Elevated Soluble ACE2 Activity in Children and Adults After SARS-CoV-2 Exposure Irrespective of Laboratory-Confirmed Infection
ABSTRACT
The pivotal role of the cell entry receptor ACE2 for SARS-CoV-2 infection is well-established. When ACE2 is shed from cell surface into plasma as soluble ACE2 (sACE2), it can effectively neutralize SARS-CoV-2. This longitudinal prospective cohort study analyzed sACE2 activity in 1192 participants, aged 4 months to 81 years, 3 and 12 months after SARS-CoV-2 household exposure. Following SARS-CoV-2 exposure, participants exhibited significantly elevated sACE2 activity, irrespective of confirmed infection, with the highest levels observed in exposed children. Longitudinal analysis revealed a decline in sACE2 levels over time, reaching levels comparable to age- and sex-matched pre-pandemic controls. An increase in sACE2 activity was also confirmed in vitro in Calu-3 (human lung) cells within hours of SARS-CoV-2 exposure, providing a direct link between SARS-CoV-2 exposure and elevated sACE2. This study, therefore, challenges the dichotomy of categorizing SARS-CoV-2 exposed participants as infected or not infected solely on currently established diagnostic assays. It demonstrates lasting host responses independent of B- and T-cell memory and may help to keep SARS-CoV-2 infections in balance and contribute to successful virus clearance in children and adults lacking humoral and cellular immune responses following SARS-CoV-2 exposure.
Trial Registration: German Registry for Clinical Studies; Identifier: D 00021521.
1 Introduction
The emergence of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) brought the world to a standstill in the spring of 2020. The lack of preexisting immunity to this virus and its ability to evade and dysregulate the immune system, coupled with widespread transmission, created a global public health crisis. Driven by the high clinical need for a better understanding of the most severe COVID-19 cases, there has been a disproportionate focus on severely affected elderly patients with symptomatic COVID-19. A better understanding of the physiological virus−host interaction in asymptomatic or mildly affected immune-naïve adults and especially children during and after the first viral encounter may be crucial for a better understanding of the history of SARS-CoV-2 and may help us to be better prepared for future emerging viruses beyond SARS-CoV-2.
One of the key features that enable SARS-CoV-2 to infect and replicate in host cells is its ability to attach to the cell surface via the host cell receptor angiotensin-converting enzyme 2 (ACE2) [1-3]. Entry mediated by ACE2 is observed for many other coronaviruses, including SARS-CoV, MERS-CoV, HCoV-NL63, and bat coronaviruses [4-6]. Physiologically, ACE2 acts as a key regulator of the renin−angiotensin system (RAS) [7]. ACE2 reduces cytokine release, protects against organ damage in cardiovascular, renal, hepatic, and pulmonary disease [8] and has been shown to protect against coronavirus-induced acute respiratory distress syndrome (ARDS) [9, 10]. To fulfill its regulatory role in the RAS, cellular ACE2 (cACE2) must first be transported to the cell surface, where it is cleaved by host proteases to produce an enzymatically active soluble form of ACE2 (sACE2) in the plasma [11]. sACE2 not only retains its enzymatic activity [12, 13], which correlates linearly with plasma concentration [14], but also its ability to bind to SARS-CoV-2 (reviewed in [15]).
The spike protein is able to induce shedding of membrane-bound ACE2 into plasma, and an increase in sACE2 has been demonstrated for SARS-CoV and HCoV-NL63, which also depend on ACE2 for viral entry [16, 17]. Preclinical studies have shown effective neutralization of SARS-CoV-2 and other ACE2-tropic viruses by recombinant sACE2, providing an immune-cell-independent and mutational-resistant broad-spectrum defense mechanism that inhibits SARS-CoV-2 infection locally in the respiratory tract, but also systemically in blood vessels and secondary organs (reviewed in [15]).
SARS-CoV-2-specific B- and T-cell responses may be absent, especially in asymptomatic or mildly infected individuals [18, 19]. Children, in particular, more often lack B- and T-cell responses [18, 19] but clear SARS-CoV-2 rapidly [20], making it more difficult to detect infections with currently established detection methods. Therefore, reliable surrogate markers are needed to close this diagnostic gap.
Increased sACE2 serum concentration and activity have been reported during [21-27] and up to 4 months after [21, 28, 29] SARS-CoV-2 infection in adults, but no studies have determined sACE2 after asymptomatic SARS-CoV-2 infection, and especially in participants with proven SARS-CoV-2 household exposure but no evidence of infection. We, therefore, investigated sACE2 longitudinally in a cohort of children and adults with household exposure to SARS-CoV-2 during the first wave of the pandemic. This provided a unique opportunity to study the effects of SARS-CoV-2 exposure in immune-naïve adults and children.
We hypothesized that sACE2 activity would be elevated not only in participants with a proven SARS-CoV-2 infection but also in participants with a proven SARS-CoV-2 exposure but who showed no evidence of a SARS-CoV-2 infection using current detection methods. We moreover hypothesized that sACE2 activity would be particularly elevated in young children, who clear SARS-CoV-2 via immune defense mechanisms independent of B- and T-cells.
In addition, we investigated whether sACE2 expression was associated with persistent symptoms, as persistent symptoms after SARS-CoV-2 infection (long COVID) have been attributed to sACE2 (reviewed in [30]).
2 Methods
2.1 Study Design and Conduct
This study is part of a multicentre, non-interventional, prospective, observational cohort study of households with at least one child and one SARS-CoV-2-infected index case as confirmed by reverse transcriptase polymerase chain reaction (RT-PCR) in the German state of Baden-Württemberg (German Registry for Clinical Studies [DRKS], study ID 00021521). The study was initiated by the University Children's Hospitals of Heidelberg, Freiburg, Tübingen, and Ulm. Results on household transmission [31, 32], immune responses [18, 33-37], and persistent symptoms [38] have been published previously. Participants recruited at the Heidelberg, Tübingen, and Ulm study sites were included in this substudy. Households were eligible for enrollment if they met all of the following inclusion criteria: (i) ≥ 1 household member with an RT-PCR-proven SARS-CoV-2 infection from a nasopharyngeal or oropharyngeal swab specimen collected ≥ 2 weeks before the study visit, (ii) ≥ 1 household member < 18 years of age, (iii) residence in the state of Baden-Württemberg, and (iv) all household members were officially released from quarantine. Exclusion criteria were: (i) lack of written informed consent, (ii) insufficient knowledge of the German language. Study participants were investigated at a first-time point (T1) between May 11 to August 1, 2020, mean (SD) 3 ± 1 (range, 1−4) months after household infection and/or exposure. They were asked to complete a questionnaire on age, sex, the result of their SARS-CoV-2 RT-PCR test, including test date, COVID-19-associated symptoms (dysgeusia, cough, fever, diarrhea), and need for hospitalization. In Tübingen and Ulm, all households that agreed to be recontacted were invited to participate at a second-time point (T2). In Heidelberg, only households with infected children were recruited for T2. T2 participation occurred 12 ± 1 (range, 9−15) months after SARS-CoV-2 infection or exposure. All participants answered questions about SARS-CoV-2 (re)infection and vaccination between T1 and T2 and persistent moderate or severe symptoms (including fatigue, reduced exercise tolerance, shortness of breath, sleep disturbances, low mood/anxiety, dysgeusia/dysosmia, impaired concentration, memory impairment, chest tightness, hair loss) still present at T2 (see [38] for more details). An age- and sex-matched healthy control group of children and adults who had blood drawn for routine laboratory analysis, for example, before elective minor surgery in early 2020, with routine laboratory results within normal limits and excess serum available were included as pre-pandemic control samples. None of these controls had another acute or chronic disease or received any medication.
The study protocol was approved by the Institutional Review Boards and Ethics Committees of the Medical Faculties Heidelberg (S-294/2020), Tübingen (293/2020BO2), and Ulm (152/20). The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants and their parents or guardians, with assent from children if appropriate for their age. The study was designed, analyzed, and reported according to the STROBE guidelines (https://www.strobe-statement.org).
2.2 Detection of SARS-CoV-2-Reactive Antibodies and Infection History
Three commercially available immunoassays were used as per the manufacturer's instructions to test all available specimens for the presence of Anti-SARS-CoV-2 S1 IgG (EI2606-9601G; EuroImmun), RBD IgG (11207377; Siemens), and Nucleocapsid pan-Ig (09203095190; Roche). Participants were defined as seropositive if at least two of these three tests were positive and as seronegative if at least two out of three tests were negative. Participants were defined as “infected” if they either reported a positive RT-PCR test for SARS-CoV-2 before T1 or were seropositive at T1. Participants were defined as “not infected” if they both did not report having had a positive SARS-CoV-2 RT-PCR test before T1 and were seronegative at T1. Participants who self-reported a SARS-CoV-2 infection or who seroconverted between T1 and T2 were excluded from analysis at T2.
2.3 SARS-CoV-2 Specific T-Cell Response
In a subset of participants from Tübingen, PBMCs were collected and SARS-CoV-2 specific T-cell responses were determined using an interferon-γ enzyme-linked immunospot assay (IFN-γ ELISPOT) assay after 12 days of in vitro stimulation as described previously [18, 39]. Briefly, PBMCs were seeded in 48-well plates on day 0. Human leukocyte antigen (HLA) class I or HLA-DR isotype-restricted epitope compositions (EC) were added to the PBMC cultures on Day 1. Cultures were supplemented with interleukin-2 (Novartis) and interleukin-7 (PeproTech) on Days 2, 5, 7, and 9. PBMCs were harvested on Day 12, counted, and analyzed by IFN-γ ELISPOT in replicates. PBMCs were seeded into 96-well microtiter plates (Millipore) coated with anti-IFN-γ (clone 1-D1K, 2 µg mL−1, MabTech). ECs for HLA class I and HLA-DR were added to each well. Ten percent DMSO was used as a negative control and PHA (10 µg mL−1, Sigma-Aldrich) as a positive control. After 24 h of incubation, the cells were removed, and the wells were washed. IFN-γ spots were labeled by incubation with biotinylated anti-IFN-γ detection antibody (clone 7-B6-1, 0.3 µg mL−1, MabTech) for 2 h and visualized by addition of ExtrAvidin alkaline phosphatase (1:1000 dilution, Sigma-Aldrich) and substrate BCIP/NBT (Sigma-Aldrich). The reaction was stopped after 7 min, the plates were dried and spot counts were determined using an ImmunoSpot S6 Ultra-V Analyzer (C.T.L.). Mean spot counts of replicates were calculated and normalized to 5 × 105 cells. Intensities of T-cell responses were calculated by subtracting the normalized mean spot counts of the EC-stimulated samples and the corresponding negative controls. T-cell responses were considered positive if the normalized mean spot count was ≥ 3 times that of the corresponding negative control and the intensity was ≥ 10. The normalized mean spot count for the positive control had to be ≥ 100.
2.4 Incubation of Human Lung Cells With SARS-CoV-2 Spike Pseudovirus
The human lung cell line Calu-3 was used for this experiment. Cells were seeded into a 96-well plate at a density of 30 000 cells per well in 100 µL DMEM supplemented with 20% fetal calf serum (FCS) and 2 mM sodium pyruvate. After 24 h, cells were washed with PBS and subsequently exposed to SARS-CoV-2 Spike (S)-pseudotyped lentiviral particles (SCoV-2-Spike), generated in 293 T cells (human kidney) in Opti-MEM using a standard second-generation lentiviral vector protocol with a synthetic codon-optimized SARS-CoV-2 S gene (Wuhan-Hu-1 strain) [40, 41]. As a negative control for SARS-CoV-2 spike-mediated entry, pseudotyped lentiviral particles were generated in the same manner as previously described, but instead of a spike-encoding plasmid, an empty vector was used. Supernatants were collected 16 h after exposure to determine the activity of the sACE2 enzyme.
2.5 sACE2 Plasma Enzyme Activity
The sACE2 plasma enzyme activity was determined in all available T1 and T2 serum samples from Tübingen and Heidelberg and in T1 samples from Ulm (limited serum volumes at T2) centrally at the study site in Heidelberg. We also determined sACE2 plasma enzyme activity in pre-pandemic control samples and in the supernatant of Calu-3 (human lung) cells after 16 h of exposure to pseudovirus with and without SARS-CoV-2 Spike. T1 samples from Heidelberg and pre-pandemic control samples were analyzed in June 2020. All other samples (Tübingen [T1 and T2], Ulm [T1], and Heidelberg [T2]) were analyzed in June 2021. Therefore, the Heidelberg samples were measured 2 months after collection at T1 and T2 to avoid bias from different sample storage times, whereas all Tübingen samples were measured together to control for batch effects. The pre-pandemic control samples were measured 4−6 months after collection, which is longer than the Heidelberg samples (2 months) but shorter than the T1 samples from Tübingen and Ulm (1 year). Plasma sACE2 enzyme activity was determined using the commercially available SensoLyte 390 ACE2 activity assay kit (72086; AnaSpec) according to the manufacturer's protocol. The fluorescence of Mc-Ala was monitored at excitation/emission 330 nm/390 nm. All reactions were performed in 96-well, clear, flat-bottomed polystyrene microplates (82.1581.001; Sarstedt, Nümbrecht, Germany).
2.6 Statistical Analysis
Analyses were performed using GraphPad Prism version 9 (GraphPad Software, San Diego, CA, USA). Binary or categorical variables are presented as absolute frequencies (n) and relative frequencies (%) and assessed by a chi-squared test. Continuous variables are presented as mean with SD. Differences in continuous variables between two groups were compared by two-tailed unpaired or paired t-test and between more than two groups by ordinary one-way ANOVA. Linear regression analysis was used to analyze whether sACE2 at T1 (Models 1 and 3) and T2 (Model 2) was influenced by the variables sex, age, and SARS-CoV-2 infection (Models 1 and 2) and additionally by SARS-CoV-2 vaccination (Model 2) or anti-SARS-CoV-2 Ig (anti-N pan-Ig, anti-S1-RBD IgG, and anti-S1 IgG), symptoms, and hospitalization during the acute SARS-CoV-2 infection (Model 3). To investigate risk factors predicting moderate/severe persistent symptoms still present at T2, we used a logistic regression model (Model 4) with the same independent variables as in Model 1 or 2 and additionally sACE2 at T1. No imputation was performed. No a priori formulated hypotheses were tested; therefore, all p values and confidence intervals (CIs) are reported as descriptive measures.
3 Results
3.1 Participants and Characteristics
We enrolled 1376 participants from 338 households with at least one child and one SARS-CoV-2 infected index case as confirmed by RT-PCR. One hundred and sixty-five participants with insufficient sample volume/quality for sACE2 and SARS-CoV-2 serological testing and three participants with an unclear SARS-CoV-2 infection history were excluded (Figure 1). In accordance with previous reports [42], participants with diabetes mellitus (n = 14) and with chronic kidney disease (n = 2) had increased sACE2 activity (Figure S1), and were therefore excluded. A total of 1192 participants aged 4 months to 81 years were available for final analysis. Five hundred and ninety-four participants were defined as infected (SARS-CoV-2 RT-PCR and/or antibody positive). Five hundred and ninety-eight participants were SARS-CoV-2 seronegative without a documented positive RT-PCR and therefore defined as only exposed at T1 (Table 1; Figures S2 and S3). SARS-CoV-2 infection was more common in participants aged ≥ 12 years (adolescents and adults) than in those aged < 12 years (children) (492/857 [57.4%] vs. 102/335 [30.4%]; p < 0.0001), and infected adolescents and adults were more likely to be symptomatic than infected children (417/492 [84.8%] vs. 55/102 [53.9%]; p < 0.0001). Hospitalization was rare in both groups (14/492 [2.8%] vs. 2/102 [2.0%]).
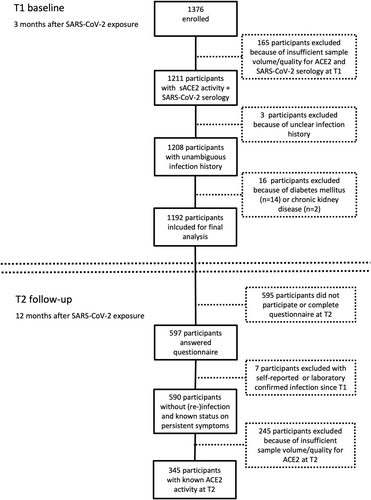
| Pre-pandemic control cohort | Participants included at T1 | Participants included at T2 | Participants with sACE2 data at T2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | < 12 years | ≥ 12 years | All | < 12 years | ≥ 12 years | All | < 12 years | ≥ 12 years | All | < 12 years | ≥ 12 years | |
| Total no of participants | 154 | 46 | 108 | 1192 | 335 | 857 | 590 | 160 | 430 | 345 | 89 | 256 |
| Age, years mean ± SD (range) | 26.7 ± 20.7 (0−79) | 7.2 ± 3.5 (0−11) | 35.1 ± 19.7 (12−79) | 28.4 ± 18.4 (0−81) | 6.8 ± 3.1 (0−11) | 36.9 ± 14.6 (12−81) | 29.1 ± 18.5 (0−81) | 7.2 ± 3.0 (0−11) | 37.3 ± 14.8 (12−81) | 29.9 ± 18.7 (0−81) | 7.0 ± 3.1 (0−11) | 37.7 ± 15.0 (12−81) |
| Sex | ||||||||||||
| Female, n (%) | 81 (52.6) | 22 (47.8) | 59 (54.6) | 606 (50.8) | 166 (49.6) | 440 (51.3) | 301 (51.0) | 74 (46.3) | 227 (52.8) | 182 (52.8) | 43 (48.3) | 139 (54.3) |
| Male, n (%) | 73 (47.4) | 24 (52.2) | 49 (45.4) | 586 (49.2) | 169 (50.4) | 417 (48.7) | 289 (49.0) | 86 (53.8) | 203 (47.2) | 163 (47.2) | 46 (51.7) | 117 (45.7) |
| SARS-CoV-2 infection | ||||||||||||
| No, n (%) | 154 (100) | 46 (100) | 108 (100) | 598 (50.1) | 233 (69.6) | 365 (42.6) | 229 (38.8) | 89 (55.6) | 140 (32.6) | 109 (31.6) | 44 (49.4) | 65 (25.4) |
| Yes, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 594 (49.9) | 102 (30.4) | 492 (57.4) | 361 (61.1) | 71 (44.4) | 290 (67.4) | 236 (68.4) | 45 (50.6) | 191 (74.6) |
| SARS-CoV-2 vaccination | ||||||||||||
| No, n (%) | 154 (100) | 46 (100) | 108 (100) | 1192 (100) | 335 (100) | 857 (100) | 520 (88.1) | 160 (100) | 360 (83.7) | 316 (91.6) | 89 (100) | 227 (88.7) |
| Yes, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 70 (11.9) | 0 (0.0) | 70 (16.3) | 29 (8.4) | 0 (0.0) | 29 (11.3) |
Of the 1192 participants included for analysis at T1, 597 completed a questionnaire on persistent symptoms still present at T2; 12 months after SARS-CoV-2 household exposure. Seven participants were excluded due to self-reported or laboratory-confirmed SARS-CoV-2 (re)infection between T1 and T2. Of the 590 included participants, 106 participants (18.0%) reported moderate or severe symptoms that were still present 11−12 months after SARS-CoV-2 exposure. As previously reported [38], persistent symptoms were more frequent in infected than in exposed participants (86/361 [23.8%] vs. 20/229 [8.7%]; p < 0.0001), in adolescents and adults than in children (100/430 [23.3%] vs. 6/160 [3.8%]; p < 0.0001), and in females than in males (67/301 [22.3%] vs. 39/289 [13.5%]; p = 0.0056).
In a subset of 345 participants sACE2 was measured longitudinally at both T1 and T2 (Figure 1). In addition, we included pre-pandemic samples from SARS-CoV-2 unexposed controls (n = 154). The sex and age distributions were balanced between groups (Table 1).
In an age- and sex-representative subset (n = 67) of the 598 exposed participants without evidence of a SARS-CoV-2 infection (Supporting Information S1: Table 1), we determined T-cell responses against multiple SARS-CoV-2 specific antigens 3 months after the SARS-CoV-2 household exposure. Of the 67 participants, 38 participants (56.7%) had a SARS-CoV-2 specific T-cell response, while 29 (43.3%) had no cellular response.
3.2 sACE2 Enzyme Activity 3 and 12 Months After SARS-CoV-2 Exposure
At 3 months after SARS-CoV-2 exposure (T1), mean sACE2 enzyme activity was significantly higher in participants than in SARS-CoV-2 unexposed controls (120.1 ± 28.1 vs. 92.5 ± 35.1 µU/mL; p < 0.0001; Figure 2A). Elevated sACE2 enzyme activity was observed not only in participants with SARS-CoV-2 infection (seropositive and/or positive RT-PCR) but also in participants only exposed to SARS-CoV-2 and without evidence of infection (seronegative and no positive RT-PCR). Infected and exposed participants had similar sACE2 levels at T1 (119.6 ± 28.8 vs. 120.6 ± 27.4 µU/mL; p = 0.82; Figure 2B), also with different definitions of seropositivity (Figure S4).
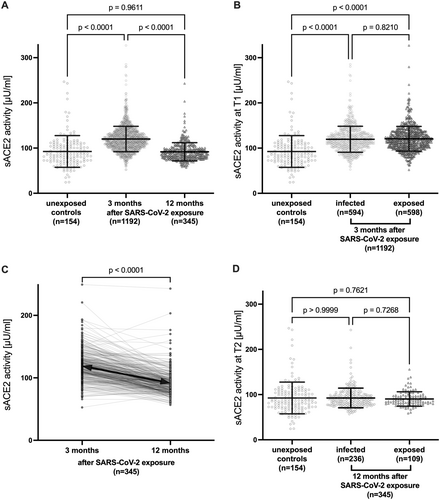
We were able to determine sACE2 longitudinally at 3 months (T1) and 12 months (T2) after SARS-CoV-2 household exposure in a representative subset (n = 345) of the full cohort (sACE2 at T1: 119.4 ± 24.3 vs. 120.1 ± 28.1 µU/mL; p = 0.66). In this longitudinal cohort, mean sACE2 decreased significantly from T1 to T2 (119.4 ± 24.3 vs. 91.8 ± 20.2 µU/mL; p < 0.0001; Figure 2C) to levels observed in unexposed pre-pandemic controls (91.8 ± 20.2 vs. 92.5 ± 35.1 µU/mL; p = 0.96; Figure 2A,D). This effect was observed independently at both study sites (Figure S5) and in infected and exposed participants who had similar sACE2 levels also at T2 (Figure 2D).
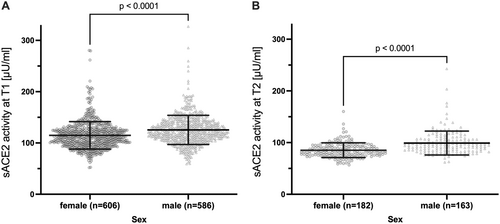
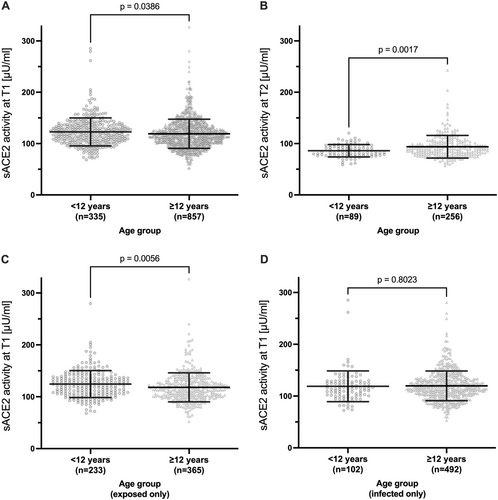
As shown in pre-pandemic cohorts [43, 44], mean sACE2 differed between sexes at both time points, with slightly but significantly higher sACE2 activity in males than in females at T1 (Figure 3A and Supporting Information S1: Table 2) and T2 (Figure 3B and Supporting Information S1: Table 3). Children had slightly higher sACE2 than adolescents and adults at T1 (Figure 4A and Supporting Information S1: Table 2), but a stronger decline from T1 to T2 (Figure S6) resulting in lower sACE2 at T2 (Figure 4B and Supporting Information S1: Table 3). The difference at T1 was driven by significantly higher sACE2 in exposed children (Figure 4C). There was a trend of a higher sACE2 activity in patients vaccinated to SARS-CoV-2 at T2 (p = 0.074, Supporting Information S1: Table 3). In a subgroup analysis of infected participants only (n = 594), sex (p < 0.0001) but not age, anti-SARS-CoV-2 Ig, and symptoms or hospitalization during the acute SARS-CoV-2 infection were significantly associated with sACE2 at T1 (Supporting Information S1: Table 4).
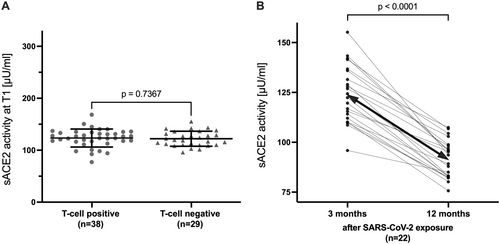
In a representative subset (n = 67) of the full cohort of exposed participants without evidence of a SARS-CoV-2 infection (sACE2 at T1: 122.8 ± 16.1 vs. 120.6 ± 27.4 Uµ/mL; p = 0.52), we determined T-cell responses against multiple SARS-CoV-2 antigens 3 months after SARS-CoV-2 household exposure. Mean sACE2 did not differ between T-cell responders and nonresponders 3 months after household exposure (123.4 ± 17.3 vs. 122.1 ± 14.5 Uµ/mL; p = 0.74; Figure 5A). Of the 29 nonresponders, 22 also had sACE2 measured at T2, and sACE2 decreased from T1 to T2 in all 22 participants (124.1 ± 14.4 vs. 91.4 ± 9.47 Uµ/mL; p < 0.0001; Figure 5B).
3.3 Relationship of sACE2 With Long COVID Symptoms 1 Year After SARS-CoV-2 Exposure
Whether sACE2 is associated with the development of long COVID is an ongoing debate [30]. We therefore analyzed whether sACE2 at T1 (3 months after SARS-CoV-2 exposure) predicted moderate or severe persistent symptoms present 1 year after SARS-CoV-2 exposure (Supporting Information S1: Table 5). The risk of moderate or severe persistent symptoms still reported 1 year after SARS-CoV-2 was associated with a laboratory-confirmed SARS-CoV-2 infection (OR 2.68 [95% CI 1.60−4.67]; p = 0.003), female sex (OR 1.79 [95% CI 1.13−2.86]; p = 0.014), and age ≥ 12 years (OR 6.40 [95% CI 2.95−16.78]; p < 0.0001). It was not associated with sACE2 activity (OR 1.00 [95% CI 0.996−1.004]; p = 0.95), and moreover, sACE2 at T1 and at T2 did not differ significantly between infected participants with and without persistent symptoms (Figure S7).
3.4 sACE2 After SARS-CoV-2-Spike Pseudovirus Exposure In Vitro
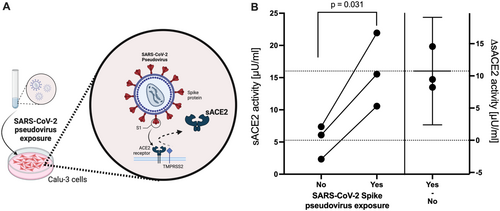
To investigate whether ACE2 shedding may be a direct effect of cells being exposed to SARS-CoV-2 spike, as demonstrated for SARS-CoV and HCoV-NL63 [16, 17], we employed a pseudovirus-based assay using lentiviral particles that bear the SARS-CoV-2 spike surface protein (SCoV-2 spike). ACE2-expressing Calu-3 (human lung) cells exposed to SCoV-2 spike pseudovirus for 16 h demonstrated significantly higher sACE2 activity in the supernatant than cells exposed to lentiviral particles without SARS-CoV-2 spike (16.0 ± 5.70 vs. 5.27 ± 2.62 Uµ/mL; p = 0.031; Figure 6).
4 Discussion
The main finding of this study is that enzyme activity of the soluble form of the SARS-CoV-2 cell entry receptor ACE2 (sACE2) was significantly elevated in participants after SARS-CoV-2 household exposure, irrespective of confirmed infection. Longitudinal analysis showed a significant decline in sACE2 activity from 3 to 12 months after exposure, reaching levels comparable to those in unexposed pre-pandemic controls.
We hypothesized that SARS-CoV-2 exposure can induce shedding of membrane-bound ACE2 into plasma as sACE2. Consistent with this, not only infected but also exposed adults, as well as adolescents and children without evidence of a SARS-CoV-2 infection, showed at least equally elevated levels of sACE2 after SARS-CoV-2 exposure. Despite exposure to SARS-CoV-2, virus-specific humoral or cellular immune responses can be absent. In particular, young children clear SARS-CoV-2 rapidly [20] and more often lack a measurable B- and/or T-cell response [18]. In the present study, exposed children without evidence of a SARS-CoV-2 infection had the highest sACE2 enzyme activity after SARS-CoV-2 exposure. This may indicate successful and rapid SARS-CoV-2 neutralization by sACE2 without the need for a B- or T-cell response to clear SARS-CoV-2 in children.
In this current study, an increase in sACE2 activity was confirmed within hours of exposure to SCoV-2 spike pseudovirus in vitro, as previously demonstrated for SARS-CoV and HCoV-NL63 [16, 17]. ACE2 shedding as well as elevated sACE2 after SARS-CoV-2 exposure may help to keep SARS-CoV-2 infections in balance. sACE2 has been shown to promote infections at low concentrations, while at higher concentrations, it reduces the likelihood of infection and severe disease in three synergistic ways (reviewed in [15]). First, by reducing the abundance of membrane-bound ACE2, thereby reducing the ability of coronaviruses to bind and infect host cells. Second, by neutralizing SARS-CoV-2 extracellularly in the respiratory tract, blood, and secondary organs as sACE2 retains its SARS-CoV-2 binding site. Third, as a key regulator of the RAS to prevent increased inflammation and organ damage as its enzymatic activity remains intact after shedding. This limits the local and systemic spread of infection and inflammation.
Trained immunity and a long-term increase in the host's innate immune response following SARS-CoV-2 exposure can persist for months (reviewed in [45]), which could be associated with elevated sACE2 levels several weeks after SARS-CoV-2 household exposure. Another possible explanation for elevated sACE2 levels is persistent SARS-CoV-2 virus, RNA, or protein triggering continuous sACE2 shedding. Although SARS-CoV-2 primarily infects the respiratory tract, it can also replicate in other organs that express ACE2. The highest ACE2 expression in the human body is found in the intestine [46, 47]. In one meta-analysis, viral RNA was found in 50% (2690/5334) of fecal samples from patients with acute COVID-19, with the highest prevalence of 86% (127/148) observed in children [48]. While SARS-CoV-2 infection is usually cleared from the respiratory tract within 2 weeks, clearance from other sites can be delayed and prolonged fecal RNA shedding as well as organ persistence of RNA or viral antigens have been reported in adults and children for several months (reviewed in [49]).
Previous studies have reported elevated sACE2 concentration and activity during [21-27] and up to 4 months after [21, 28, 29] (severe) SARS-CoV-2 infections. sACE2 activity has been associated with disease severity [22, 25, 27] and discussed as a result of organ dysfunction. We did not find higher sACE2 in participants with symptomatic infection during the acute SARS-CoV-2 infection or in participants with moderate or severe persistent symptoms (long COVID). Risk factors for severe COVID-19, such as age, male sex, obesity, diabetes mellitus, and chronic renal and cardiac disease, are associated with high sACE2 activity independent of SARS-CoV-2 [42-44, 50].
Our study has limitations. As discussed above, we most likely missed to detect SARS-CoV-2 infections, especially in children. For practical reasons, we could not evaluate sACE2 during the potential infection by SARS-CoV-2. During the first pandemic wave, household members were required to home-quarantine during the household infection and were not sequentially tested due to the unavailability of rapid antigen tests and only limited RT-PCR test capacities. Another limitation is that we did not have serum samples from participants before household exposure to define baseline sACE2 levels. Instead, we used age- and sex-matched pre-pandemic controls for comparison. We cannot exclude the possibility that increased sACE2 activity was observed independently of SARS-CoV-2 exposure due to other uncontrolled factors (e.g., isolation, stress, or inflammation). However, blood samples were taken during general lockdowns at T1 and T2 and additionally in Heidelberg at the same time of the year (May) and day (morning). We were able to demonstrate increased sACE2 activity within hours of exposure to SCoV-2 spike pseudovirus in vitro, but we did not study continuous SARS-CoV-2 or spike protein persistence in clinical samples or continuous spike-induced ACE2 shedding in vitro. An indirect mechanism is presumably involved in the persistent release of ACE2 3 months after SARS-CoV-2 exposure.
A major strength is the timing of the study, which provided a unique opportunity to study the host responses of a diverse immune-naïve cohort. Households were recruited through health authorities during the first wave of the pandemic when household members were SARS-CoV-2 naïve before household exposure. The multicentre cohort of 1192 participants aged 4 months to 81 years, with mostly mild or asymptomatic infections and exposures, and no overrepresentation of severe COVID-19, was a good representation of SARS-CoV-2 infections and exposures in the general population.
In conclusion, this study provides new insights into host responses after SARS-CoV-2 exposure. We observed that sACE2 enzyme activity after SARS-CoV-2 exposure was elevated independent of confirmed infection by currently established assays, and hypothesize that this mechanism may contribute to SARS-CoV-2 clearance. This study therefore challenges the dichotomy of categorizing SARS-CoV-2 exposed participants as infected or not infected solely on currently established diagnostic assays.
Author Contributions
Maximilian Stich designed the study, procured funding, collected and analyzed data, and drafted the first version of the manuscript. Vladimir Gonçalves Magalhães, Friederike Bürger, Kerstin Mohr, Armin Rabsteyn, Peter Lang, Eva-Maria Jacobsen, Barbara Müller, Jürgen G. Okun, Ralf Bartenschlager, and Marco Binder designed and performed laboratory analysis and provided key resources. Sven F. Garbade did statistical analysis. Kathrin Jeltsch, Anneke Haddad, and Sebastian F. N. Bode collected and analyzed data. Roland Elling, Hans-Georg Kräusslich, Georg Friedrich Hoffmann, Aleš Janda, Hanna Renk, and Burkhard Tönshoff designed the study, procured funding, and provided key resources. All authors revised the manuscript for important intellectual content, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work.
Acknowledgments
We are especially indebted to all households who participated in this study. We thank the following staff members for data and sample collection, laboratory analyses and excellent organizational support: Jürgen Grulich-Henn, Kristine Chobanyan-Jürgens, Andreas Ziegler, Michal Fischer, Iris Schelletter, Florian Gleich (from Heidelberg), Andrea Evers-Bischoff, Andrea Bevot, Theda Himpel, Corinna Engel (from Tübingen), and Maria Zernickel, Carmen Blum, Andrea Hänsler, Gudrun Kirsch, and Ulrike Tengler (from Ulm). The study was funded by the Ministry of Science, Research and the Arts in Baden-Württemberg, Germany, within the framework of the special funding line for COVID-19 research, part of the measures to combat the SARS-CoV-2 pandemic in the field of medical research. Maximilian Stich was funded by the DZIF clinical leave stipend of the German Center for Infection Research. The funder of the study had no role in the design and/or conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. Open Access funding enabled and organized by Projekt DEAL.
Ethics Statement
The study protocol was approved by the Institutional Review Boards and Ethics Committees of the Medical Faculties Heidelberg (S-294/2020), Tübingen (293/2020BO2), and Ulm (152/20). The study was conducted in accordance with the Declaration of Helsinki.
Consent
Informed consent was obtained from all participants and their parents or guardians, with assent from children if appropriate for their age.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Deidentified data will be made available upon publication to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. Proposals should be submitted to [email protected].




