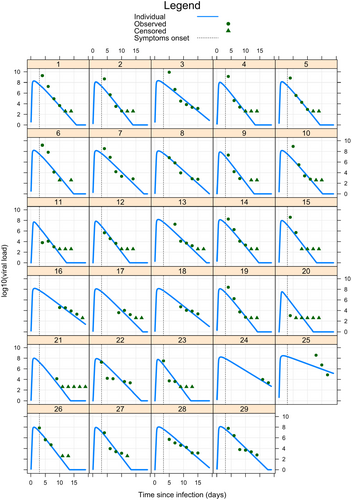
A total of 17 subjects (59%) were sampled at all 6 time-points over a period of 11 days. Of the 29 subjects, 26 (90%) had complete data or no more than 1 missing sample. Using the TCL model, we constructed both population-average (Figure 3) and individual (Figure 4) viraemia-time profiles for all 29 subjects. The population-average profile revealed a peak viral load at ~1.87 day prior to symptoms onset, with significant variability observed at the individual level. Estimated population parameters (Supporting Information S2: Tables S2 and S3) were calculated using a nonlinear mixed effects model based on the TCL model. From these parameters, descriptive statistics of CHIKV viraemia across the 29 patients were derived and reported in Table 2.
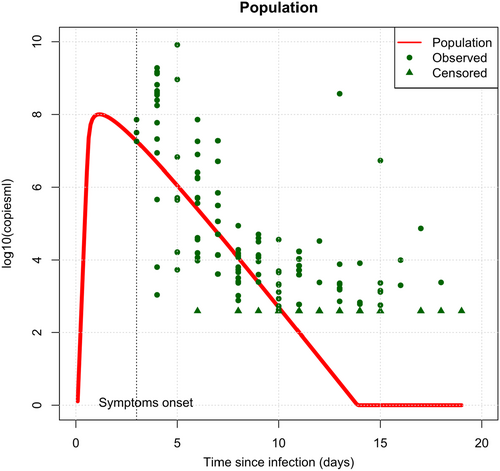
| Parameter—median (range) | Since infection | Since symptoms onset |
|---|---|---|
| Maximum log10 viral load | 8.10 (7.54, 8.48) | 8.10 (7.54, 8.48) |
| Time of maximum viral load (days) | 1.13 (1.09, 1.53) | −1.87 (−1.91, −1.47)a |
| Time of first detectable viral load (days) | 0.28 (0.20, 0.37) | −2.72 (−2.80, −2.63)a |
| Time of last detectable viral load (days) | 10.94 (8.35, 19.00) | 7.94 (5.35, 16.00) |
| Duration of viraemia (days) | 10.72 (7.98, 18.80) | 10.72 (7.98, 18.80) |
- a Negative values indicate event before symptoms onset.
Significant differences in the viral kinetics were identified between adults and children. Infants displayed a significantly lower target cell infection rate, but higher viral replication rate and maximum viral load leading to a shorter time to first detectable viraemia (Supporting Information S2: Table S4). The resulting higher peak viraemia in children (Supporting Information S2: Figure S3) did not translate into any differences in the frequency or number of symptoms. Viral kinetics were not associated with sex or number of symptoms (analyzed as a binary variable </≥ the median). Restricting this analysis to the number of acute musculoskeletal symptoms reported (arthralgia, arthritis, myalgia, and stiffness) also showed no statistically significant association with viral kinetics (Supporting Information S2: Table S4).
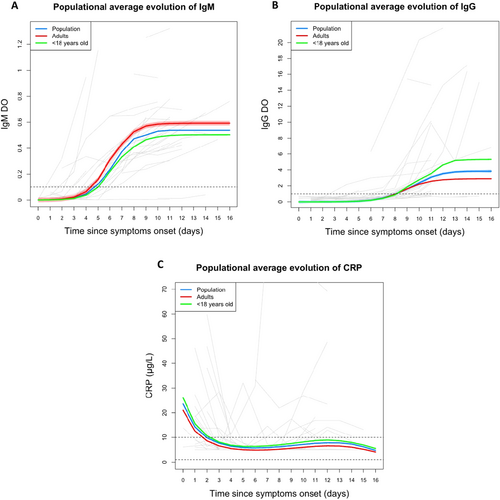
3.3 Kinetics of IgM, IgG, and CRP
IgM was first detectable in the majority of patients between 4 and 8 days post-symptom onset, with earlier detection in adults compared to children (p = 0.036; Figure 5A). In contrast, IgG was generally detectable after the first week, with no significant difference in time to detection between age groups (Figure 5B). Analysis by age category showed a more rapid appearance of IgM in adults with no difference in time to IgG appearance. In contrast, the amplitude of the IgM response was similar in the two age groups while the amplitude of the IgG response was greater in children (Table 3).
| Overall | < 18 years old | Adults | pb | |
|---|---|---|---|---|
| N = 29a | N = 15a | N = 14a | ||
| IgM | ||||
| Time of first detectable IgM after symptoms onset (days) | 4.80 (0.60, 6.50) | 5.10 (2.70, 6.30) | 4.10 (0.60, 6.50) | 0.036 |
| Unknown | 2 | 0 | 2 | |
| IgM amplitude | 0.54 (0.02, 1.31) | 0.50 (0.37, 0.71) | 0.59 (0.02, 1.31) | 0.4 |
| IgG | ||||
| Time of first detectable IgG after symptoms onset (days) | 8.10 (4.00, 10.60) | 8.10 (4.00, 9.70) | 8.10 (4.00, 10.60) | > 0.9 |
| Unknown | 4 | 1 | 3 | |
| IgG amplitude | 3.8 (0.7, 21.7) | 5.3 (1.0, 21.7) | 2.9 (0.7, 16.0) | 0.016 |
| CRP | ||||
| Time of CRP < 10 µg/L after symptoms onset (days) | 1.85 (0.80, 3.50) | 2.10 (1.50, 3.50) | 1.60 (0.80, 2.90) | 0.022 |
| Unknown | 3 | 2 | 1 | |
| CRP value (µg/L) at time of symptom onset | 24 (14, 100) | 26 (20, 74) | 21 (14, 100) | 0.033 |
- a Median (range).
- b Wilcoxon rank sum test; Wilcoxon rank sum exact test.
Elevated CRP levels (> 10 µg/L) were found in approximately half of patients at the first sample (17/29), typically during the first 3 days after symptom onset (Figure 5C). Most later samples showed normal serum concentrations, though a few patients had elevated levels in the second week. Only three individuals had markedly elevated CRP at multiple timepoints (Supporting Information S2: Figure S4).
It was noted that two individuals showed almost no detectable IgM or IgG during the sampling period (Supporting Information S2: Figure S4). The first was a 24-year-old female experiencing arthralgia and other symptoms throughout the 10-day follow-up, but with a maximum viral load of only 1.09 × 103 copies/mL and no elevation of CRP. The second was a 70-year-old female experiencing few symptoms in this period, although viral load started high at 3.7 × 108 declining to 7.3 × 104 copies/mL 4 days later with elevated CRP between 8 and 14 mg/L.
3.4 Simulations of Antiviral Strategies
Simulation models indicated that an antiviral agent achieving a 99% reduction in target cell entry, such as an anti-CHIKV monoclonal antibody, when administered within the first 3 days of symptom onset, reduced the area under the viraemia curve (AUC) by 18%–23%, with no significant impact on the maximum viral load (data available upon request). However, prophylactic administration 3 days prior to symptom onset resulted in a 99% reduction in AUC, a 98% reduction in maximum viral load, and effectively prevented detectable viraemia.
4 Discussion
This study provided key insights into the dynamics of viraemia, immune response, and the potential impact of mAb treatment and prevention of CHIKV infection.
4.1 Viraemia and Transmission Dynamics
Consistent with existing studies, our model suggests that viraemia can be detectable ~2–3 days before symptom onset. Presymptomatic viraemia is a critical factor in the transmission of CHIKV, similar to asymptomatic carriers, who might unknowingly contribute to outbreaks. Our findings align with previous reports by Riswari et al. [29], who observed prefebrile viraemia in clinical cases. This Indonesian study also highlighted variability in the timing of peak viral loads, with some patients showing detectable viraemia at Days 5–6 prior to symptom onset, potentially indicating a longer incubation period.
We observed that viral load typically peaks within 48–72 h post-symptom onset, with prolonged viraemia lasting up to 18 days in certain individuals. These results are consistent with prior population-level studies, which observed viraemia persisting beyond the first week of symptoms [25, 26]. Further research is needed to assess transmissibility, particularly during the extended period of detectable viremia.
4.2 Clinical Signs and Disease Progression
Our findings indicate that viraemia levels did not significantly correlate with the number or severity of symptoms in the acute infection phase, including musculoskeletal symptoms such as arthralgia and myalgia. This aligns with studies reporting no direct relationship between viral load and symptom severity, suggesting that symptom expression may be more strongly influenced by individual immune responses or other host factors [32, 33]. However, contrasting evidence highlights that higher viraemia levels and prolonged viraemia duration are associated with more severe manifestations, including neurological complications, some rare haemorrhagic features, and persistent joint pain. Animal models also suggest a potential link between CHIKV RNA detection in joint tissues and chronic arthralgia, indicating that localized viral persistence may contribute to disease severity and sequelae [34-37]. Furthermore, older age has been identified as a significant risk factor for severe disease, including atypical presentations and higher mortality during outbreaks [38-40]. These discrepancies underscore the need for further research into the interplay between host immune response, age, underlying conditions, viral load, and the persistence of CHIKV in tissues to better understand the determinants of symptom severity and disease progression.
4.3 Immune Response and Antibody Dynamics
In terms of immune response, anti-CHIKV IgM antibodies were first detectable between 4 and 8 days post-symptom onset, with adults showing earlier detection compared to children (p = 0.036). This finding is in line with previous studies [25, 41], which demonstrated a delay in IgM appearance in pediatric cases. The delayed IgM response in children, coupled with their higher viral replication rates and peak viral loads, could potentially extend the period of viraemia, making younger individuals more infectious for longer periods.
Interestingly, while there was no significant difference in the time to IgG detection between adults and children, children exhibited a stronger IgG response. This could reflect a more robust long-term immune response in pediatric cases, though the clinical significance of this remains unclear.
4.4 C-Reactive Protein (CRP) as an Inflammatory Marker
Our study found that CRP, a marker of systemic inflammation, was transiently elevated in about half of the patients during the acute phase of CHIKV infection, without strong associations with viral load or symptom severity. This aligns with previous studies suggesting CRP as an acute-phase inflammatory biomarker without clear predictive value for chronic complications [42, 43].
However, contrasting evidence indicates that elevated CRP during the acute phase may correlate with higher risk of chronic outcomes like postviral arthritis [44, 45]. These discrepancies highlight the need for further research to determine CRP's role in predicting long-term complications, accounting for variations in study populations and methodologies.
4.5 Monoclonal Antibodies as a Therapeutic Strategy
Our simulations of antiviral strategies demonstrated the significant potential of mAb treatments. Administering mAbs early—particularly within 3 days of symptom onset—was shown to reduce viraemia by up to 23%. These findings are consistent with recent advances in mAb therapy for CHIKV, where clinical and experimental studies have demonstrated their ability to neutralize the virus and reduce disease severity in animal models [17, 22, 23, 46, 47]. Moreover, other studies have confirmed the safety and efficacy of mAbs in reducing viraemia and alleviating joint inflammation [17, 48, 49].
Importantly, our simulation results indicated that by incorporating mAb prophylaxis into public health interventions, the risk of transmission during the presymptomatic phase could be significantly minimized. This approach may provide a powerful tool to curb outbreaks, limit community transmission, and protect public health. However, further clinical study is needed to understand its effectiveness in real world.
5 Study Limitations
Several limitations should be considered. First, the use of symptom onset as a reference point for assessing viral kinetics may introduce inaccuracies, as many CHIKV symptoms are subjective and nonspecific and our questionnaire did not capture the level of severity, which may have limited the analysis on the association of higher viraemia and disease severity. The study was also limited to a single outbreak in Cambodia, and the results may not be generalizable to other strains or geographic regions. Although the modeling framework allowed robust inference based on densely sampled individual data, the relatively small sample size may still limit the generalizability of our findings. Finally, while we focused on the acute phase of CHIKV infection, we did not assess the association between viral kinetics as well as other factors and the development of long-term musculoskeletal symptoms, an area that requires further investigation.
6 Conclusion
This study enhances our understanding of CHIKV viral kinetics, providing valuable insights based on serial sampling of each individual CHIVK-confirmed patient into the dynamics of viraemia, antibody responses, and the potential role of mAbs in controlling infection. The early detection of viraemia before symptom onset underscores the need for rapid diagnostic tools and early therapeutic intervention. Future studies should aim to explore the relationship between viral kinetics, immune response, and long-term clinical outcomes to optimize treatment strategies for CHIKV.
Author Contributions
Tey Putita Ou: CHIKV assay validation, laboratory supervision, writing – review and editing of the manuscript. Saraden In: data acquisition in the lab, review and editing of the manuscript. Sreymom Ken: data acquisition in the lab, review and editing of the manuscript. Sopheak Sorn: conducting community investigation, interview, sampling, review and editing of the manuscript. Kunthy Nguon: conducting community investigation, interview, sampling, review and editing of the manuscript. Sowath Ly: supervision of community investigation and review and editing of the manuscript. Claude Flamand: supervision of community investigation and review and editing of the manuscript. Nicolas Voirin: performing statistic and modeling analysis, writing – review and editing of the manuscript. Hugh Watson: conceptualization, project administration, writing – original draft. Marie Mandron: conceptualization, funding acquisition, writing – review and editing of the manuscript. Veasna Duong: conceptualization, project administration, data acquisition, writing – review and editing of the manuscript.
Acknowledgments
This study was supported with funding from the United States Defense Advanced Projects Agency (DARPA), Grant TIA HR0011-17-3-0002.
Ethics Statement
The study received ethical approval from the Cambodian National Ethics Committee for Health Research (Approval Date: 26th October 2020, NECHR No. 262).
Consent
All participants provided informed consent for the use of their blood samples and anonymized clinical data. For pediatric patients, parental consent was required.
Conflicts of Interest
Marie Mandron and Hugh Watson are employees of Evotec.
Open Research
Data Availability Statement
All deidentified patient's clinical informations and laboratory test results are securely store at IPC's archived storage room and electronic copy is stored on secured database with protected password accessible only by staff involved in the project. These data are available upon request. Mathematical modeling codes are available in Supporting Methods and upon request.




