Global burden of vaccine-associated hepatobiliary and gastrointestinal adverse drug reactions, 1967–2023: A comprehensive analysis of the international pharmacovigilance database
Sooji Lee and Kyeongmin Lee contributed equally to this study.
Abstract
Although previous studies have focused on hepatobiliary and gastrointestinal adverse drug reactions (ADRs) associated with COVID-19 vaccines, literature on such ADRs with other vaccines is limited, particularly on a global scale. Therefore, we aimed to investigate the global burden of vaccine-associated hepatobiliary and gastrointestinal ADRs and identify the vaccines implicated in these occurrences. This study utilized data from the World Health Organization (WHO) international pharmacovigilance database to extract reports of vaccine-associated hepatobiliary and gastrointestinal ADRs from 1967 to 2023 (total reports = 131 255 418). Through global reporting counts, reported odds ratios (ROR) with 95% confidence interval (CI), and information components (IC) with IC0.25, the study examined the association between 16 vaccines and the incidence of hepatobiliary and gastrointestinal ADRs across 156 countries. Of the 6 842 303 reports in the vaccine-associated ADRs, 10 786 reports of liver injury, 927 870 reports of gastrointestinal symptoms, 2978 reports of pancreas and bile duct injury, and 96 reports of intra-abdominal hemorrhage between 1967 and 2023 were identified. Most hepatobiliary and gastrointestinal ADRs surged after 2020, with the majority of reports attributed to COVID-19 messenger RNA (mRNA) vaccines. Hepatitis A vaccines exhibited the highest association with liver injury (ROR [95% CI]: 10.30 [9.65–10.99]; IC [IC0.25]: 3.33 [3.22]), followed by hepatitis B, typhoid, and rotavirus. Specifically, ischemic hepatitis had a significant association with both Ad5-vectored and mRNA COVID-19 vaccines. Gastrointestinal symptoms were associated with all vaccines except for tuberculosis vaccines, particularly with rotavirus (11.62 [11.45–11.80]; 3.05 [3.03]) and typhoid (11.02 [10.66–11.39]; 3.00 [2.96]). Pancreas and bile duct injury were associated with COVID-19 mRNA (1.99 [1.89–2.09]; 0.90 [0.83]), MMR (measles, mumps, and rubella), and papillomavirus vaccines. For intra-abdominal hemorrhage, inactivated whole-virus COVID-19 vaccines (3.93 [1.86–8.27]; 1.71 [0.41]) had the highest association, followed by COVID-19 mRNA (1.81 [1.42–2.29]; 0.77 [0.39]). Most of these ADRs had a short time to onset, within 1 day, and low mortality rate. Through a global scale database, the majority of ADRs occurred within 1 day, emphasizing the importance of healthcare workers' vigilant monitoring and timely management.
1 INTRODUCTION
Vaccines make a significant contribution to reducing the incidence and mortality rates of infectious diseases, serving as an essential measure for establishing herd immunity.1 While the benefits of vaccines in protecting vulnerable populations such as infants and immunocompromised individuals are undeniable,2 they may also be accompanied by some adverse drug reactions (ADRs). The most common ADRs are generally mild and temporary, including fever, local muscle pain, fatigue, chills, and nausea.3 Although gastrointestinal ADRs like nausea are frequently reported, hepatobiliary ADRs such as hepatotoxicity are relatively less understood. Particularly during the COVID-19 pandemic, concerns about the safety of COVID-19 vaccination have arisen, with a few case reports indicating liver injury associated with COVID-19 vaccines.4 However, large-scale studies investigating the consequences and associations of such injuries remain scarce. Apart from liver injury, pancreatic injury or inflammation of the bile duct may also result in symptoms like epigastric or abdominal pain, potentially serving as underlying causes for unknown pain following vaccination.5
In contrast to previous studies primarily focused on the effects of COVID-19 messenger RNA (mRNA) vaccines, this research broadens its scope to encompass all vaccine types, examining 19 types of hepatobiliary and gastrointestinal ADRs using extensive data from the World Health Organization (WHO). The aim of this study is to impartially present the potential adverse effects following various vaccination regimes, which is often overlooked in current literature. Hepatobiliary and gastrointestinal ADRs have received less attention compared to other ADRs, but identifying which vaccines are most associated with specific ADRs and understanding the causes of hepatobiliary injury postvaccination can help determine whether the benefits or risks of vaccination outweigh each other. Our study aims to improve understanding of potential complications associated with a variety of vaccines by providing comprehensive data on the global burden and clinical manifestations observed in patients.
2 METHODS
2.1 Data sources
VigiBase, managed by the WHO for Drug Monitoring Cooperation in Sweden, is a global database. It has been collecting data since its establishment in 1967 until June 2023 and is developed and maintained by the Upsala Monitoring Center.6 This comprehensive repository contains over 131 255 418 individual safety reports (ICSRs) related to suspected ADRs. These reports originate from more than 156 countries actively participating in WHO international drug monitoring programs.7-9 Despite potential data heterogeneity regarding the relationship between medications/drugs and reported ADRs, conducting extensive quantitative testing based on comprehensive data is essential for effective drug monitoring. The Institutional Review Boards of Kyung Hee University Medical Center and the Uppsala Monitoring Center (WHO Collaborating Centre) have approved the use of confidential and electronically processed patient data.
2.2 Selection of cases
Vaccine-associated hepatobiliary and gastrointestinal ADRs data were compiled from the beginning of 1969 until 2023, and vaccines were organized into 16 distinct categories: (1) diphtheria, tetanus toxoids, pertussis, polio, and Hemophilus influenza type b (DTaP-IPV-Hib) vaccines; (2) meningococcal vaccines; (3) pneumococcal vaccines; (4) typhoid vaccines; (5) encephalitis vaccines; (6) influenza vaccines; (7) hepatitis A (HAV) vaccines; (8) hepatitis B (HBV) vaccines; (9) measles, mumps, and rubella (MMR) vaccines; (10) rotavirus diarrhea vaccines; (11) zoster vaccines; (12) papillomavirus vaccines; (13) smallpox; (14) COVID-19 mRNA vaccines; (15) Ad5-vectored COVID-19 vaccines; and (16) inactivated whole-virus COVID-19 vaccines. Using the Medical Dictionary for Regulatory Activities (MedDRA) 26.0, we collected all the adverse events of deduplicated vaccinators around the world regarding the preferred terms (Supporting Information S1: Table S1). Following the WHO causality assessment recommendations, all the vaccines were only considered “suspected” for the computation of the disproportional association with hepatobiliary and gastrointestinal ADRs.
2.3 Data collection
In this study, we meticulously documented suspected cases of vaccine-associated hepatobiliary and gastrointestinal ADRs, conducting a comprehensive investigation. Our data set primarily relied on ICSRs collected from various sources, including patients, healthcare professionals, and pharmaceutical companies, within the postmarket setting. It encompassed patient demographics (i.e., age [0–11, 12–17, 18–44, 45–64, ≥65 years, and unknown] and sex), administrative information (i.e., reporting regions [African, America, South-East Asia, Europe, Eastern Mediterranean, and Western Pacific], reporting years [1967–1979, 1980–1989, 1990–1999, 2000–2009, 2010–2019, and 2020–2023], reporter qualifications [health professionals, nonhealth professionals, and unknown], and study categories [study-related, nonstudy-related, and unknown]). Information regarding vaccines (i.e., type of vaccine and single suspected vaccine), and adverse drug reaction information (i.e., time to onset [TTO] of reaction).2 All voluntary reports indicated at least one suspected vaccine in hepatobiliary and gastrointestinal ADRs.
2.4 Statistical analysis
Report and nonreport groups were established from the data set, after which each vaccine in VigiBase underwent disproportionality analysis to determine any significant associations with reports of hepatobiliary and gastrointestinal ADRs. This analysis utilized two common indicators in pharmacovigilance: the information component (IC) and the reporting odds ratio (ROR).2 The IC was computed for report-nonreport analysis using the Bayesian method, comparing the event rates of hepatobiliary and gastrointestinal ADRs associated with a specific vaccine to those of all other vaccines in VigiBase. An IC0.25 value greater than 0 (IC0.25 > 0) is the conventional threshold for detecting statistical signals, with IC0.25 representing the lower limit of the 95% confidence interval (CI). A positive value of IC0.25 (IC0.25 > 0) serves as the conventional threshold for detecting statistical signals.10, 11 In cases where the entire database is not utilized as a comparator metric, sensitivity analyses are conducted, and the ROR serves as the preferred measure of disproportionality.2, 9 The ROR is a measure of association derived from the number of adverse reactions and the contingency table of the vaccine. An important association between the drug and a certain adverse drug reaction is indicated when the 95% lower CI of the ROR is >1.0.10 Statistical significance was determined with a two-sided p value < 0.05.12 All analyses were performed using SAS (version 9.4; SAS Inc.).
3 RESULTS
3.1 Overall analysis
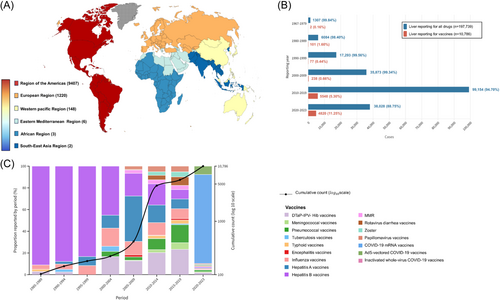
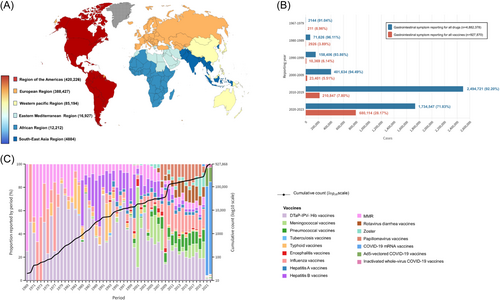
Of the 6 842 303 reports in the vaccine-associated database (Table 1), 10 786 reports (male, n = 5291) of liver injury, 927 870 reports (male, n = 258 422) of gastrointestinal symptoms, 2978 reports (male, n = 1299) of pancreas and bile duct injury, and 96 reports (male, n = 29) of hemorrhage in the Vigibase between 1967 and 2023 were identified. As depicted in Figures 1 and 2 and Supporting Information S1: Figures S1 and S2, the world is divided into six geographical areas. Most reports were originated from the American region, followed by contributions from the European, Western Pacific, Eastern Mediterranean, African, and Southeast Asia regions in cases of liver injury and pancreas and bile duct injury. In the case of gastrointestinal symptoms, the European region reported the highest incidence, followed by the American, Western Pacific, Eastern Mediterranean, African, and Southeast Asia regions. Intra-abdominal hemorrhage was predominantly reported in the American region, succeeded by Europe and the Western Pacific regions. The COVID-19 mRNA vaccine accounted for the highest number of reports among hepatobiliary and gastrointestinal ADRs (liver injury: 35.31%; gastrointestinal symptoms: 48.19%; pancreas and bile duct injury: 54.59%; and intra-abdominal hemorrhage:73.38%). Most ADRs were reported to have a short TTO, occurring within 1 day.
| Variables | Liver injury (n = 10 786) | Gastrointestinal symptom (n = 927 870) | Pancreas and bile duct injury (n = 2978) | Intra-abdominal hemorrhage (n = 96) |
|---|---|---|---|---|
| Region reporting, n (%) | ||||
| African region | 3 (0.03) | 12 212 (1.32) | 5 (0.17) | 0 (0.00) |
| Region of the Americas | 9407 (87.21) | 420 226 (45.29) | 1902 (63.87) | 68 (70.83) |
| South-East Asia region | 2 (0.02) | 4884 (0.53) | 6 (0.20) | 0 (0.00) |
| European region | 1220 (11.31) | 388 427 (41.86) | 707 (23.74) | 20 (20.83) |
| Eastern Mediterranean region | 6 (0.06) | 16 927 (1.82) | 5 (0.17) | 0 (0.00) |
| Western Pacific region | 148 (1.37) | 85 194 (9.18) | 353 (11.85) | 8 (8.33) |
| Reporting year, n (%) | ||||
| 1969–1979 | 2 (0.02) | 211 (0.02) | 0 (0.00) | 0 (0.00) |
| 1980–1989 | 101 (0.94) | 2926 (0.32) | 228 (0.74) | 0 (0.00) |
| 1990–1999 | 77 (0.71) | 10 369 (1.12) | 24 (0.81) | 0 (0.00) |
| 2000–2009 | 238 (2.21) | 23 401 (2.52) | 48 (1.61) | 0 (0.00) |
| 2010–2019 | 5548 (51.44) | 210 847 (22.72) | 797 (26.76) | 4 (4.17) |
| 2020–2023 | 4820 (44.69) | 680 114 (73.30) | 2087 (70.08) | 92 (95.83) |
| Unknown | 0 (0.00) | 2 (0.00) | 0 (0.00) | 0 (0.00) |
| Reporter qualification, n (%) | ||||
| Health professional | 2431 (22.54) | 467 895 (50.43) | 1165 (39.12) | 14 (14.58) |
| Nonhealth professional | 8348 (77.40) | 459 974 (49.57) | 1813 (60.88) | 76 (79.17) |
| Unknown | 7 (0.06) | 1 (0.00) | 0 (0.00) | 6 (6.25) |
| Studies, n (%) | ||||
| Study related | 10 738 (99.55) | 890 524 (95.98) | 2947 (98.96) | 96 (100.00) |
| Nonstudy related | 48 (0.45) | 37 138 (4.00) | 30 (1.01) | 0 (0.00) |
| Unknown | 0 (0.00) | 208 (0.02) | 1 (0.03) | 0 (0.00) |
| Sex, n (%) | ||||
| Male | 5291 (49.05) | 258 422 (27.85) | 1299 (43.62) | 29 (30.21) |
| Female | 5445 (50.48) | 658 815 (71.00) | 1654 (55.54) | 67 (69.79) |
| Unknown | 50 (0.47) | 10 633 (1.15) | 25 (0.88) | 0 (0.00) |
| Age, n (%) | ||||
| 0–11 years | 2360 (21.88) | 158 693 (17.10) | 342 (11.48) | 0 (0.00) |
| 12–17 years | 821 (7.61) | 49 041 (5.29) | 172 (5.78) | 1 (1.04) |
| 18–44 years | 2744 (25.44) | 334 153 (36.01) | 727 (24.41) | 15 (15.63) |
| 45–64 years | 2097 (19.44) | 217 376 (23.43) | 706 (23.71) | 17 (17.71) |
| ≥65 years | 1712 (15.87) | 99 297 (10.70) | 503 (16.89) | 38 (39.58) |
| Unknown | 1052 (9.75) | 69 310 (7.47) | 528 (17.73) | 25 (26.04) |
| Delay (TTO), days, median (IQR) | 1 (1–1) | 1 (1–2) | 1 (0–1) | 0 (0–1) |
| Mortality, n (%) | 0 (0.0) | 85 (0.01) | 1 (0.03) | 0 (0.0) |
| Drug class, n (%) | ||||
| DTaP-IPV-Hib vaccines | 1301 (12.06) | 84 074 (9.06) | 101 (3.39) | 0 (0.00) |
| Meningococcal vaccines | 233 (2.16) | 22 684 (2.44) | 21 (0.71) | 0 (0.00) |
| Pneumococcal vaccines | 731 (6.78) | 28 189 (3.04) | 50 (1.68) | 0 (0.00) |
| Tuberculosis vaccines | 33 (0.31) | 564 (0.06) | 12 (0.40) | 0 (0.00) |
| Typhoid vaccines | 160 (1.48) | 4952 (0.53) | 18 (0.60) | 0 (0.00) |
| Encephalitis vaccines | 44 (0.41) | 2923 (0.32) | 11 (0.37) | 0 (0.00) |
| Influenza vaccines | 696 (6.45) | 46 104 (4.97) | 173 (5.81) | 3 (3.13) |
| Hepatitis A vaccines | 923 (8.56) | 8273 (0.89) | 42 (1.41) | 0 (0.00) |
| Hepatitis B vaccines | 1206 (11.18) | 17 894 (1.93) | 88 (2.96) | 0 (0.00) |
| MMR vaccines | 230 (2.13) | 20 836 (2.25) | 259 (8.7) | 0 (0.00) |
| Rotavirus diarrhea vaccines | 318 (2.95) | 24 423 (2.63) | 15 (0.50) | 0 (0.00) |
| Zoster vaccines | 249 (2.31) | 16 296 (1.76) | 68 (2.28) | 2 (2.08) |
| Papillomavirus vaccines | 337 (3.12) | 24 952 (2.69) | 149 (5.00) | 1 (1.04) |
| COVID-19 mRNA vaccines | 3953 (36.65) | 507 078 (54.65) | 1741 (58.46) | 76 (79.17) |
| Ad5-vectored COVID-19 vaccines | 372 (3.45) | 97 743 (10.53) | 181 (6.08) | 7 (7.29) |
| Inactivated whole-virus COVID-19 vaccines | 0 (0.00) | 20 885 (2.25) | 49 (1.65) | 7 (7.29) |
| Single drug suspected, n (%) | 10 783 (99.97) | 927 758 (99.99) | 2976 (99.93) | 96 (100.00) |
- Abbreviations: DTaP-IPV-Hib, diphtheria, tetanus toxoids, pertussis, polio, and Hemophilus influenza type b; IQR, interquartile range; MMR, measles, mumps, and rubella; TTO, time to onset; WHO, World Health Organization.
3.2 Disproportionality analysis of vaccine-associated liver injury
As shown in Table 2, HAV vaccines showed the highest association with liver injury (ROR, 10.30 [95% CI, 9.65–10.99]; IC, 3.33 [IC0.25, 3.22]), followed by HBV vaccines (ROR, 7.43 [95% CI, 7.02–7.87]; IC, 2.87 [IC0.25, 2.77]), typhoid vaccine (ROR, 6.46 [95% CI, 5.53–7.55] IC, 2.66 [IC0.25, 2.39]), rotavirus vaccines (ROR, 2.68 [95% CI, 2.40–2.99]; IC, 1.41 [IC0.25, 1.23]), pneumococcal vaccines (ROR, 1.84 [95% CI, 1.71–1.98]; IC, 0.88 [IC0.25, 0.75]), papillomavirus vaccines (ROR, 1.73 [95% CI, 1.56–1.93]; IC, 0.79 [IC0.25, 0.61]), COVID-19 mRNA vaccines (ROR, 1.72 [95% CI, 1.67 to 1.78]; IC, 0.72 [IC0.25, 0.67]), encephalitis vaccines (ROR, 1.46 [95% CI, 1.09–1.97]; IC, 0.54 [IC0.25, 0.04]), influenza vaccines (ROR, 1.34 [95% CI, 1.24–1.44]; IC, 0.41 [IC0.25, 0.29]), and DTaP-IPV-Hib vaccines (ROR, 1.11 [95% CI, 1.05–1.17]; IC, 0.15 [IC0.25, 0.06]). The ROR, IC values, and characteristics of reports for six specific ADRs (aspartate transaminase [AST], alanine transaminase [ALT], bilirubin, ammonia, ischemic hepatitis, and hepatic failure) are presented in Table 3 and Supporting Information S1: Table S2. Age and sex-categorized values can be found in Supporting Information S1: Tables S6–S11.
| Liver injury | Gastrointestinal symptoms | Pancreas and bile duct injury | Intra-abdominal hemorrhage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccines | Drug | Observed | IC (IC0.25) | ROR (95% CI) | Observed | IC (IC0.25) | ROR (95% CI) | Observed | IC (IC0.25) | ROR (95% CI) | Observed | IC (IC0.25) | ROR (95% CI) |
| DTaP-IPV-Hib | 812 141 | 1301 | 0.15 (0.06) | 1.11 (1.05–1.17) | 84 074 | 1.54 (1.53) | 3.17 (3.15–3.19) | 101 | −2.36 (−2.69) | 0.19 (0.16–0.23) | 0 | NA | NA |
| Meningococcal | 160 715 | 233 | 0.10 (−0.12) | 1.07 (0.94–1.22) | 22 684 | 2.07 (2.05) | 4.83 (4.76–4.90) | 21 | −2.18 (−2.91) | 0.22 (0.14–0.33) | 0 | NA | NA |
| Pneumococcal | 274 186 | 731 | 0.88 (0.75) | 1.84 (1.71–1.98) | 28 189 | 1.52 (1.50) | 3.10 (3.06–3.14) | 50 | −1.81 (−2.28) | 0.28 (0.21–0.37) | 0 | NA | NA |
| Tuberculosis | 34 441 | 33 | −0.60 (−1.18) | 0.66 (0.47–0.92) | 564 | −1.14 (−1.28) | 0.44 (0.41–0.48) | 12 | −0.87 (−1.85) | 0.54 (0.30–0.94) | 0 | NA | NA |
| Typhoid | 17 021 | 160 | 2.66 (2.39) | 6.46 (5.53–7.55) | 4952 | 3.00 (2.96) | 11.02 (10.66–11.39) | 18 | 0.67 (−0.12) | 1.62 (1.02–2.58) | 0 | NA | NA |
| Encephalitis | 20 806 | 44 | 0.54 (0.04) | 1.46 (1.09–1.97) | 2923 | 1.97 (1.91) | 4.43 (4.26–4.61) | 11 | −0.27 (−1.30) | 0.82 (0.46–1.48) | 0 | NA | NA |
| Influenza | 368 978 | 696 | 0.41 (0.29) | 1.34 (1.24–1.44) | 46 104 | 1.84 (1.82) | 3.99 (3.96–4.03) | 173 | −0.42 (−0.67) | 0.75 (0.64–0.86) | 3 | −2.22 (−4.29) | 0.19 (0.06–0.59) |
| Hepatitis A | 63 173 | 923 | 3.33 (3.22) | 10.30 (9.65–10.99) | 8273 | 1.87 (1.84) | 4.09 (4.00–4.19) | 42 | 0.05 (−0.46) | 1.04 (0.77–1.40) | 0 | NA | NA |
| Hepatitis B | 110.384 | 1206 | 2.87 (2.77) | 7.43 (7.02–7.87) | 17 894 | 2.14 (2.11) | 5.07 (4.99–5.15) | 88 | 0.26 (−0.09) | 1.20 (0.98–1.48) | 0 | NA | NA |
| MMR | 223 329 | 230 | −0.53 (−0.74) | 0.69 (0.61–0.79) | 20 836 | 1.35 (1.32) | 2.71 (2.67–2.75) | 259 | 0.81 (0.61) | 1.76 (1.56–1.99) | 0 | NA | NA |
| Rotavirus diarrhea | 82 471 | 318 | 1.41 (1.23) | 2.68 (2.40–2.99) | 24 423 | 3.05 (3.03) | 11.62 (11.45–11.80) | 15 | −1.78 (−2.66) | 0.28 (0.17–0.47) | 0 | NA | NA |
| Zoster | 216 070 | 249 | −0.30 (−0.51) | 0.81 (0.72–0.92) | 16 296 | 1.10 (1.07) | 2.25 (2.21–2.28) | 68 | −1.00 (−1.40) | 0.50 (0.39–0.63) | 2 | −1.97 (−4.57) | 0.21 (0.05–0.86) |
| Papillomavirus | 134 328 | 337 | 0.79 (0.61) | 1.73 (1.56–1.93) | 24 952 | 2.37 (2.35) | 6.20 (6.12–6.29) | 149 | 0.78 (0.51) | 1.72 (1.47–2.02) | 1 | −2.09 (−5.88) | 0.17 (0.02–1.20) |
| COVID-19 mRNA | 4 009 826 | 3953 | 0.72 (0.67) | 1.72 (1.67–1.78) | 507 078 | 2.21 (2.20) | 7.07 (7.05–7.10) | 1741 | 0.90 (0.83) | 1.99 (1.89–2.09) | 76 | 0.77 (0.39) | 1.81 (1.42–2.29) |
| Ad5-vectored COVID-19 | 1 226 581 | 372 | −1.09 (−1.26) | 0.47 (0.42–0.52) | 97 743 | 1.42 (1.41) | 2.95 (2.93–2.97) | 181 | −0.77 (−1.02) | 0.58 (0.50–0.67) | 7 | −1.02 (−2.32) | 0.47 (0.22–0.99) |
| Inactivated whole-virus COVID-19 | 162 570 | 0 | NA | NA | 20 885 | 2.23 (2.21) | 5.44 (5.36–5.52) | 49 | 0.37 (−0.10) | 1.30 (0.98–1.72) | 7 | 1.71 (0.41) | 3.93 (1.86–8.27) |
- Note: Bold style indicates when the value of IC0.25 is >0.0 or the lower end of the ROR 95% CI is >1.0. This means it is statistically significant; thus, numbers in bold indicate statistical significance.
- Abbreviations: CI, confidence interval; DTaP-IPV-Hib, diphtheria, tetanus toxoids, pertussis, polio, and Hemophilus influenza type b vaccines; IC, information component; MMR, measles, mumps, and rubella; NA, not available; ROR, reported odds ratio.
| Vaccines | Liver injury | Gastrointestinal symptoms | Pancreas and bile duct injury | Hemorrhage | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AST | ALT | Bilirubin | Ammonia | Ischemic hepatitis | Hepatic failure | Nausea and vomiting | Constipation | Diarrhea | Abdominal distension | Abdominal pain | Perforation | Amylase | Lipase | Epigastric pain | Pancreatitis | Cholangitis | Retroperitoneal hemorrhage | Intra-abdominal hemorrhage | |
| DTaP-IPV-Hib | 0.50 (0.36) | 0.00 (−0.14) | −0.15 (−0.42) | 0.85 (0.33) | NA | −1.21 (−1.84) | 1.65 (1.64) | −0.21 (−0.30) | 1.75 (1.73) | 0.11 (0.01) | 1.07 (1.03) | 0.39 (−0.03) | −0.68 (−1.29) | −1.51 (−2.33) | −3.68 (−5.09) | −2.92 (−3.43) | −1.55 (−2.97) | NA | NA |
| Meningococcal | 0.11 (−0.26) | −0.12 (−0.47) | 1.04 (0.62) | −1.65 (−5.43) | NA | −3.08 (−6.86) | 2.3 (2.28) | −0.43 (−0.65) | 1.89 (1.83) | 0.80 (0.59) | 1.75 (1.67) | −1.45 (−3.52) | −1.44 (−3.51) | −1.47 (−3.54) | −2.18 (−4.25) | −2.39 (−3.41) | −1.40 (−5.18) | NA | NA |
| Pneumococcal | 1.21 (1.03) | 0.71 (0.51) | 0.53 (0.15) | 1.85 (1.23) | NA | −0.30 (−1.09) | 1.48 (1.46) | 0.28 (0.15) | 2.01 (1.97) | 0.71 (0.55) | 1.13 (1.06) | 0.93 (0.34) | −1.18 (−2.48) | −1.02 (−2.24) | −3.03 (−5.10) | −2.00 (−2.64) | −0.31 (−1.87) | NA | NA |
| Tuberculosis | −1.27 (−2.57) | −1.39 (−2.61) | 1.06 (0.19) | 0.03 (−3.75) | NA | −0.40 (−3.00) | −1.03 (−1.20) | −2.45 (−3.43) | −1.35 (−1.69) | −1.36 (−2.34) | −0.96 (−1.37) | −0.06 (−2.65) | −0.78 (−4.57) | NA | NA | −0.32 (−1.34) | NA | NA | NA |
| Typhoid | 2.76 (2.34) | 2.66 (2.26) | 2.64 (1.96) | NA | NA | 0.89 (−1.18) | 2.89 (2.83) | 0.87 (0.45) | 3.13 (3.03) | 1.80 (1.36) | 3.88 (3.77) | NA | 0.71 (−1.89) | 1.17 (−0.90) | 0.63 (−1.44) | 0.37 (−0.77) | 0.74 (−3.04) | NA | NA |
| Encephalitis | 0.49 (−0.38) | 0.6 (−0.15) | 0.88 (−0.34) | NA | NA | −0.54 (−4.32) | 2.25 (2.18) | −1.32 (−2.16) | 1.48 (1.32) | −1.03 (−2.17) | 1.92 (1.73) | NA | −0.22 (−4.00) | NA | −0.81 (−4.59) | −0.43 (−1.85) | 1.84 (−0.23) | NA | NA |
| Influenza | 0.69 (0.49) | 0.32 (0.12) | 0.34 (−0.01) | −0.49 (−1.80) | −0.12 (−2.71) | −0.53 (−1.28) | 2.08 (2.06) | −0.95 (−1.13) | 1.69 (1.66) | −0.15 (−0.34) | 1.35 (1.29) | −1.78 (−3.19) | 0.32 (−0.33) | 0.57 (−0.01) | −0.73 (−1.45) | −0.73 (−1.09) | −0.68 (−2.24) | −1.55 (−3.62) | NA |
| Hepatitis A | 3.45 (3.28) | 3.38 (3.22) | 3.37 (3.08) | 0.99 (−0.77) | NA | 1.27 (0.33) | 2.02 (1.98) | −0.28 (−0.61) | 1.84 (1.76) | −0.11 (−0.57) | 1.89 (1.78) | NA | 0.81 (−0.49) | 1.13 (−0.01) | −1.47 (−4.07) | −0.18 (−0.90) | 0.36 (−2.23) | NA | NA |
| Hepatitis B | 3.12 (2.98) | 2.95 (2.81) | 2.43 (2.13) | 1.03 (−0.27) | NA | 0.37 (−0.61) | 2.25 (2.22) | 0.32 (0.13) | 1.99 (1.93) | 0.44 (0.16) | 2.43 (2.36) | 0.37 (−0.77) | 1.56 (0.83) | −0.44 (−2.00) | −1.79 (−3.86) | 0.23 (−0.23) | 1.06 (−0.35) | NA | NA |
| MMR | −0.04 (−0.35) | −0.64 (−0.99) | −1.77 (−2.71) | −0.98 (−3.05) | NA | −1.00 (−2.14) | 1.41 (1.38) | −0.41 (−0.59) | 1.71 (1.66) | −1.22 (−1.57) | 0.97 (0.89) | NA | 3.49 (3.23) | −0.77 (−1.98) | −2.77 (−4.84) | −0.08 (−0.44) | −0.72 (−2.79) | NA | NA |
| Rotavirus diarrhea | 1.66 (1.38) | 1.31 (1.02) | 1.12 (0.56) | 2.04 (1.02) | NA | 0.41 (−0.73) | 2.43 (2.39) | 2.46 (2.35) | 4.10 (4.06) | 2.99 (2.86) | 3.39 (3.33) | 3.29 (2.84) | −0.63 (−2.70) | NA | −0.99 (−2.75) | −2.35 (−3.77) | 0.07 (−2.53) | NA | NA |
| Zoster | −0.02 (−0.34) | −0.47 (−0.81) | −0.39 (−0.98) | 0.40 (−0.81) | 0.96 (−1.11) | −1.97 (−3.74) | 1.37 (1.34) | −1.01 (−1.24) | 0.98 (0.92) | −0.74 (−1.05) | 0.16 (0.04) | −3.15 (−6.93) | 0.14 (−0.77) | 0.11 (−0.79) | −1.23 (−2.36) | −1.48 (−2.08) | −1.84 (−5.62) | −1.32 (−3.91) | NA |
| Papillomavirus | 0.82 (0.52) | 0.69 (0.41) | 1.43 (1.04) | −0.77 (−3.36) | NA | −1.34 (−3.10) | 2.65 (2.63) | 0.94 (0.79) | 1.00 (0.92) | 1.11 (0.90) | 2.87 (2.82) | −2.52 (−6.31) | 1.52 (0.84) | 1.61 (0.95) | 0.12 (−0.75) | 0.61 (0.25) | −0.52 (−3.12) | NA | −0.88 (−4.67) |
| COVID-19 mRNA | 0.88 (0.80) | 0.58 (0.50) | 0.77 (0.63) | 0.07 (−0.42) | 2.20 (1.78) | 0.45 (0.22) | 2.36 (2.36) | −0.31 (−0.37) | 2.08 (2.07) | 1.28 (1.24) | 1.99 (1.98) | 0.05 (−0.31) | 0.66 (0.33) | 1.51 (1.33) | 0.35 (0.17) | 1.00 (0.89) | 1.24 (0.91) | 0.58 (0.01) | 0.94 (0.43) |
| Ad5-vectored COVID-19 | −1.19 (−1.49) | −1.10 (−1.36) | −1.07 (−1.52) | −1.71 (−3.48) | 1.11 (0.04) | −0.79 (−1.41) | 1.65 (1.64) | −1.04 (−1.16) | 0.97 (0.94) | −0.51 (−0.65) | 1.21 (1.17) | −1.73 (−2.95) | −2.65 (−4.72) | −0.84 (−1.59) | −1.02 (−1.52) | −0.44 (−0.76) | −1.32 (−2.89) | −0.30 (−1.72) | −2.36 (−6.15) |
| Inactivated whole-virus COVID-19 | NA | NA | NA | NA | NA | NA | 1.91 (1.87) | 2.31 (2.20) | 2.72 (2.67) | 0.59 (0.32) | 3.03 (2.97) | NA | NA | NA | 2.05 (1.57) | −3.62 (−7.40) | NA | NA | 2.44 (1.14) |
- Note: Bold style indicates when the value of IC0.25 is >0.0 or the lower end of the ROR 95% CI is >1.0. This means it is statistically significant; thus, numbers in bold indicate statistical significance.
- Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; DTaP-IPV-Hib, diphtheria, tetanus toxoids, pertussis, polio, and Hemophilus influenza type b; IC, information component; MMR, measles, mumps, and rubella; NA, not available.
3.3 Disproportionality analysis of vaccine-associated gastrointestinal symptoms
As shown in Table 2, most vaccines except for tuberculosis vaccines were associated with gastrointestinal symptoms. Rotavirus vaccines (ROR, 11.62 [95% CI, 11.45–11.80]; IC, 3.05 [IC0.25, 3.03]) showed the highest association with gastrointestinal symptoms, followed by typhoid vaccines (ROR, 11.02 [95% CI, 10.66–11.39]; IC, 3.00 [IC0.25, 2.96]), COVID-19 mRNA vaccines (ROR, 7.07 [95% CI, 7.05–7.10]; IC, 2.21 [IC0.25, 2.20]), papillomavirus vaccines (ROR, 6.20 [95% CI, 6.12–6.29]; IC, 2.37 [IC0.25, 2.35]), inactivated whole-virus COVID-19 vaccines (ROR, 5.44 [95% CI, 5.36–5.52]; IC, 2.23 [IC0.25, 2.21]), HBV vaccines (ROR, 5.07 [95% CI, 4.99–5.15]; IC, 2.14 [IC0.25, 2.11]), meningococcal vaccines (ROR, 4.83 [95% CI, 4.76–4.90]; IC, 2.70 [IC0.25, 2.05]), encephalitis vaccines (ROR, 4.43 [95% CI, 4.26–4.61]; IC, 1.97 [IC0.25, 1.91]), HAV vaccines (ROR, 4.09 [95% CI, 4.00–4.19]; IC, 1.87 [IC0.25, 1.84]), influenza vaccines (ROR, 3.99 [95% CI, 3.96–4.03]; IC, 1.84 [IC0.25, 1.82]), DTaP-IPV-Hib vaccines (ROR, 3.17 [95% CI, 3.15–3.19]; IC, 1.54 [IC0.25, 1.53]), pneumococcal vaccines (ROR, 3.10 [95% CI, 3.06–3.14]; IC, 1.52 [IC0.25, 1.50]), Ad5-vectored COVID-19 vaccines (ROR, 2.95 [95% CI, 2.93–2.97]; IC, 1.42 [IC0.25, 1.41]), MMR vaccines (ROR, 2.71 [95% CI, 2.67–2.75]; IC, 1.35 [IC0.25, 1.32]), and zoster vaccines (ROR, 2.25 [95% CI, 2.21–2.28]; IC, 1.10 [IC0.25, 1.07]). The ROR and IC values for six specific ADRs (nausea and vomiting, constipation, diarrhea, abdominal distention, abdominal pain, and perforation) are presented in Table 3 and Supporting Information S1: Table S3. Age and sex-categorized values can be found in Supporting Information S1: Tables S6–S11.
3.4 Disproportionality analysis of vaccine-associated pancreas and bile duct injury
Table 2 displays the association between vaccines and pancreatic and bile duct injury. COVID-19 mRNA vaccines were associated most with pancreas and bile duct injury (ROR, 1.99 [95% CI, 1.89–2.09]; IC, 0.90 [IC0.25, 0.83]), followed by MMR vaccines (ROR, 1.76 [95% CI, 1.56–1.99]; IC, 0.81 [IC0.25, 0.61]) and papillomavirus vaccines (ROR, 1.72 [95% CI, 1.47–2.02]; IC, 0.78 [IC0.25, 0.51]). Table 3 and Supporting Information S1: Table S4 displays the ROR, IC values, and characteristics of reports for five specific ADRs (amylase, lipase, epigastric pain, pancreatitis, and cholangitis). Detailed values categorized by age and sex are available in Supporting Information S1: Tables S6–S11.
3.5 Disproportionality analysis of vaccine-associated hemorrhage
Inactivated whole-virus COVID-19 vaccines exhibited the highest association with hemorrhage (ROR, 3.93 [95% CI, 1.86–8.27]; IC, 1.71 [IC0.25, 0.41]) (Table 2), followed by COVID-19 mRNA vaccines (ROR, 1.81 [95% CI, 1.42–2.29]; IC, 0.77 [IC0.25, 0.39]). Table 3 and Supporting Information S1: Table S5 exhibit the ROR, IC values, and characteristics of reports for two specific ADRs (retroperitoneal hemorrhage and intra-abdominal hemorrhage). Detailed values categorized by age and sex can be found in Supporting Information S1: Tables S6–S11.
3.6 Cumulative report analysis
The cumulative number of vaccine-associated hepatobiliary and gastrointestinal ADRs is shown in Figures 1 and 2 and Supporting Information S1: Figures S3–S6. Most reports emerged after 2020, indicating a substantial increase in hepatobiliary and gastrointestinal cases linked to the COVID-19 vaccines, notably the COVID-19 mRNA vaccine.
4 DISCUSSION
4.1 Key finding
In this study, we conducted a broad and thorough examination of the global burden of hepatobiliary and gastrointestinal ADRs associated with vaccines, utilizing extensive data from the WHO international pharmacovigilance database. Most hepatobiliary and gastrointestinal ADRs surged after 2020, with the majority of reports attributed to COVID-19 mRNA vaccines.
Symptoms such as elevated levels of AST, ALT, bilirubin, and ammonia, indicative of liver injury, as well as hepatic failure, showed higher associations with hepatitis vaccines like HAV and HBV, followed by typhoid and rotavirus vaccines. However, ischemic hepatitis exhibited a significant association specifically with COVID-19 vaccines. Gastrointestinal symptoms such as nausea and vomiting, diarrhea, and abdominal pain were associated with most vaccines, particularly the rotavirus and typhoid vaccines. Constipation and abdominal distention were associated with rotavirus and typhoid vaccines. Increased lipase and amylase levels, along with symptoms like epigastric pain and pancreatitis, indicative of pancreatic injury, revealed significant associations with MMR, typhoid, HPV, and COVID-19 mRNA vaccines. Intraabdominal hemorrhage and retroperitoneal hemorrhage were only associated with the COVID-19 vaccines. All hepatobiliary ADRs manifested with a short TTO, occurring within 1 day, and low mortality rate. Consequently, it is highly advisable for healthcare workers to monitor patients exhibiting these symptoms closely, as early intervention may be necessary.
4.2 Plausible underlying mechanisms
Previous studies have attempted to explain liver injury associated with certain vaccines. The most notable examples are hepatitis vaccines; the HAV vaccine, for instance, is known to induce hepatic injury because it can form autoantibodies, potentially leading to autoimmune hepatitis,13 while the HBV vaccine has been described to cause hepatic injury by HBsAg inducing apoptotic cell death through both caspase-dependent and intrinsic pathways.14 Additionally, the aluminum component present in HAV and HBV vaccines may rarely cause liver toxicity.14 The relationship between the live attenuated typhoid vaccine and liver injury, as well as cholangitis, is believed to be associated with the mechanism of the typhoid virus, which causes acute systemic infection affecting organs of the mononuclear phagocyte system and the gallbladder.15 The possibility of hepatotoxicity associated with the rotavirus vaccine has been raised through a few case reports, supported by the fact that the rotavirus vaccine induces viral hepatitis in mice.16 During the COVID-19 pandemic, reports of liver injury following COVID-19 vaccines raised concerns about the safety of COVID-19 vaccination.17 However, a recent study revealed that liver injury caused by COVID-19 vaccines is much lower compared to acute liver injury caused by SARS-CoV-2 infection.4 Furthermore, during the COVID-19 pandemic, despite the higher number of reported cases following COVID-19 vaccinations, previous studies have consistently shown that COVID-19 vaccines are generally safe and effective, even in individuals with pre-existing liver disease.18, 19 These findings underscore the importance of vaccination in protecting vulnerable populations from the potentially severe consequences of SARS-CoV-2 infection.
Moreover, previous studies have suggested that COVID-19 vaccine-induced liver injury exhibits distinct features from autoimmune hepatitis, implying complete recovery without the development of long-term liver injury.20 This finding is consistent with our study, where the association of COVID-19 vaccines with liver injury was found to be less significant compared to HAV, HBV, typhoid, and rotavirus vaccines. Ischemic hepatitis, unlike other reported liver injuries associated with vaccines, was linked specifically to the COVID-19 vaccine. This association may be attributed to the dysregulation caused by the spike protein encoded by the COVID-19 mRNA vaccine, which can disrupt prothrombin and fibrinogen chains, resulting in arterial or venous thrombosis in various parts of the body.21
Gastrointestinal symptoms such as nausea, vomiting, constipation, diarrhea, abdominal pain, and distention were reported with most vaccines, but notably demonstrated a strong association with rotavirus and typhoid vaccines. This is likely attributed to intussusception, one of the most well-known side effects of the rotavirus vaccine, which presents symptoms such as abdominal pain, vomiting, and abdominal distention.22 Typhoid vaccine, which exhibited pronounced association with gastrointestinal symptoms, reported that most events were mild to moderate in severity and resolved within few days.23
Several vaccines have been shown to have association with elevated lipase and amylase, symptoms like epigastric pain and pancreatitis. Live attenuated vaccines, due to their utilization of viruses with modified pathogenicity that replicate within host cells, may lead to viral pancreatitis.24 This phenomenon is particularly observed with mumps and typhoid vaccines. HPV vaccines, however, due to their molecular mimicry between the virus and self-antigens are known to cause pancreatitis.25 Regarding COVID-19 vaccines, the presence of SARS-CoV-2 receptors in the pancreas implies that endothelial damage can occur when the SARS-CoV-2 spike protein attaches to these receptors.26 Epigastric pain induced by COVID-19 vaccines may also be attributed to vaccine-induced immune-mediated thrombotic thrombocytopenia, as vein occlusion resulting from thrombosis can lead to abdominal pain.27
4.3 Clinical and policy implications
In our study, both HAV and HBV vaccines were associated with liver injury. However, it is well known that individuals with chronic hepatic failure face a heightened risk of severe hepatic injury and increased fatality rates upon contracting HAV and HBV infections.28 Therefore, even in patients with impaired liver function, HAV and HBV vaccination is recommended.29 Therefore, even in patients with impaired liver function, HAV and HBV vaccination is recommended. Previous studies reported that most inactivated vaccines have comparable safety profiles in individuals with chronic liver disease and healthy individuals.30 Vaccination is crucial in preventing infections and subsequent hepatic decompensation in individuals with chronic liver disease. Consequently, majority of studies recommend HAV, HBV, influenza, pneumococcus, and herpes zoster vaccinations for these populations, especially in the early disease stages.31 The increased reporting of adverse events following COVID-19 vaccination can be attributed to heightened awareness and anxiety surrounding newly developed vaccines such as mRNA vaccines,32 improved monitoring and surveillance systems activated during the pandemic,33 and the widespread administration of COVID-19 vaccines, which has expanded the pool of recipients and increased the likelihood of adverse event reporting. However, our large-scale study showed minimal association between COVID-19 vaccines and hepatobiliary ADRs, compared to other vaccines such as HAV, HBV, typhoid, and rotavirus vaccines. Consequently, vaccination may prevent liver and pancreatic injuries resulting from COVID-19 infection in patients with underlying hepatobiliary conditions.
Hence, healthcare workers should assess the risk of vaccine-associated liver and pancreatic injury by considering disease signatures, patient risk factors, and excluding concomitant drugs and other potential causes. Prompt diagnosis can lead to appropriate interventions to alleviate patient pain. Symptoms like abdominal pain are common after overall vaccination. Nevertheless, vigilant observation and early intervention by healthcare workers are essential to minimize severe consequences of side effects. Based on previous studies suggesting the potential benefits of corticosteroids in severe hepatitis, the initiation of corticosteroid therapy can be considered depending on the overall condition of the patient.34
4.4 Strengths and limitation
The detection of liver injury postvaccination presents challenges, particularly due to the common occurrence of elevated liver enzymes in healthy individuals. While symptomatic cases may be reported, identifying asymptomatic, transient increases in liver enzymes is difficult,35 compounded by the enzyme elevation's multifactorial nature, including factors like exercise36 or dietary changes.37 Moreover, the impact of comorbidities and concurrent medications on liver function remains poorly understood, necessitating further investigation. Hepatotropism of diverse pathogens, such as HAV, HBV, and other pathogens, can lead to liver injury. Simultaneously, HAV and HBV vaccinations are recommended for individuals at high risk of infection, such as those traveling to HAV endemic regions,38 intravenous drug users, and healthcare workers.39 Consequently, it is challenging to distinguish whether the observed hepatobiliary symptoms are due to the vaccines or the underlying risk of infection in these populations. Hepatotropism of the pathogens and the high-risk nature of the vaccinated population may influence the interpretation of our results, as the potential confounding effects of these factors cannot be easily separated from the impact of the vaccines themselves. In addition, due to the lack of data, further studies are needed to address the adverse events in individuals with liver disease. Administration of acetaminophen for common postvaccination adverse reactions such as local pain and fever introduces another layer of complexity,40 as acetaminophen itself can induce liver injury.41, 42 Additionally, reliance on voluntary reporting systems like the WHO VigiBase may introduce discrepancies in reported cases, especially in low- and middle-income countries with limited healthcare access.
Our study found that hepatobiliary and gastrointestinal symptoms typically manifested within 1 day following vaccination, which is consistent with the findings of a previous retrospective single-center study.43 However, the lack of data on recovery periods in the VigiBase database limited our ability to investigate the duration of these symptoms further. The earlier study reported a median recovery period of 5 days for gastrointestinal complications after COVID-19 vaccination, indicating a potentially short recovery time.43 In contrast, another study highlighted significant variability in the duration of gastrointestinal symptoms following COVID-19 vaccination, with persistence ranging from 1 to 2 days to more than 3 months.44 These conflicting findings underscore the need for further research to better understand the duration of hepatobiliary and gastrointestinal symptoms associated with vaccinations.
Despite these limitations, our study harnesses the robustness of extensive, long-term data from the WHO VigiBase. Unlike previous studies reliant on case reports, we comprehensively analyzed adverse events associated with all vaccines, providing insights into specific vaccine mechanisms and associated adverse events. This large-scale analysis, augmented by continuous reporting to the WHO database, contributes to a deeper understanding of vaccine safety profiles.
Further research is imperative to unravel the nuanced risks associated with hepatobiliary and gastrointestinal ADRs across different vaccines. Our study endeavors to equip healthcare professionals with nuanced insights into vaccine adverse event profiles, facilitating the formulation of targeted patient care strategies and the development of safer vaccination protocols.
5 CONCLUSION
In this comprehensive study, we examined the prevalence of hepatobiliary and gastrointestinal ADRs associated with vaccines using extensive data from the WHO international pharmacovigilance database from 1967 to 2023. Most ADRs displayed a short TTO, occurring within 1 day, highlighting the importance of vigilant monitoring by healthcare workers and timely management. These findings contribute to a more precise evaluation of the association between vaccines and hepatobiliary and gastrointestinal ADRs, aiding in the diagnosis and management of these symptoms postvaccination.
AUTHOR CONTRIBUTIONS
Dong Keon Yon had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors have approved the final version of the manuscript before submission. Study concept and design: Sooji Lee, Kyeongmin Lee, Hayeon Lee, and Dong Keon Yon. Acquisition, analysis, or interpretation of data: Sooji Lee, Kyeongmin Lee, Hayeon Lee, and Dong Keon Yon. Drafting of the manuscript: Sooji Lee, Kyeongmin Lee, Hayeon Lee, and Dong Keon Yon. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Sooji Lee, Kyeongmin Lee, Hayeon Lee, and Dong Keon Yon. Study supervision: Sooji Lee, Kyeongmin Lee, Hayeon Lee, and Dong Keon Yon. Dong Keon Yon supervised the study and is guarantor for this study. Sooji Lee and Kyeongmin Lee contributed equally to this study as first authors. Hayeon Lee and Dong Keon Yon contributed equally to this study as corresponding authors. The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
ACKNOWLEDGMENTS
VigiBase, the global database managed by the Uppsala Monitoring Centre, a division of the World Health Organization, serves as a repository for reported potential adverse events associated with medicinal products. The authors extend their appreciation to the Uppsala Monitoring Centre for providing access to and authorizing the use of the data analyzed in this study. The information provided does not represent the viewpoints of the Uppsala Monitoring Centre or the World Health Organization. This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT; RS-2023-00248157). Hayeon Lee was supported by a grant from the Health Fellowship Foundation, South Korea (2023). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Approval for using confidential and electronically processed patient data was granted by the institutional review board of Kyung Hee University Medical Center and the Upsala Monitoring Center (WHO Collaborating Centre). The requirement for written consent was waived by the ethics committee owing to the population-level data set.
Open Research
DATA AVAILABILITY STATEMENT
Data are available on reasonable request. Study protocol, statistical code: available from DKY (email: [email protected]). Data set: available from the Uppsala Monitoring Centre or World Health Organization through a data use agreement.




