Global Temporal Trends in Mother-to-Child Transmission Disease Incidence Among Women of Child-Bearing Age: An Analysis of Global Burden of Disease Study 2021 Data
Qiubai Jin, Mingxiao Zhou, and Meiqi Sun contributed equally to this work and share first authorship.
ABSTRACT
Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS), syphilis, hepatitis B and hepatitis C are the four major Mother-to-child transmission (MTCT) diseases, represent a significant public health challenge worldwide. Understanding the disease burden among women of child-bearing age (WCBA) helps to implement effective screening and treatment programs to control the MTCT diseases globally. Data on HIV/AIDS, syphilis, hepatitis B, and hepatitis C was collected from the Global Burden of Disease (GBD) 2021 database spanning 1992–2021. We analyzed the temporal trends of four MTCT diseases with joinpoint regression and further evaluated age-period-cohort effects using the age-period-cohort model. The age-standardized incidence rates per 100,000 population for HIV/AIDS, syphilis, hepatitis B, and hepatitis C in 2021 were 34.73 (95% uncertainty interval [UI]: 30.03, 40.54), 279.19 (95% UI: 174.59, 459.54), 908.39 (95% UI: 494.99, 1640.44), and 77.44 (95% UI: 41.57, 126.12). Joinpoint regression revealed ongoing declines in HIV/AIDS and hepatitis B rates among WCBA, contrasting with increasing trends for hepatitis C and syphilis post-2012. Age effects for HIV/AIDS, syphilis, and hepatitis B peaked in the 15–29 age group, while hepatitis C peaked in the 45–49 age group. Period effects showed increased incidence rates for syphilis and hepatitis C since 2012, peaking between 2017–2021, while HIV/AIDS and hepatitis B showed a general decline. Cohort effects for all four diseases generally followed a fluctuating downward trend. For hepatitis B, the incidence rate is declining in all 21 GBD regions. At national level, only the United Kingdom and Greece have seen a slight increase in incidence rates compared to 30 years ago, but the increase is minimal. For other MTCT diseases, Eastern Europe has the highest increase in incidence rates of HIV/AIDS and hepatitis C among WCBA, which requires special attention. Tropical Latin America is the region with the greatest increase in syphilis incidence rates. Specifically, at the national level, the countries with the highest increase in incidence rates for HIV/AIDS, syphilis, and hepatitis C are Pakistan, Greece, and Ukraine, respectively. Globally, while HIV/AIDS and hepatitis B incidence in WCBA has decreased, negative age, period, and cohort effects persist in certain countries. Post-2012, hepatitis C and syphilis incidence in WCBA has risen, underscoring the need to refine management strategies against MTCT diseases.
1 Introduction
Mother-to-child transmission (MTCT) diseases are diseases that are transmitted from mother to baby during pregnancy, delivery and lactation, including human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), syphilis, hepatitis B, and hepatitis C, etc. These diseases can seriously affect the normal development of the fetus and the health of the newborn. For example, more than 90% of all new HIV infections in infants and young children are transmitted from mother to child [1]. Syphilis during pregnancy is the second leading cause of fetal death [2]. For hepatitis B, infants born to hepatitis B surface antigen-positive mothers without preventive measures have a 70%–90% probability of developing chronic hepatitis B [3]. Although hepatitis C is not generally considered to be a major MTCT disease, the risk of vertical transmission (about 6%) should not be ignored [4].
The World Health Organization (WHO) in its global health sector strategy for 2022–2030 emphasizes the goal of eliminating the epidemics of HIV/AIDS, viral hepatitis, and sexually transmitted infections by 2030 [5]. Eliminating MTCT is a crucial strategic action for preventing and reducing new infections of these four diseases, which can protect the health of women and children and improve the quality of the birth population. Women of child-bearing age (WCBA) are the key population for preventing the MTCT of HIV/AIDS, syphilis, hepatitis B, and hepatitis C. Understanding the disease burden among WCBA helps to implement effective screening and treatment programs to control the MTCT of these four diseases globally.
The Global Burden of Disease (GBD) database, a collaborative effort by over 11,500 experts worldwide, offers a comprehensive view of global health trends [6]. This study will utilize the GBD database to investigate the temporal trends in the incidence rates of HIV/AIDS, syphilis, hepatitis B, and hepatitis C among WCBA globally from 1992 to 2021, particularly identifying countries and regions with the most significant increases in MTCT disease incidence over the past 30 years. We applied joinpoint regression and age-period-cohort models to reveal patterns of disease growth in specific areas and combined our findings with existing literature to explore the possible causes of these increases and provide targeted recommendations. The study aims to guide resource allocation to high-risk areas and inform public health policies for reducing MTCT disease transmission.
2 Methods
2.1 Data Source and Related Definition
This research is a secondary analysis of the 2021 GBD study. In this study, data regarding HIV/AIDS, syphilis, the total burden of hepatitis B, and the total burden of hepatitis C among WCBA were collected from the GBD database from 1992 to 2021, updated in May 2024. The total burden of hepatitis B (hereinafter referred to as hepatitis B in this research) was defined as the sum of acute hepatitis related to hepatitis B, chronic hepatitis B including cirrhosis, and liver cancer related to hepatitis B. The same applies to the total burden of hepatitis C. And WCBA was defined as 15–49 years by WHO [7]. The GBD also employed the Sociodemographic Index (SDI) to gauge national development, reflecting factors like income, education, and fertility rates. With scores from 0 to 1, the SDI in 2021 sorts nations into 5 quintiles, indicating health outcomes linked to socioeconomic status. Besides, to ensure equitable regional comparisons, we employed the direct standardization method to adjust the data for age. We utilized the ageadjust. direct function from the epitools package in R software to carry out the age standardization [8]. The standard population we used was the global standard population estimated by the GBD for the year 2021 [6].
2.2 Joinpoint Regression
To analyze incidence trends of four MTCT diseases among WCBA, we performed a joinpoint regression. Joinpoint regression is a statistical method that analyzes changes in trends within time-series data and identifies significant shifts, known as ‘joinpoints’. It employs a grid search algorithm to detect unknown joinpoints and fits multiple linear segments to model the changing trends, making it particularly suitable for public health studies on disease incidence and mortality over time [9]. We set the maximum number of joinpoints to five, and determined the number, location, and corresponding p-values of joinpoints by Monte Carlo permutation test [10]. Using the joinpoint model, we also calculated the annual percent change (APC) and average annual percent change (AAPC) in the incidence of the four MTCT diseases from 1992 to 2021 at global, regional, and national levels, respectively.
2.3 Age-Period-Cohort Model
To further evaluate the impact of age, period, and cohort effects on the incidence of the four MTCT diseases among WCBA, we selected the regions and countries with the largest AAPCs from 21 GBD regions and five SDI quintiles for inclusion in the age-period-cohort model. Age-period-cohort model is particularly valuable in health and social sciences for its ability to attribute disease trends to age-related natural history, contemporary time-related medical and social factors, and early-life health behaviors and social exposure on disease trends [11]. It can dissect the distinct impacts of age, period, and cohort effects on disease incidence, which makes it a powerful tool for understanding how various factors contribute to changes in epidemiological measures over time.
Generally, the age-period-cohort model fits a log-linear Poisson model over a Lexis diagram of observed rates and quantifies the additive effects of age, period, and birth cohorts [12]. However, due to the linear relationship between age, period, and cohort (birth cohort = period − age), it is statistically impossible to estimate their independent effects, known as the nonidentifiability problem [13]. To circumvent this issue, this study produced estimable APC parameters and functions without imposing arbitrary constraints on model parameters. The related methodological details are available in prior publications [12].
We used the incidence estimates for MTCT disorders from the GBD 1992–2021 as inputs for the age-period-cohort model. Following the requirements of this model, the datasets were divided into seven 5-year age groups (15–19, 20–24… 45–49), six 5-year calendar periods (1992–1996, 1997–2001… 2017–2021) and 12 partially overlapping 10-year birth cohorts (1942–1951, 1947–1956… 1997–2006) [14].
In age-period-cohort model, age effects capture the variation in disease risk associated with aging. Period effects assess how risk changes across all ages over a specific time frame, often due to influences like economic conditions or public health measures. Cohort effects consider the distinct experiences of groups born during the same period, which can be shaped by the social, economic, and health events of their time. These factors help explain differences in disease risk among age groups, and the choice of reference periods or cohorts is arbitrary without affecting the analysis [15].
Statistical tests were two-sided and p < 0.05 is considered significant. Joinpoint 5.2.0 software was used for joinpoint regression analysis. For age-period-cohort model analysis, we employed an online software available at http://analysistools.nci.nih.gov/apc/. For data visualization, we used the ggplot2 package in R 4.2.3.
3 Results
3.1 Descriptive Analysis at Global, Regional, and National Levels
In 2021, the global numbers of new cases for hepatitis B, syphilis, hepatitis C, and HIV/AIDS among WCBA were documented as 17,815,299, 5,355,439, 1,521,932, and 670,804, respectively. Correspondingly, their age-standardized incidence rates (ASIRs) per 100,000 population were 908.39 (95% UI: 494.99, 1640.44), 279.19 (95% UI: 174.59, 459.54), 77.44 (95% UI: 41.57, 126.12), and 34.73 (95% UI: 30.03, 40.54). Hepatitis B constituted the highest proportion of all incident cases (70.24%) globally, followed by syphilis (21.11%), hepatitis C (6.00%), and HIV/AIDS (2.65%).
From the scope of SDI quintiles, the low SDI quintile had the highest ASIR of hepatitis B (ASIR = 1656.80; 95% UI: 865.18, 2795.87), syphilis (ASIR = 568.25; 95% UI: 302.17, 938.33), hepatitis C (ASIR = 128.09; 95% UI: 67.57, 204.67), and HIV/AIDS (ASIR = 39.45; 95% UI: 32.13, 49.18) (Table 1).
| 1992 | 2021 | 1992–2021 | |||
|---|---|---|---|---|---|
| Location | Incident cases (95% CI) | Age-standardised incidence rate per 100,000 (95% CI) | Incident cases (95% CI) | Age-standardised incidence rate per 100,000 (95% CI) | AAPC of incidence rate (95% CI) |
| HIV/AIDS | |||||
| Global | 1076492.44 (968201.14 to 1207173.27) | 75.44 (66.41 to 85.68) | 670804.13 (596593.96 to 762161.34) | 34.73 (30.03 to 40.54) | −2.68 (−2.92 to −2.43) |
| High SDI | 17147.81 (11856.92 to 23268.81) | 7.5 (4.95 to 10.38) | 21789.89 (11605.13 to 31997.48) | 9.08 (4.69 to 14.05) | 0.65 (−0.15 to 1.47) |
| High-middle SDI | 9975.73 (8680.12 to 11377.33) | 3.43 (2.88 to 4.09) | 50530.03 (39303.15 to 67252.76) | 16.53 (10.82 to 27.06) | 5.52 (4.86 to 6.18) |
| Middle SDI | 157017.88 (137260.36 to 178510.35) | 32.52 (27.45 to 38.08) | 185427.80 (158204.62 to 212802.63) | 30.69 (25.25 to 36.5) | −0.32 (−0.92 to 0.29) |
| Low-middle SDI | 416958.03 (375463.38 to 465966.58) | 140.75 (122.14 to 161.42) | 201843.84 (168488.15 to 247814.09) | 39.45 (32.13 to 49.18) | −4.30 (−4.48 to −4.11) |
| Low SDI | 474451.75 (389032.72 to 569994.46) | 393.34 (315.53 to 482.35) | 210618.54 (157932.46 to 276386.44) | 76.12 (55.64 to 103.45) | −5.45 (−5.66 to −5.24) |
| Syphilis | |||||
| Global | 3707369.61 (2594300.47 to 4930060.11) | 257.11 (140.5 to 417.63) | 5355439.77 (3717325.88 to 7240281.19) | 279.19 (147.59 to 459.54) | 0.28 (0.04 to 0.52) |
| High SDI | 214027.71 (149677.00 to 291606.54) | 93.96 (48.73 to 158.03) | 216230.28 (151386.06 to 293381.59) | 91.99 (47.68 to 155.01) | −0.10 (−0.35 to 0.14) |
| High-middle SDI | 276676.68 (192535.51 to 376399.73) | 95.26 (49.79 to 159.79) | 329243.68 (231640.16 to 441592.68) | 114.59 (59.44 to 191.81) | 0.59 (0.32 to 0.86) |
| Middle SDI | 1118445.35 (763471.06 to 1517169.24) | 228.79 (121.92 to 376.68) | 1399907.67 (979371.64 to 1895219.45) | 232.96 (122.76 to 384.22) | 0.03 (−0.12 to 0.17) |
| Low-middle SDI | 1158407.58 (799383.80 to 1552211.44) | 383.76 (204.45 to 630.06) | 1749377.03 (1201232.77 to 2371568.06) | 337.39 (177.13 to 557.64) | −0.42 (−0.82 to −0.03) |
| Low SDI | 936464.24 (680919.03 to 1201145.92) | 738.7 (425.74 to 1160.11) | 1656353.16 (1160829.97 to 2227205.49) | 568.25 (302.17 to 938.33) | −0.91 (−1.10 to −0.72) |
| Hepatitis B | |||||
| Global | 18766690.74 (13168240.26 to 24572196.24) | 1341.14 (814.42 to 2137.7) | 17815299.28 (12553428.14 to 24290226.39) | 908.39 (494.99 to 1640.44) | −1.34 (−1.40 to −1.28) |
| High SDI | 1350156.11 (1030992.92 to 1709469.76) | 589.6 (374.76 to 917.72) | 798391.60 (567678.96 to 1098935.30) | 306.42 (169.92 to 551.83) | −2.23 (−2.42 to −2.05) |
| High-middle SDI | 4081427.74 (2854491.06 to 5379211.22) | 1415.03 (848.39 to 2262.42) | 2303519.06 (1493093.63 to 3384681.23) | 694.55 (354.15 to 1294.67) | −2.48 (−2.62 to −2.34) |
| Middle SDI | 7782582.89 (5469864.58 to 10263415.25) | 1659.28 (997.66 to 2664.36) | 5885192.15 (4038638.65 to 8154919.28) | 927.87 (494.92 to 1690.73) | −2.00 (−2.18 to −1.82) |
| Low-middle SDI | 3355380.44 (2403979.94 to 4358765.85) | 1168.06 (709.97 to 1839.91) | 4565005.74 (3304856.47 to 6103020.32) | 906.45 (503.73 to 1622.56) | −0.86 (−0.92 to −0.81) |
| Low SDI | 2186041.14 (1535625.77 to 2921147.89) | 1840.5 (1093.94 to 2934.65) | 4253164.71 (2987489.89 to 5709994.36) | 1566.8 (865.18 to 2795.87) | −0.55 (−0.59 to −0.52) |
| Hepatitis C | |||||
| Global | 1110859.42 (779188.77 to 1488173.60) | 82.15 (44.85 to 133.92) | 1521932.00 (1068304.88 to 2079625.67) | 77.44 (41.57 to 126.12) | −0.19 (−0.27 to −0.12) |
| High SDI | 151268.67 (106914.90 to 201532.96) | 63.89 (35.62 to 100.55) | 150580.16 (105278.16 to 199933.65) | 58.07 (32.32 to 92.07) | −0.32 (−0.38 to −0.26) |
| High-middle SDI | 171878.14 (118293.60 to 234726.66) | 60.98 (32.27 to 102.06) | 158423.55 (107943.73 to 218285.63) | 50.3 (26.67 to 83.26) | −0.68 (−0.82 to −0.54) |
| Middle SDI | 325543.91 (221947.90 to 451888.48) | 71.45 (37.51 to 119.92) | 415724.42 (287101.46 to 578596.74) | 66.34 (35.21 to 109.09) | −0.25 (−0.30 to −0.21) |
| Low-middle SDI | 306197.61 (222182.14 to 405731.83) | 113.23 (64.52 to 180.23) | 472234.47 (331462.38 to 642459.78) | 95.14 (51.49 to 155.18) | −0.58 (−0.69 to −0.47) |
| Low SDI | 155019.46 (107926.25 to 209955.08) | 141.42 (75.2 to 231.81) | 323711.60 (226732.69 to 439319.49) | 126.09 (67.57 to 204.67) | −0.40 (−0.42 to −0.37) |
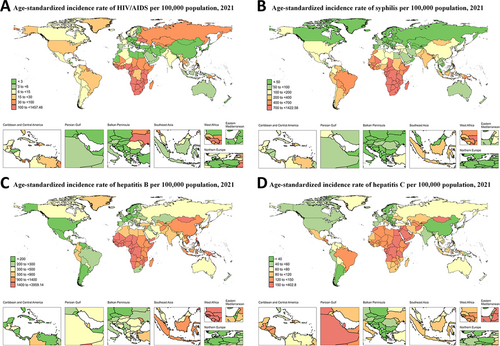
At the regional level, the highest ASIR of hepatitis B (ASIR = 2557.61; 95% UI: 1310.60, 4695.60), hepatitis C (ASIR = 235.29; 95% UI: 130.05, 376.02), and syphilis (ASIR = 1121.39; 95% UI: 599.45, 1898.21) were all observed in Central Sub-Saharan Africa. For HIV/AIDS, the highest ASIR was in Southern Sub-Saharan Africa (ASIR = 552.09; 95% UI: 420.95, 701.98) (Tables S1–S4, Figure 1).
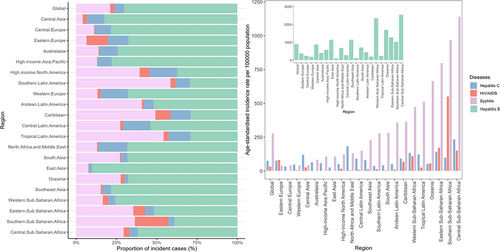
Among 204 countries and territories, the highest ASIR of hepatitis B, syphilis, HIV/AIDS, and hepatitis C were observed in Zimbabwe (ASIR = 3959.12, 95% UI: 1962.16, 7370.91), Equatorial Guinea (ASIR = 1422.58; 95% UI: 743.06, 2365.70), Lesotho (ASIR = 1257.45; 95% UI: 704.17, 2026.53), and Egypt (ASIR = 402.70, 95% UI: 221.76, 660.14) (Tables S5–S8, Figure 2).
3.2 Global Trends by Joinpoint Regression
Globally, the overall trend of HIV/AIDS ASIR among WCBA decreased between 1992 and 2021 (AAPC: −2.68 [−2.92 to −2.43]), whereas the local trends were inconsistent across different joinpoints. Specifically, the local trends of HIV/AIDS ASIR went through two phases of increase in 1992–1997 (APC: 1.93 [1.47 to 2.40]) and 2008–2014 (APC: 0.57 [0.23 to 0.91]), one phase of slow decrease in 2014–2019 (APC: −3.50 [−4.04 to −2.96]), two of rapid decrease in 1997–2005 (APC: −6.96 [−7.20 to −6.71]) and 2019–2021 (APC: −6.55 [−8.66 to −4.40]), and no changes in 2005–2008 (Figure 3).
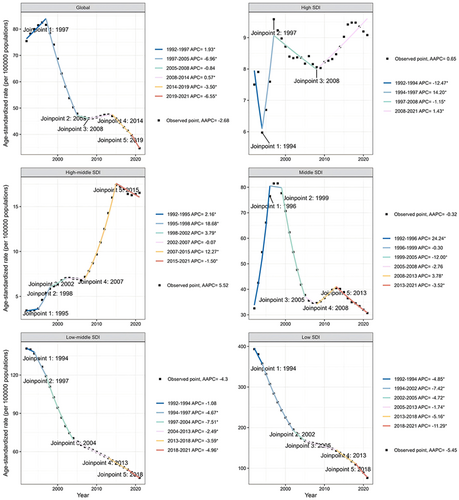
The global trend of syphilis ASIR among WCBA increased between 1992 and 2021 (AAPC: 0.28 [0.04 to 0.52]). The local trends of syphilis ASIR went through one phase of increase in 1992–2001 (APC: 0.57 [0.40 to 0.75]), two of decrease in 2001–2006 (APC: −0.85 [−1.44 to −0.24] and 2006–2010 (APC: −3.19 [−4.09 to −2.29]), two of rapid increase in 2015–2019 (APC: 1.80 [0.91 to 2.70]) and 2019–2021 (APC: 6.22 [4.23 to 8.25]), and no changes in 2010–2015 (Figure S1).
The global trend of hepatitis B ASIR among WCBA decreased between 1992 and 2021 (AAPC: −2.68 [−2.92 to −2.43]). The local trends of hepatitis B went through four continuous phases of decrease in 1992–2001 (APC: −0.61 [−0.68 to −0.55]), 2001–2010 (APC: −1.14 [−1.21 to −1.07]), 2010–2019 (APC: −1.92 [−1.99 to −1.85]), and 2019–2021 (APC: −2.93 [−3.70 to −2.16]) (Figure S2).
The global trend of hepatitis C ASIR among WCBA decreased between 1992 and 2021 (AAPC: −0.19 [−0.27 to −0.12]). The local trends of hepatitis C ASIR went through four discontinuous phase of decrease in 1992–1996 (APC: −0.89 [−1.07 to −0.71]), 1996–2000 (APC: −1.72 [−2.00 to −1.44]), 2005–2011 (APC: −0.33 [−0.46 to −0.21]), and 2011–2014 (APC: −1.01 [−1.56 to −0.45]), and two of increase in 2000–2005 (APC: 1.18 [1.00 to 1.36]) and 2014–2021 (APC: 0.58 [0.51 to 0.66]) (Figure S3).
3.3 Global Trends by SDI by Joinpoint Regression
Between 1992 and 2021, the incidence of HIV/AIDS (AAPC: 5.52 [4.86 to 6.18]), and syphilis (AAPC: 0.59 [0.32 to 0.86]) was increasing in high-middle SDI quintiles, and the incidence of hepatitis B (AAPC: −2.48 [−2.62 to −2.34]) and hepatitis C (AAPC: −0.68 [−0.82 to −0.54]) is decreasing. There were no significant changes in HIV/AIDS and syphilis incidence in high and middle SDI quintiles. The incidence of hepatitis B (AAPC: −2.23 [−2.42 to −2.05]) and hepatitis C (AAPC: −0.32 [−0.38 to −0.26]) was decreasing in high SDI quintiles. And the incidence of hepatitis B (AAPC: −2.00 [−2.18 to −1.82]) and hepatitis C (AAPC: −0.25 [(−0.3 to −0.21]) in middle SDI quintiles was also decreasing. For low-middle SDI quintiles, the incidence of HIV/AIDS (AAPC: −4.30 [−4.48 to −4.11]), syphilis (AAPC: −0.42 [−0.82 to −0.03]), hepatitis B (AAPC: −0.86 [−0.92 to −0.81]), and hepatitis C (AAPC: −0.58 [−0.69 to −0.47]) was declining. The incidence of HIV/AIDS (AAPC: −5.45 [−5.66 to −5.24]), syphilis (AAPC: −0.91 [−1.10 to −0.72]), hepatitis B (AAPC: −0.55 [−0.59 to −0.52]), and hepatitis C (AAPC: −0.40 [−0.42 to −0.37]) has declined in the Low SDI quintiles (Table 1, Figure 3, Figures S1–S3).
3.4 Regional Trends by Joinpoint Regression Analysis
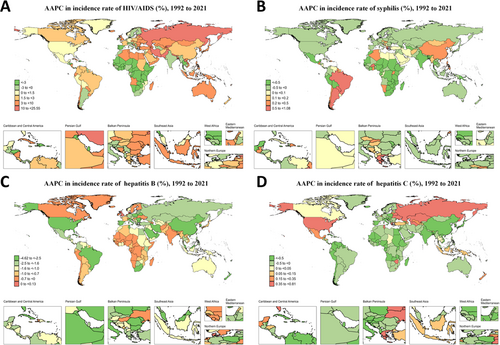
Between 1992 and 2021, the greatest increases in HIV/AIDS, syphilis, and hepatitis C incidence among WCBA were in Eastern Europe (AAPC 11.2 [10.14 to 12.27]), Tropical Latin America (AAPC 0.94 [0.70 to 1.18]), and Eastern Europe (AAPC 0.73 [0.61 to 0.84]) (Figures S4, S5, and S7). Encouragingly, the incidence of hepatitis B is declining in all 21 GBD regions. Comparatively, the regions with the largest decreases in HIV/AIDS, syphilis, hepatitis B, and hepatitis C incidence among WCBA from 1992 to 2021 were in Eastern Sub-Saharan Africa (AAPC −6.10 [−6.37 to −5.84]), Southern Sub-Saharan Africa (AAPC −2.12 [−2.28 to −1.97]), Tropical Latin America (AAPC −2.83 [−2.97 to −2.69]), and High-income Asia Pacific (AAPC −2.12 [−2.28 to −1.97]). Other regional level of AAPC values were shown in Tables S1–S4.
3.5 National Trends by Joinpoint Regression Analysis
Between 1992 and 2021, the countries with the most significant increases in incidence rates of HIV/AIDS, syphilis, hepatitis B, and hepatitis C among WCBA were Pakistan (AAPC 25.55 [22.29 to 28.90]), Greece (AAPC 1.07 [0.77 to 1.38]), the United Kingdom (AAPC 0.12 [0.11 to 0.14]), and Ukraine (AAPC 0.80 [0.70 to 0.90]). Conversely, the regions with the most significant decreases in incidence rates for these diseases were Burundi (AAPC −15.37 [−16.02 to −14.72]), Malawi (AAPC −2.74 [−2.97 to −2.51]), Niue (AAPC −4.62 [−5.20 to −4.03]), and Japan (AAPC −2.62 [−3.05 to −2.18]). Between 1992 and 2021, only the United Kingdom and Denmark had an AAPC greater than 0 for hepatitis B globally. The temporal trends of their ASIR are displayed in Figure S6. For the other MTCT diseases, temporal trends of the countries with the highest AAPC in each SDI quintile are shown in Figures S4, S5, and S7. The AAPC values for other countries are presented in Tables S5–S8 and Figure 4.
3.6 Temporal Changes in Age Distribution
Globally, in 2021, the highest age-specific incidence rates of HIV/AIDS and hepatitis B among WCBA were in the 25–29 age group, accounting for 25.6% and 17.9% respectively. For syphilis, the group with the highest age-specific incidence rate in 2021 was WCBA aged 20–24 (25.6%). The highest age-specific incidence rate of hepatitis C in 2021 was found in WCBA aged 35–39 (16.2%). The age-specific incidence rate of HIV/AIDS and syphilis for WCBA aged 15–24 has been decreasing annually, while for those aged 25–49, the rate has been on an upward trend. The age-specific incidence rates of hepatitis B and C among WCBA (15–29 years old) have been decreasing annually, while for those aged 30–49, the rates have been gradually increasing. Each disease shows similar patterns across the five SDI quintiles as the global trends. The age distribution of the SDI quintiles were showed in Figure 5 and Figures S8–S10. The proportions in the bar chart of Figure 5 were calculated based on the number of incident cases. Additionally, the age distribution of the exemplary regions and countries that were specifically analyzed in the subsequent age-period-cohort model is shown in Figures S11–S14.
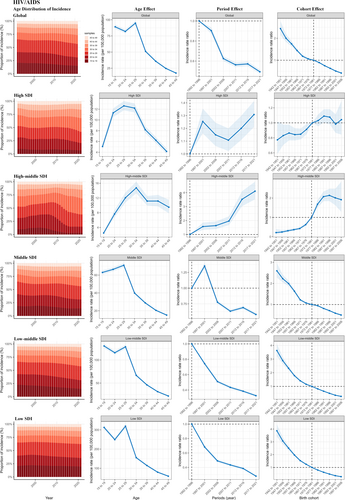
3.7 Age, Period, and Cohort Effects
For HIV/AIDS, there was a similar pattern of age effects across middle, low-middle, and low SDI regions, with women aged 15–29 years having the highest risk. And the risk for those aged 30–49 years decreased with age. Age effects presented a rising first and then declining risk of incidence among high and high-middle SDI regions, and the risk was highest in those aged 25–34 years. During 1992–2021, the low-middle and low SDI regions had the most notable decrease of period risks, while the middle-SDI region has significantly reduced the period risk until after 2001. In contrast, for the high and high-middle SDI regions, the period effects have generally shown a fluctuating upward trend over the past 30 years. As for the cohort effects, there was a gradual improvement in the incidence of middle, low-middle, and low SDI regions in successive birth cohorts. The cohort effects presented a fluctuating rising risk of incidence among high and high-middle SDI regions, with the highest risk in the birth cohort of 1982–1991 and 1987–1996 (Tables S9–S11, Figure 5).
The age effects of syphilis show a similar pattern across different SDI quintiles, with the risk increasing and then decreasing with age, and with the highest risk in those aged 20–24 years. In middle, low-middle, and low SDI regions, period effects have led to a pattern of syphilis incidence risk that first declines and then rises, and the turning point occurred between 2007 and 2016. Period effects generally showed a decreasing risk of syphilis incidence across high, middle, low-middle, and low SDI regions. The cohort effects of syphilis showed progressive improvement across high, middle, low-middle, and low SDI regions in successive birth cohorts. For the high-middle SDI region, cohort effects resulted in a stable and then rapidly increasing risk of incidence, peaking in the birth cohort of 1997–2006 (Tables S9–S11, Figure S8).
The age effects, period effects, and cohort effects of hepatitis B show a similar pattern across different SDI quintiles. In the five SDI quintiles, the age effect led to an increase and then a decrease in the risk of incidence, with the highest risk occurring in individuals aged 20–29 years. The period effects of the different SDI regions have generally shown a fluctuating downward trend over the past 30 years, with the highest risk in the period of 1992–1996. There has also been a gradual decline in cohort effects across different regions, reflecting a progressive improvement in the incidence of hepatitis B (Tables S9–S11, Figure S9).
For hepatitis C, age effects in the 20–49 age group across different SDI quintiles show a gradual increase in risk with increasing age. In high, high-middle, and middle SDI regions, period effects led to fluctuating downward trend in incidence risk. In low-middle and low SDI regions, period effects led to a decrease and then an increase in the risk of incidence, with the turning point occurring in 2012–2016. The cohort effect showed a fluctuating but overall decreasing trend across the SDI quintiles (Tables S9–S11, Figure S10).
We also conducted an analysis using the age-period-cohort model for regions with the highest AAPC in each MTCT disease and for countries with the highest AAPC in each SDI quintile, and the age, period, and cohort effects on the incidence of MTCT diseases in these exemplary regions and countries were shown in Tables S9–S11 and Figures S11–S14.
4 Discussion
MTCT diseases are characterized by their difficulty to prevent and treat, posing challenges to global health policies and systems. Updating global incidence data for these diseases among WCBA is crucial for informing policy and enhancing prevention strategies. Our study offers extensive incidence estimates across global, regional, and national levels from 1992 to 2021.
Among the 21 GBD regions, Eastern Europe has the highest increase in HIV/AIDS incidence among WCBA between 1992 and 2021. Our joinpoint regression found that Russian Federation had the fourth largest increase in HIV/AIDS incidence among WCBA from 1992 to 2021. Intravenous drug use is the primary transmission mode, with about 40% of users were women [16]. The lack of opioid substitution therapy (OST) and poorly executed needle and syringe exchange programmes (NSEPs) intensifies the intravenous transmission of HIV/AIDS Women face higher infection risks during condomless intercourse [17]. However, there are no government-funded condom distribution programs, and some organizations that promote ‘traditional values’ even discourage condom use [18]. Additionally, around 48% of female sex workers are HIV positive, and law enforcement's use of condoms as evidence of sex work may further deter their use [16, 19]. Russia still faces severe stigmatization regarding HIV/AIDS, obstacles within the health sector for HIV testing and treatment, a high number of undiagnosed individuals, and those who refuse treatment, all of which require urgent attention [20].
Despite having the largest increase in HIV/AIDS incidence among high-SDI countries, Estonia has actually seen significant improvements since 2001. Before 2000, about 74% of HIV/AIDS cases were linked to intravenous drug use [21]. To combat this, Estonia launched NSEPs in 1997 and introduced OST in 1999 [22, 23]. As a result, the share of HIV transmission linked to intravenous drug use fell to around 23% by 2015, serving as a model for other Eastern European countries [21]. In 2010, Estonia also implemented directly observed treatment to improve medication adherence among HIV/AIDS patients. Our joinpoint regression found 2001 and 2010 as key years for a drop in HIV/AIDS incidence among WCBA in Estonia, which we suspect is closely related to the implementation of the aforementioned policies.
Armenia saw the largest HIV/AIDS incidence increase among WCBA in middle SDI regions, and its region, Central Asia, ranked third in this increase. Economic hardships have led nearly 20% of Armenians to work abroad [24]. Seasonal labor migrants are the sole group contributing to the rising HIV/AIDS infection rates in Armenia, with most new female HIV/AIDS cases from 2011 to 2015 being partners of these migrants [25]. Fortunately, our joinpoint analysis indicated that, after a prolonged rise, the HIV/AIDS incidence rate among WCBA in Armenia experienced a turning point in 2013, supported by the age-period-cohort model. This change is largely attributed to the national strategic plan launched in 2013, focusing HIV/AIDS services on seasonal labor migrants with targeted testing, education, condom distribution, and mobile health care [26]. This intervention serves as a model for addressing HIV/AIDS in resource-limited settings and for countries with significant seasonal labor migrant populations, like Nepal, Ethiopia, and other Central Asian nations [27, 28].
In low-middle and low SDI quintiles, HIV/AIDS incidence among WCBA is generally declining, but some countries still struggle. Pakistan and the Solomon Islands are examples of poor HIV/AIDS prevention in low-middle and low SDI regions, respectively. Pakistan and the Solomon Islands showed similar HIV/AIDS trends among WCBA from 1992 to 2021. Poverty and inequality in low-middle and low SDI countries can limit HIV/AIDS awareness and access to healthcare and prevention. A survey in Solomon Islands indicated that most participants were unaware of HIV transmission routes beyond sexual contact [29]. Pakistan faces significant challenges in controlling HIV transmission through intravenous means, due to a widespread misconception that injections are more effective than oral medications, as well as the reuse of syringes [30, 31]. These factors increase the risk of bloodborne HIV transmission, which is an urgent issue for the Pakistani government to address.
Globally, syphilis incidence has risen sharply over the past 20 years, with Tropical Latin America seeing the steepest increase [32]. Czechia and Greece exemplify high and high-middle SDI countries where syphilis control has faltered. With the spread of highly active antiretroviral therapy (HAART) in wealthy countries towards the end of the 20th century, concerns about sexually transmitted diseases (STDs), including HIV/AIDS, have diminished. The therapeutic optimism associated with HAART has led to an increase in unprotected sex and has exacerbated the spread of syphilis [33, 34]. Additionally, our age-period-cohort model analysis shows that in the Czechia and Greece, age effects have caused syphilis risk to first rise and then fall, with the highest risk among women aged 20 to 29. For instance, a survey of young people aged 18 to 30 in Greece revealed that only 40.4% of respondents reported frequently using condoms during sexual intercourse [35]. This proportion may be even lower among sexually active populations in some less developed countries and regions, indicating a global need to focus more on increasing condom use and sex education to tackle the challenge of STDs.
Our joinpoint model indicated that syphilis incidence among WCBA in Brazil (Middle SDI) rose steadily from 2005 to 2021, along with increasing rates among pregnant women and congenital syphilis cases [36]. While improved monitoring and rapid testing may explain some of this increase, they do not fully account for the rising incidence [37]. Economic and healthcare disparities play a significant role in the incidence of syphilis in Brazil [36]. The Northeast region, which has the highest syphilis incidence, also has the highest illiteracy rate [37]. Sociologically vulnerable groups, such as women with brown and black skin, are at a higher risk of contracting syphilis and face greater challenges in accessing quality prenatal care [36]. The Brazilian government has implemented a series of measures to improve public health [38]. However, as a developing country with a large population, ethnic diversity, and uneven economic development, Brazil still has a long way to go in eliminating social inequalities.
Democratic People's Republic of Korea (DPRK) had the highest increase in syphilis incidence among WCBA in low-middle SDI regions, yet it rarely shares its health data internationally. A study of DPRK travelers to China from 2015 to 2017 found rising syphilis rates, primarily among women aged 30–50 [39]. Most travelers are economically well-off and have better access to medical resources, suggesting that syphilis transmission may be more severe within DPRK. Given that STDs are a taboo topic, we recommend that the DPRK enhance public health education to raise awareness and prevention of these diseases.
Burkina Faso has the fastest-growing syphilis incidence rate among low-SDI countries. In Burkina Faso, the joinpoint analysis showed that syphilis incidence among WCBA rose rapidly from 2000 to 2005, then slowed and started declining after 2009. This decline is linked to sustained prevention campaigns for STDs that have increased public awareness and changed behaviors since 2005, along with intensified systematic screening and penicillin treatment supported by the international community during the same period [40, 41]. Despite the declining incidence rate, Burkina Faso still has a long way to go in eliminating syphilis.
Globally, only Denmark and the United Kingdom showed a slight increase in hepatitis B incidence in WCBA between 1992 and 2021. Despite this, many high-incidence countries have seen declines due to hepatitis B vaccines. The joinpoint model detects even minor changes in these countries with initially low incidence rates. The actual increase in hepatitis B incidence in the UK and Denmark is minimal, and we recommend they take appropriate detection and prevention measures to prevent broader public health issues. Notably, despite the global decline in incidence, 254 million people still have hepatitis B, with a significant number in countries like Bangladesh, China, Ethiopia, India, and Nigeria. In the Western Pacific, only 23% of diagnosed patients receive treatment, indicating the need for ongoing efforts to reduce incidence and improve treatment rates [42].
Eastern Europe has also seen the highest increase in hepatitis C incidence globally from 1992 to 2021. Our joinpoint model indicates that Ukraine (high-middle SDI) exemplifies unfavorable hepatitis C prevention and control in Eastern Europe. Most new hepatitis C virus infections can be attributed to injecting drug use [43, 44]. Due to the lack of national funding for hepatitis C diagnosis in Ukraine, the cost of testing is often borne by the patients, which may delay the start of treatment [45]. Additionally, WHO recommends postponing hepatitis C treatment until after delivery and breastfeeding cessation. In Ukraine, WCBA often breastfeed for over 2 years and may become pregnant soon after birth, limiting their access to timely treatment [45]. Our age-period-cohort model also revealed a notably high risk of hepatitis C among females aged 15 to 19 years, related to an illicit drug use rate of up to 18% in adolescents, highlighting the need for significant government intervention [46].
Tunisia, Zimbabwe, and Solomon Islands exemplify unfavorable hepatitis C prevention and control among middle, low-middle, and low SDI countries, respectively. Our joinpoint and age-period-cohort models showed similar trends in hepatitis C incidence among WCBA in these countries, with drug use being a significant transmission route. Most injection drug users in Tunisia have low levels of education and are generally unemployed, and the absence of OST and low-coverage NSEPs make it difficult to effectively carry out Hepatitis C prevention and control efforts [47]. Women who use drugs in North Africa, including Tunisia, face significant social and cultural stigma, complicating Hepatitis C control [48]. Zimbabwe has not implemented prevention measures for injecting drug users and female sex workers, who often experience social discrimination and criminalization [49]. Public healthcare in Zimbabwe cannot perform hepatitis C virus polymerase chain reaction quantitative test, forcing patients to seek costly private options, and treatment coverage is low due to limited drug supply [47]. The Solomon Islands struggles with insufficient medical resources for Hepatitis C screening and monitoring [50]. Social-cultural barriers and limited medical resources exacerbate the disadvantaged position of women in hepatitis C prevention in these countries.
Additionally, we've found that Monaco has the highest increase in Hepatitis C incidence among high-SDI countries. However, due to its small population size and scarcity of relevant literature, we cannot currently analyze the reasons for poor Hepatitis C control in Monaco, only issuing a caution.
This study presents for the first time the incidence of four major MTCT diseases among WCBA based on GBD 2021 estimates, offering crucial data for policymakers to craft health policies and allocate resources effectively. However, the limitations of this study should not be overlooked. Firstly, the limited availability of primary data from some low- and middle-income countries means that modeling estimates had to be used for regions lacking direct data, which may affect the accuracy of our estimates. Therefore, when interpreting information from the GBD database and formulating public health strategies, it is advisable to conduct a comprehensive analysis in conjunction with other authoritative data sources like WHO. By comparing and analyzing the results from different data sources, mutual verification and supplementation can be achieved, thereby enhancing the accuracy of the assessment. Secondly, data from 1990 to 1991 were excluded due to the age-period-cohort model's requirement for continuous 5-year age groups. Thirdly, joinpoint regression and age-period-cohort models do not explain the root causes of incidence changes, leading to a reliance on literature reviews that could introduce subjectivity and bias into our analysis. Additionally, focusing on the incidence among newborns is crucial for assessing the severity of MTCT diseases. However, the GBD database does not provide data on syphilis incidence in newborns. Given the resource constraints of this study, we have decided to concentrate on analyzing the incidence rates among WCBA. In the future, we plan to conduct a dedicated study on the disease burden of HIV/AIDS, hepatitis B, and hepatitis C in newborns, to provide information for the prevention and treatment of MTCT diseases from another perspective.
5 Conclusion
This study found that from 1992 to 2021, the global incidence rates of HIV/AIDS and hepatitis B showed a downward trend, while those of hepatitis C and syphilis increased after 2012. From a regional and national perspective, the incidence rates of HIV/AIDS and hepatitis C in Eastern Europe increased the most, which requires special attention. The rise in syphilis incidence rates was most significant in Tropical Latin America. At the national level, Pakistan, Greece, and Ukraine were the countries with the fastest-growing incidence rates of HIV/AIDS, syphilis, and hepatitis C among WCBA, respectively. Although prevention and control efforts have achieved success in some regions, the disease burden in many areas is still increasing. In the future, targeted health education, screening, and precise interventions should continue to be strengthened to ensure that high-risk regions and groups have access to sufficient medical resources and to reduce the prevalence of MTCT diseases.
Author Contributions
Ping Song, Shuanglin Zhou, and Qiubai Jin conceived the study. Qiubai Jin, Mingxiao Zhou, Meiqi Sun, and Bobiao Ning analysed the GBD data. QBJ, MXZ, and Mingyue Liu contributed to the statistical analysis and interpretation of data. Qiubai Jin and Mingxiao Zhou drafted the manuscript, and other authors critically revised the manuscript. All authors have read and approved the final version of the manuscript.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 82474521). We acknowledge the Institute for Health Metrics and Evaluation (University of Washington), the GBD Collaborators, and all staff who provided the data necessary for this study. The opinions expressed here are those of the authors and do not necessarily represent the official position of the organizations with which they are affiliated.
Ethics Statement
The present investigation, which leverages data extracted from the GBD database, does not involve any direct interaction with human subjects. Given that the data set consists of anonymized records accessible to the public domain, this research was exempt from obtaining ethical approval.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in Global Burden of Disease (GBD) Results Tool at https://vizhub.healthdata.org/gbd-results/. These data were derived from the following resources available in the public domain: - Global Health Data Exchange (GHDx), https://vizhub.healthdata.org/.




