The origin and spread of HIV-1 CRF59_01B epidemic in China: A molecular network and phylogeographic analysis
Huanchang Yan, Hao Wu, and Shunming Li are contributed equally to this study.
Abstract
Human immunodeficiency virus type 1 CRF59_01B, identified in China in 2013, has been detected nationwide, exhibiting notably high prevalence in Guangzhou and its vicinity. This study aimed to unravel its origin and migration. A data set was established, incorporating all available CRF59_01B pol gene sequences and their metadata from Guangzhou and the public database. Bayesian phylogeographic analysis demonstrated that CRF59_01B originated in Shenzhen, the neighboring city of Guangzhou, around 1998 with posterior probability of 0.937. Molecular network analysis detected 1131 transmission links and showed a remarkably high clustering rate (78.9%). Substantial inter-city transmissions (26.5%, 300/1131) were observed between Shenzhen and Guangzhou while inter-region transmissions linked Guangzhou with South (46) and Southwest (64) China. The centre of Guangzhou was the hub of CRF59_01B transmission, including the inflow from Shenzhen (3.57 events/year) and outflow to the outskirts of Guangzhou (>2 events/year). The large-scale analysis revealed significant migration from Shenzhen to Guangzhou (5.08 events/year) and North China (0.59 events/year), and spread from Guangzhou to Central (0.47 events/year), East (0.42 events/year), South (0.76 events/year), Southwest China (0.76 events/year) and Shenzhen (1.89 events/year). Shenzhen and Guangzhou served as the origin and the hub of CRF59_01B circulation, emphasizing inter-city cooperation and data sharing to confine its nationwide diffusion.
1 INTRODUCTION
China has the most complex cocirculation of human immunodeficiency virus type 1 (HIV-1) genotypes, featured by iterative recombination and replacement of prevalent strains.1-3 Subtype B, subtype C and CRF01_AE were demonstrated to be the initial inflow HIV-1 genotypes in China.1, 4 During the 1990s, CRF07_BC and CRF08_BC emerged as a result of the recombination of subtype B and subtype C in China's HIV-1 gateway of Yunnan, then spreading nationwide through drug trafficking routes,5-8 while CRF07_BC achieved a stable proportion in the late 2000s within the men who have sex with men (MSM) population.9-11 Since 2010s, CRF55_01B and CRF59_01B have been successively identified, reshaping the HIV epidemic in China.12, 13 Their proportions were increasing, whereas CRF55_01B followed a transmission route originating from Shenzhen, extended to Guangdong, and subsequently spread nationwide.14 However, the origin and migration of CRF59_01B remain unclear.
CRF59_01B is the second CRF01_AE and subtype B recombinant strain identified among MSM in China.13 It exhibited a significant prevalence (>2%) in Shenzhen and Guangzhou,15, 16 two major cities in Guangdong, while displaying sporadic transmission in other five provinces and one municipality.13, 15, 17-21 The current study aimed to unravel the origin and spread of HIV-1 CRF59_01B epidemic in China through molecular network and phylogeographic analysis. Our results confirm Guangdong as the origin and hub of CRF59_01B epidemic, and highlight the necessary of continuously monitoring the emergence and transmission of HIV-1 genotypes.
2 MATERIALS AND METHODS
2.1 CRF59_01B sequences
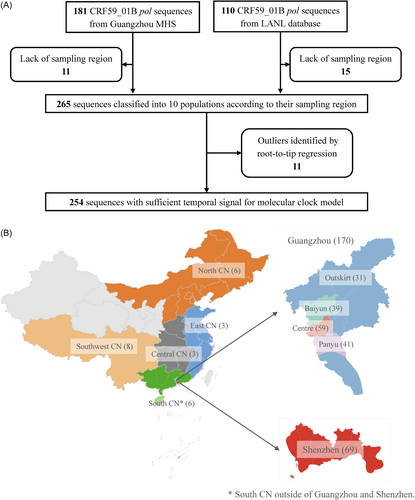
181 CRF59_01B pol gene region sequences (HXB2: 2253−3821 nt, 1568 bp) were collected from newly diagnosed people living with HIV (PLWH) between 2008 and 2020 through HIV molecular surveillance in Guangzhou, China. Sample collection, sequencing and subtyping have previously been described.22, 23 Additional 110 CRF59_01B sequences (HXB2: 2253−3821 nt) were retrieved from the Los Alamos National Laboratory (LANL) HIV sequence database. CRF59_01B sequences without sampling location or sampling year information were excluded from the complete data set (Figure 1). According to the sampling regions, the CRF59_01B sequences were classified into 10 populations, that is, Baiyun district, Panyu district, the city centre of Guangzhou (GZ centre), the outskirts of Guangzhou (GZ outskirts), Shenzhen (a nearby city to Guangzhou within the Pearl River Delta), North China (North CN), Central China (Central CN), East China (East CN), South China (South CN) and Southwest China (Southwest CN) (Figure 1; Table S1). This classification divided Guangzhou into four regions (city centre, city outskirts, and the other two districts between them) to obtain a high-resolution interpretation of spatio-temporal history regarding CRF59_01B circulation. When assessing the overall profile (e.g., the overall export of CRF59_01B), Guangzhou was analyzed as a whole region. We termed these two classifications as the small-scale and large-scale analysis, respectively.
Demographic (age at diagnosis, gender, marital status, education, and occupation), social (HIV infection route, and the total number of reported contacts through sexual behavior or needle sharing) and clinical (HIV/AIDS status at diagnosis and current treatment status) characteristics were extracted from the electronic follow-up records from China Information Management System for Comprehensive Prevention and Control of AIDS, with the permission of Guangzhou Center of Disease Control and Prevention (CDC). This study was approved by the Institutional Review Board of School of Public Health, Sun Yat-sen University (No. 2023073).
2.2 Molecular network inference
To profile the geographic transmission pattern, molecular network of CRF59_01B was constructed at a pairwise genetic distance (GD) threshold of 0.012 substitution/site using HIV-TRACE (TRAnsmission Cluster Engine).24 CRF59_01B pol sequences were initially aligned by MAFFT v7.25 Pairwise GD was then calculated by employing Tamura-Nei 93 model.26 Each PLWH was assigned according to the sampling region and represented by a node in the molecular network while nodes were linked if the pairwise GD is 0.012 substitution/site or less. The molecular network was visualized using Cytoscape 3.7.027 and spatialized to generate a transmission intensity matrix according to the numbers of links between regions.
2.3 Temporal structure test
We conducted regression of sampling time versus root-to-tip GD to examine the temporal structure in the data set. The tree topology and branch lengths were inferred using maximum likelihood (ML) method in IQ-TREE 1.6.12.28 The nucleotide substitution model was selected for phylogenetic reconstruction based on the Bayesian information criterion using ModelFinder.29 Node support in the ML phylogeny was estimated using an ultrafast bootstrap with 1000 replicates and a Shimodaira-Hasegawa approximate likelihood-ratio test with 1000 replicates.30 The regression analysis was performed on the ML tree using TempEst 1.5.3.31 The outliers with poor temporal signals were subsequently removed from the final data set (Figure 1).31
2.4 Bayesian phylogeographic analysis
Bayesian discrete phylogeographic analysis was performed on the final data set using BEAST v1.10.4 to investigate the spatiotemporal dynamic of CRF59_01B in China.32 Bayesian stochastic search variable selection (BSSVS) procedure incorporating an asymmetric substitution model was used to infer viral migration.33 Additionally, a robust counting (Markov jumps) approach was applied to estimate the expected location-state transitions along the phylogenetic branches.34 Simultaneously, Bayesian coalescent phylogeny construction was done by using Markov Chain Monte Carlo (MCMC) integration,32 to estimate the evolution rate and the time to the most recent common ancestor (tMRCA). We selected a general time-reversible nucleotide substitution model with a gamma distribution prior on each relative substitution rate.35 A relaxed uncorrelated lognormal molecular clock model was used to infer the timescale of CRF59_01B evolution, further specified with a gamma distribution prior on the mean clock rate (shape = 0.001, scale = 1000).36 A skygrid demographic model was implemented to infer the changes in effective population size of CRF59_01B over time.37 The MCMC chain length was set to 3 × 108 steps, logging every 5000 iterations.
The MCMC output was inspected with Tracer v1.7.2.38 After discarding the first 10% of samples as burn-in using LogCombiner v1.10.4, we checked the convergence by ensuring that effective sample sizes for parameters exceeded 200. A maximum clade credibility tree (MCC) with discrete geographical traits was inferred from the posterior set of trees in TreeAnnotator v1.10.4, with a 10% burn-in of measured states. The MCC tree was visualized in the ggtree R package.39 To reveal when and from where CRF59_01B entered a particular region, we defined an introduction as the oldest node on a branch which transitioned into this region and counted all of its descendants as part of the same introduction irrespective of further exports or re-introductions. The location of the parent node of the introduction branch indicated where CRF59_01B imported, while the time of the transition node gave a conservative time estimation of introduction.40 To avoid an accident introduction, we required at least 3 local infections present in the branch. In addition, statistical support for each pairwise diffusion between regions was evaluated by Bayes factors (BF) summarized in SpreaD3 0.9.6.41 Migration pathways were considered significant if supported by BF ≥ 3 and posterior probability ≥ 0.50.
2.5 Associating geographic traits with inferred tree topology
Association of geography with phylogeny was tested using a Bayesian Tip-association Significance Testing (BaTS).42 By assigning a geographic trait to each PLWH and subsequently reconstructing the ancestral states of nodes in the phylogeny, we determined whether a geographic trait is more likely to be shared by PLWH whose viruses are closely related than expected by chance alone, that is, when compared to random distribution on the same phylogeny. To address phylogenetic uncertainty, 1000 trees were subsampled every 300 000 iterations from the posterior set of trees using LogCombiner and analyzed using 100 null replicates per each tree. The phylogeny-trait association was measured by three statistics: association index (AI) statistic, parsimony score (PS) statistic, and monophyletic clade (MC) statistics for each discrete-trait. In brief, the AI statistic is an observed-to-expected ratio that measures the frequency of the most common branch tip trait subtended by internal nodes.43 The PS statistic calculates the PSs for the traits supplied by the operator.44 The MC statistic evaluates the number of tips with a given trait value in the largest MC identified in subsampled trees.45 The statistics were calculated and tested using BaTS v0.9 beta.42 p < 0.05 means statistically significant.
2.6 Statistical analysis
Joinpoint regression analysis was conducted to examine the trend in counts of newly diagnosed CRF59_01B infections in Guangzhou using Joinpoint v5.0.2.46 Chi-square test was performed to compare the clustering rates between groups of different infection routes. PLWH in Guangzhou and those involved in the introductions of CRF59_01B to Guangzhou were mapped according to their available residence, respectively. Descriptive analysis was used to characterize PLWH that involves in the largest introduction to Guangzhou. Univariate and multivariate logistic models were used to identify potential factors related to the largest introduction of CRF59_01B to Guangzhou. Statistical analysis was performed using R v4.2.3.
3 RESULTS
3.1 Rapid increase of CRF59_01B infections
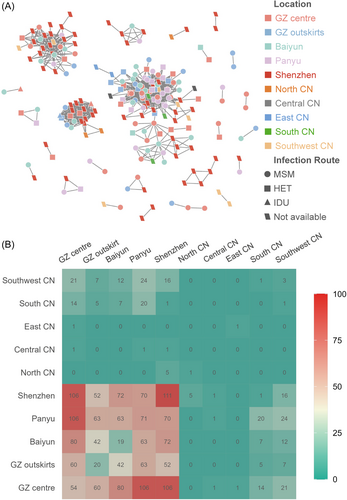
During 2008 and 2020, a total of 181 HIV-1 CRF59_01B pol sequences were obtained from individuals newly diagnosed with HIV-1 infection in Guangzhou. Joinpoint regression analysis of these CRF59_01B sequences indicated that the number of people infected with HIV-1 CRF59_01B significantly increased in Guangzhou with an average annual percent change of 25.63 (95% confidence interval [CI]: 15.14−36.55) while the annual percent change [APC] between 2011 and 2015 was as high as 94.72 (95% CI: 45.71−187.63, [Figure S1A]). To further investigate the change of CRF59_01B epidemic, a data set of 265 HIV-1 CRF59_01B pol sequences were established, including the above 170 HIV-1 CRF59_01B sequences obtained from Guangzhou and 95 CRF59_01B sequences retrieved from LANL database, and the detailed information of CRF59_01B infections were collected (Figure 1 and Table S1). The data set was used to construct the molecular transmission network with a pairwise GD threshold of 0.012 substitutions/site. 78.9% of these sequences formed 25 transmission clusters of 2-100 sequences, comprising 1131 potential transmission links (Figure 2A and Figure S2A). Of note, the clustering rate for non-MSM was significantly higher than that for MSM (87.8% vs. 77.7%, χ2 = 28.8, p < 0.001). Most of the clustered sequences were sampled from Shenzhen (61/209, 29.2%), or the city centre (47/209, 22.5%) and Panyu district (32/209, 15.3%) of Guangzhou. Furthermore, the transmission intensity matrix shows that the transmission links were predominantly from CRF59_01B-infected PLWH in Guangzhou and Shenzhen city (1117/1131, 98.8%).
3.2 Origin and evolution of CRF59_01B
Root-to-tip regression excluded 11 CRF59_01B sequences that are incongruent in their genetic divergence and phylogenetic position and 254 CRF59_01B sequences with a root-to-tip correlation coefficient of 0.47 were included in the molecular clock model to infer time-scaled tree.
Bayesian phylogenetic analysis showed that the tMRCA of CRF59_01B was 1998.6 with a 95% highest probability density [HPD] interval of 1994.6−2001.9 in a small-scale analysis where Guangzhou was coded into 4 regions (see Section 2). The mean substitution rate was estimated as 2.75 × 10−3 (95% HPD interval: 2.22 × 10−3−3.29 × 10−3) substitutions/site/year. The large-scale study where Guangzhou was coded as a whole region (see Section 2) revealed the estimated tMRCA of 1997.5 [1992.9−2001.5], along with the mean substitution rate of 2.75 × 10−3 [2.23 × 10−3−3.30 × 10−3] substitutions/site/year. The time-scaled Bayesian skygrid demographic plot illustrated a rapid increase of CRF59_01B population since its emergence although a slight decline was observed after 2018 (Figure 3B).
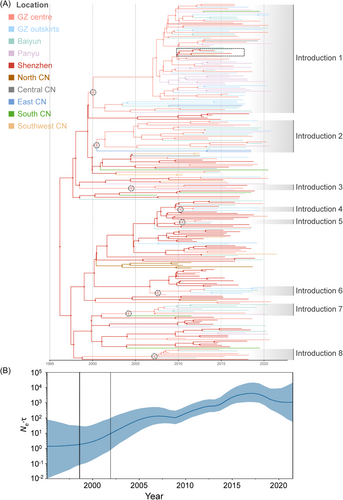
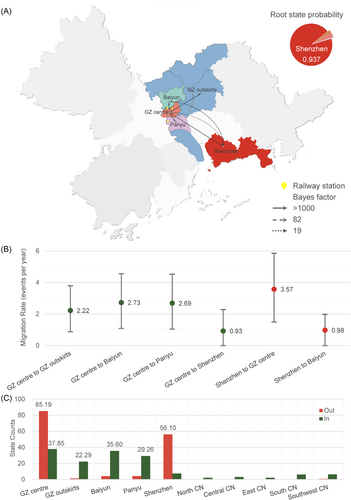
Both small and large scale BSSVS analysis indicated that the root of the phylogenetic trees of CRF59_01B was in Shenzhen city with a posterior probability of 0.937 (Figure 3A and Figure 4A) and 0.721 (Figure 5A), respectively. Furthermore, the global phylogeny-geography association for CRF59_01B was statistically supported by AI (15.227, p < 0.001) and PS (114.708, p < 0.001, Table S2), indicating that CRF59_01B sequences were phylogenetically clustered by region. The association tested by the MC statistic was significant for the outskirts (p = 0.010), Baiyun (p = 0.050) and Panyu (p = 0.010) district of Guangzhou, Shenzhen city (p = 0.010), as well as North (p = 0.010), East (p = 0.010), and Southwest China (p = 0.010). The geographic structure further confirmed the circulation of CRF59_01B in these regions (Table S2). Taken together, the Bayesian phylogeographic analysis indicated that CRF59_01B originated in Shenzhen around 1998, and rapidly spread in Shenzhen and transmitted to other regions in China.
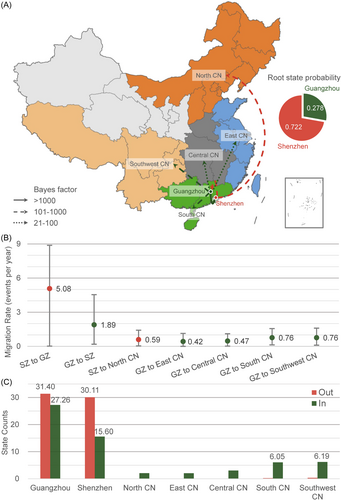
3.3 CRF59_01B migration between Shenzhen and Guangzhou
Molecular network analysis exhibited 300 inter-city transmission links between Shenzhen and Guangzhou while 35.3% (106/300) of the links associated with GZ centre (Figure 2B and Figure S2B). The small-scale phylogeographic analysis revealed the migration of CRF59_01B from Shenzhen to the city centre (BF = 2581, posterior probability = 0.997, mean rate = 3.57 events/year) and Baiyun district (BF = 82, posterior probability = 0.908, mean rate = 0.98 events/year) of Guangzhou while the migration of CRF59_01B was also identified from the centre of Guangzhou to Shenzhen (BF = 19, posterior probability = 0.694, mean rate = 0.93 events/year, Figure 4A,B and Table S3). The migration pathways between Guangzhou and Shenzhen were strongly supported in the large-scale analysis (Figure 5A,B and Table S3). By counting the location transitions along the phylogeny, we confirmed the major import of CRF59_01B from Shenzhen to Guangzhou (Figure 4C and Figure 5C). The time-scaled MCC tree showed that eight introductions (see Section 2) occurred from Shenzhen to the centre of Guangzhou during 2004 and 2011 (Figure 3A and Table S4). Notably, six of them appeared around 2007 or 2010. And the largest introduction occurred around 2004.2 [2001.2−2007.4], and transmitted back to Shenzhen around 2010.8 [2009.2−2012.5]. This introduction caused HIV-1 infection of 71 PLWH in Guangzhou, 3 in Shenzhen, 1 in Central CN, 1 in East CN, 1 in South CN and 1 in Southwest CN. Our analysis confirmed the frequent transmission of CRF59_01B between Shenzhen and Guangzhou since 2004 while the city centre of Guangzhou served as the entry point of CRF59_01B.
3.4 CRF59_01B spread within Guangzhou
The molecular transmission network showed that 51.1% (578/1131) of the potential transmission links of CRF59_01B were within Guangzhou (Figure S2B). Among them, a lot of inter-region links were observed between GZ centre and other regions of Guangzhou (Baiyun [80], Panyu district [106] and GZ outskirts [60]) while few links were within GZ centre (54) (Figure 2B). The phylogeographic analysis further revealed inter-region migration pathways from GZ centre to Baiyun (BF = 497095, posterior probability > 0.999, mean rate = 2.73 events/year), Panyu (BF = 99412, posterior probability > 0.999, mean rate = 2.69 events/year), and GZ outskirts (BF = 82842, posterior probability > 0.999, mean rate = 2.22 events/year), indicating the major migration pathway of CRF59_01B from the centre to the other regions of Guangzhou, rather than its transmission within the city centre.
Eight introductions of CRF59_01B from Shenzhen resulted in 123 infections in Guangzhou and primarily located in the city centre (36/123, 29.3%) and Panyu district (33/123, 26.8%, Figure S1C). Logistic regression analysis identified the factors associated with CRF59_01B infections, including residence, marital status (married vs unmarried: odds ratio [OR] = 2.33, 95% confidence interval [95% CI]: 1.18−4.67), number of closed contact and engagement in commercial sex (OR = 2.75, 95% CI: 1.12−7.23, Table S5). Compared with the city centre of Guangzhou, the multivariate model indicated that residence of Panyu district (OR = 6.90, 95% CI: 1.81−30.40) and GZ outskirts (OR = 4.76, 95% CI: 1.21−20.86) was more likely to be a risk factor related to CRF59_01B infection. Both univariate and multivariate models confirmed that fewer closed contacts were less likely to be infected with CRF59_01B since OR was 0.89 (95% CI: 0.79−0.98) and 0.84 (95% CI: 0.70−0.97), respectively.
3.5 CRF59_01B expansion nationwide
The molecular network showed that CRF59_01B transmission was more frequently observed between Guangzhou and Central CN (2 vs. 1), East CN (1 vs. 0), South CN (46 vs. 1) and Southwest CN (64 vs. 16) than that between Shenzhen and these regions in China (Figure S2B) while CRF59_01B infections in North CN exclusively linked with Shenzhen. The Markov jump method confirmed the exports of CRF59_01B from Guangzhou and Shenzhen to other regions in China (Figure 5C). The 7 nationwide migration pathways included migration from Shenzhen to Guangzhou (mean rate = 5.08 events/year, BF = 158, posterior probability = 0.968), from Guangzhou to Shenzhen (mean rate = 1.89 events/year, BF = 15820, posterior probability > 0.999), from Guangzhou to South CN (mean rate = 0.76 events/year, BF = 796, posterior probability = 0.993), from Shenzhen to North CN (mean rate = 0.59 events/year, BF = 428, posterior probability = 0.988), from Guangzhou to Southwest CN (mean rate = 0.76 events/year, BF = 156, posterior probability = 0.967), from Guangzhou to Central CN (mean rate = 0.47 events/year, BF = 77, posterior probability = 0.936) and from Guangzhou to East CN (mean rate = 0.42 events/year, BF = 37, posterior probability = 0.874) (Figure 5A,B and Table S3). The results of tMRCAs showed that CRF59_01B spread from Shenzhen to North CN since 2004.3 [2001.7−2006.8] and from Guangzhou to Southwest CN and East CN since 2009.0 [2007.7−2010.2] and 2013.6 [2010.0−2017.1], respectively. Thus, CRF59_01B spread from Shenzhen to North CN, while it expanded from Guangzhou to Central, East, South, and Southwest China.
4 DISCUSSION
By integrating molecular network and phylogeographic methods, we revealed that Shenzhen city might be the origin and Guangzhou city the transmission hub of emerging CRF59_01B. Since its emergence in Shenzhen around 1998, CRF59_01B was frequently introduced to the city centre of Guangzhou since 2004, and then spread to other regions in China, resulted in nationwide expansion, highlighting the importance of collaborative efforts between these two cities in HIV prevention and control. Our results also characterized CRF59_01B infection with the capability of rapid growth, remarkable clustering rate and wide expansion in general population, indicating more frequent transmission of CRF59_01B among local PLWH and the need of cost-effective intervention policies.47
Frequent inter-city transmission of CRF59_01B between Shenzhen and Guangzhou was observed in the molecular network analysis and its direction was further determined through Bayesian phylogeographic analysis. The significant migration pathways between Guangzhou and Shenzhen presented the spatiotemporal pattern of CRF59_01B epidemic, which might be closely associated with the convenient railway connection between Guangzhou and Shenzhen since the opening of Guangzhou-Shenzhen Railway system between the city centre of Guangzhou and Shenzhen in 2007 and Guangzhou South Railway Station in Panyu district of Guangzhou in 2010 (Figure 4). Both Guangzhou and Shenzhen are the major driver of economic and technologic development in the Pearl River Delta. Improved connection and exchange between the two cities may speed the transmission of HIV-1 infections and expansion of the emerging CRF59_01B. These findings highlight the need of molecular monitoring system and information sharing of HIV-1 infection in Guangzhou and Shenzhen.
Of note, Guangzhou was not only the entry point, but also the transmission hub of CRF59_01B, exporting the strain to other regions and triggering the local transmission within Southwest and East China. The large-scale phylogeographic analysis revealed CRF59_01B introductions from Guangzhou to Central, East, South and Southwest China and from Shenzhen to North China. These results are consistent with the migration pattern of CRF55_01B infection, suggesting CRF59_01B and CRF55_01B might share the same migration pathways alongside Beijing−Guangzhou and Beijing−Kowloon railway system.14 The small-scale phylogeographic analysis pinpointed the city centre of Guangzhou as the major entry point for introductions from Shenzhen, and the transit hub for the local diffusion. Thus, prevention efforts should be focused on the city centre of Guangzhou to curb CRF59_01B transmission in the current stage.
Both small-scale and large-scale analysis indicated that CRF59_01B emerged around 1998, 3 years earlier than a previous estimate of 2001.13 This difference might be due to the significantly larger data set used to improve reliability of inference in our study, but only 7 CRF59_01B sequences used in the previous study conducted in 2014. Although CRF59_01B likely originated earlier than CRF55_01B (2003),14 it has been detected at a relatively low frequency in China.13, 15, 17-20 However, the number of newly diagnosed CRF59_01B infections rapidly increased in Guangzhou around 2012. The increasing pattern of CRF59_01B population is very similar to CRF55_01B epidemic, which rapidly increased around 2008.14 Furthermore, for CRF59_01B, the clustering rate, a proxy for transmissibility48 reached an exceptionally high level (78.9%) at a GD threshold of 0.012 substitutions/site/year. These results indicate the remarkable transmission fitness of CRF59_01B. Genetic mutations may impact viral virulence, transmissibility and disease progression.49, 50 Further investigation about the potential mechanisms to affect viral fitness and disease progression is essential.51
Although CRF59_01B was first identified among MSM, a considerable number of PLWH acquired CRF59_01B through non-MSM infection routes in Guangzhou probably because a substantial proportion of Chinese MSM married or had sex with women,52 suggesting that the epidemic have spread to the general population. In contrast to other CRFs, CRF59_01B infection exhibited high clustering rate among non-MSM subjects in Guangzhou.23 There is an urgent need to implement prevention strategy to effectively address and curb CRF59_01B transmission among both MSM and non-MSM.
Our study has several limitations. First, CRF59_01B sequences retrieved from LANL HIV sequence database may not represent all CRF59_01B infections nationwide and may potentially affect location state inference for parent nodes in phylogeographic analysis. However, to the best of our knowledge, this study established the largest CRF59_01B data set to date to comprehensively understand the spatiotemporal dynamic. Further analysis utilizing continuously accumulated data in the future may help mitigate potential bias introduced by sampling. Second, the difference of geographic categories between the small-scale analysis and the large-scale analysis may lead to variation in nodal location state estimates based on the trait of tips. This study presented regional migration within the Pearl River Delta from the small-scale analysis and nationwide migration patterns from the large-scale analysis. Thirdly, the study did not account for the impact of social mobility improved by the recent development of public transportation, especially within Guangzhou, thereby potentially introducing bias in the estimation of migration rates. Further research should include data on social and individual mobility to improve our understanding of the migration dynamic of HIV-1 strains.
In conclusion, this study employs molecular network and phylogeographic methods to identify the origin and transmission patterns of CRF59_01B. The results highlight the importance of continuously monitoring of HIV-1 transmission and precise prevention strategy at regional level to prevent HIV-1 epidemic.
AUTHOR CONTRIBUTIONS
Yu Liu, Zhigang Han, Huanchang Yan, Jing Gu, and Yuantao Hao contributed to conceptualizing and study design. Hao Wu, Shunming Li, Yefei Luo, Yuzhou Gu, Yanshan Cai, and Zhigang Han collected the data. Huanchang Yan, Jiahang Wang, and Rui Luo analyzed the data. Huanchang Yan, Shixing Tang, Yuantao Hao, Jing Gu, and Yu Liu interpreted the data. All authors contributed to drafting and final review of the manuscript and approved the final manuscript.
ACKNOWLEDGMENTS
We would like to thank Wenjing Wu and Mingyu Chen for their suggestions. This work was supported by the Guangdong Basic and Applied Basic Research Foundation (2021A1515011591 to Yu Liu), the Guangdong Medical Science and Technology Research Foundation (A2021104 to Yu Liu and A2023393 to Hao Wu), the Key Project of Medicine Discipline of Guangzhou (2021-2023-11 to Zhigang Han), the Nation Natural Science Foundation of China (71774178 to Zhigang Han), the Guangzhou Science and Technology Project (2023B03J259 to Hao Wu and Zhigang Han), and the Guangzhou Medical Science and Technology Grant (20211A011056 to Yefei Luo).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from corresponding authors. The data are not publicly available due to privacy or ethical restrictions.




