Period prevalence of uveitis in human T-lymphotropic virus 1 carriers versus noncarriers in a highly endemic area: The Nagasaki Islands Study
Abstract
The magnitude of the effect of human T-lymphotropic virus 1 (HTLV-1) infection on uveitis remains unclear. We conducted a cross-sectional study in a highly endemic area of HTLV-1 in Japan. The study included 4265 residents (men, 39.2%), mostly middle-aged and older individuals with a mean age of 69.9 years, who participated in our surveys between April 2016 and September 2022. We identified HTLV-1 carriers by screening using chemiluminescent enzyme immunoassays and confirmatory tests, and the proportion of carriers was 16.1%. Participants with uveitis were determined from the medical records of all hospitals and clinics where certified ophthalmologists practiced. We conducted logistic regression analyses in an age- and sex-adjusted model to compute the odds ratio (OR) and 95% confidence interval (CI) of uveitis according to HTLV-1 infection status. Thirty-two (0.8%) participants had uveitis. For HTLV-1 carriers, the age- and sex-adjusted OR (95% CI) of uveitis was 3.27 (1.57–6.72) compared with noncarriers. In conclusion, HTLV-1 infection was associated with a higher risk of uveitis among mostly middle-aged and older Japanese residents in a highly endemic HTLV-1 area. Our findings suggest that physicians who treat HTLV-1 carriers should assess ocular symptoms, and those who diagnose patients with uveitis should consider HTLV-1 infection.
1 INTRODUCTION
Human T-lymphotropic virus 1 (HTLV-1) is a retrovirus that enters CD4+ T cells via cell-to-cell transmission, integrates the viral deoxyribonucleic acid (DNA) into the host genome, and is likely to persist via clonal expansion in the chronic stages of infection,1-3 leading to multiple HTLV-1-associated diseases, such as adult T cell leukemia, HTLV-1-associated myelopathy/tropical spastic paraparesis, and uveitis.1, 2, 4 In Japan, the number of HTLV-1 carriers was estimated to be at least 1.08 million in 2006–20075 and approximately 0.66 million in 2020–2021.6 Although routine HTLV-1 antibody testing was incorporated into prenatal pregnancy screening throughout Japan in 2010,7 most asymptomatic carriers may not be aware of their own infection. This virus is highly endemic to the Kyushu district in southwestern Japan.1, 2, 5-8
Uveitis is an inflammatory disorder that affects intraocular tissues and causes ocular symptoms, such as floaters, blurred vision, ocular pain, photophobia, and burning.1, 2, 9 Prior studies into the pathogenesis of HTLV-1-associated uveitis have stated that accumulated HTLV-1-infected T cell-stimulated inflammatory cytokines in the eye can damage ocular tissues, HTLV-1-infected retinal pigment epithelial cells can disturb immune homeostasis in the eye, and epitopes of HTLV-1 antigens can cross-react with retinal antigens.1, 10
Although HTLV-1-associated uveitis is an accepted clinical entity, the magnitude of the risk of uveitis in HTLV-1 carriers compared with that in non-carriers remains to be investigated. Two cross-sectional studies in Brazil compared the prevalence of uveitis in HTLV-1 carriers and noncarriers.11, 12 One of the studies did not find a significant association between HTLV-1 infection and uveitis due to a small number of cases,11 and the other study estimated the prevalence ratio but was limited because HTLV-1 carriers and noncarriers were sampled separately, without matching methods, which could lead to biased estimates.12 Several other studies have compared patients with uveitis and other eye diseases at single-center or multicenter ophthalmic institutions in specific regions and estimated the prevalence of HTLV-1 carriers among patients with uveitis.13-16 However, those studies only assessed data for patients who visited these institutions. Hence, a study that assesses the magnitude of the risk of uveitis in HTLV-1 carriers compared with that in noncarriers is warranted.
Therefore, we aimed to compare the risk of uveitis in HTLV-1 carriers with that in noncarriers, based on data from a population-based cohort study (the Nagasaki Islands Study [NaIS]),17, 18 which included mostly middle-aged and older individuals in a highly endemic area of HTLV-1, the Kyushu district in southwestern Japan. The estimated proportion of HTLV-1 carriers in this cohort was approximately 15%–20%.17 If the risk of uveitis in HTLV-1 carriers is high, the findings will be important for physicians who treat HTLV-1 carriers and those who treat patients with uveitis.
2 MATERIALS AND METHODS
2.1 Study design, setting, and participants
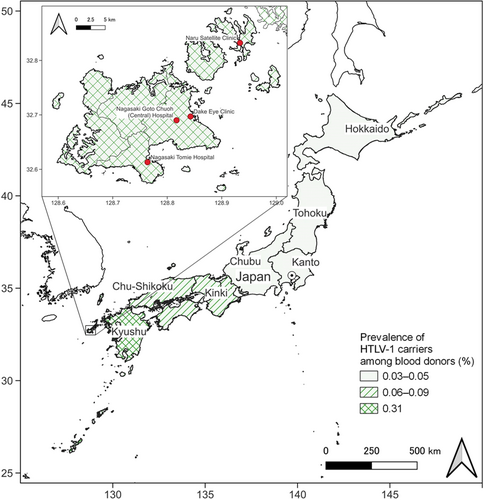
We performed a cross-sectional study comparing HTLV-1 carriers and noncarriers using data from the NaIS, a population-based open-cohort study in Goto City (located on the remote islands of Nagasaki Prefecture, Japan) (Figures 1 and 2).17, 18 The Nagasaki Prefecture is located in the Kyushu district, a highly endemic area of HTLV-1.1, 2, 5-8 Prenatal HTLV-1 screening commenced in Nagasaki Prefecture locally in 1987, before the rest of Japan.7 Rather than the incidence or the cumulative incidence, we calculated and compared the period prevalence of uveitis because it was difficult to determine the onset of uveitis, due to its frequently recurring clinical course,1, 9, 10 and to distinguish new cases from prevalent cases.
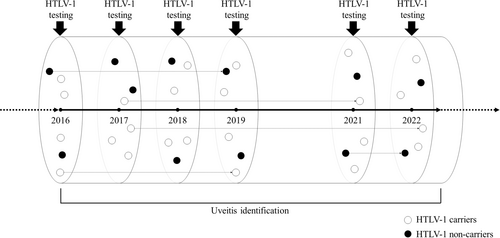
The NaIS commenced in 2014, enrolling residents who participated in our surveys along with the annual standardized checkups organized by the municipality for mostly middle-aged and older residents.17, 18 Our surveys were conducted annually, except in 2020, due to the coronavirus disease 2019 pandemic. This study included 4265 residents who participated in the surveys between April 2016 and September 2022 (Figure 1); that is, we calculated a 6.5-year prevalence. Of these, 3087 participants completed our survey once, 1030 participants completed it twice, and 148 participants completed it three or more times. Data on anti-HTLV-1 antibodies were unavailable until 2015.
From the NaIS data set, we obtained data on age, sex, presence of anti-HTLV-1 antibodies, and uveitis diagnosis. Participant age was calculated as of July 1, 2019, midway through the recruitment period, and was used for analysis. Other data on the possible etiologies of uveitis include sarcoidosis, Vogt–Koyanagi–Harada disease, human leukocyte antigen (HLA)-B27-associated uveitis, herpetic iritis, Behçet's disease, Posner–Schlossman syndrome, diabetic iritis, and cytomegalovirus retinitis were obtained. Data on family history or hometown, which could affect HTLV-1 infection, were unavailable.
The study protocol was developed by the research team and approved by the Ethics Committee of the Nagasaki University Graduate School of Biomedical Sciences (project registration number: 14051404 [latest version: 17th edition]). This study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its subsequent amendments. All the participants provided written informed consent.
2.2 Identification of HTLV-1 carriers
In line with the methods of a prior study, we collected blood specimens from the participants at our annual surveys and identified HTLV-1 carriers by a screening chemiluminescent enzyme immunoassay (CLEIA) and confirmatory tests, consisting of a real-time polymerase chain reaction (PCR) and western blotting (WB) or a line immunoassay (LIA) for the real-time PCR-negative cases (Figures 1 and 3).17, 19 First, we used a screening CLEIA (based on the standard laboratory procedures of SRL, Inc.) to examine anti-HTLV-1 antibodies in all participants. If the result was negative, the participant was defined as a noncarrier. Second, for participants who tested positive for anti-HTLV-1 antibodies on a screening CLEIA, we conducted a confirmatory real-time PCR test using the hydrolysis probe method with the LightCycler 480 System (Roche). If the result was positive, the participant was defined as a carrier. Third, we conducted WB or a LIA as other confirmatory tests (based on the standard laboratory procedures of SRL, Inc.) for participants who tested negative for anti-HTLV-1 antibodies using real-time PCR. If the result was positive, the participant was defined as an HTLV-1 carrier; if the result was negative, the participant was defined as a noncarrier. Participants for whom WB/LIA was used for testing without real-time PCR (because of insufficient specimens) were defined as HTLV-1 carriers if the WB/LIA test result was positive. Each participant was counted as one person even if the participant was tested more than once. For participants who were tested for HTLV-1 infection using a CLEIA on at least two occasions during the study period and whose results were consistently positive, confirmatory tests for other specimens were omitted if the results of the confirmatory tests conducted on the oldest specimen were positive. For participants who were tested using a CLEIA on at least two occasions and whose results were inconsistent, confirmatory tests were conducted on CLEIA-negative specimens to determine whether the negative results were correct.
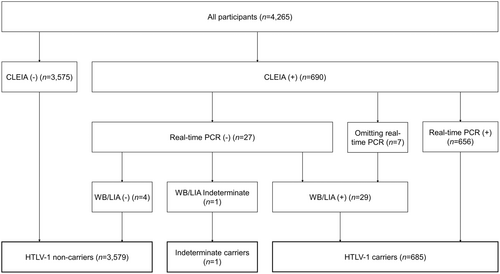
In the current study, we defined participants who were tested for HTLV-1 infection on at least two occasions during the study period and changed from HTLV-1 negative to positive (newly diagnosed carriers) as HTLV-1 carriers. In addition, indeterminate carriers, who (1) only had either real-time PCR or WB/LIA performed because of the lack of specimens, and the test results were negative, or (2) had a negative real-time PCR and an indeterminate WB/LIA, were defined as noncarriers.
2.3 Diagnosis of uveitis
We identified participants with uveitis from the medical records of all hospitals and clinics that treat eye diseases in Goto City (Nagasaki Goto Chuoh [Central] Hospital and Naru Satellite Clinic, Nagasaki Tomie Hospital, and Dake Eye Clinic) and where certified ophthalmologists practiced during the study period (Figures 1 and 2). A three-step process was used to identify the diagnosis of uveitis. First, we extracted the records of all the patients who had uveitis, during the study period, from the health insurance claims database of each hospital or clinic. We could not distinguish new cases from prevalent cases. Second, we excluded the records of patients who did not participate in our study. Third, we reviewed the remaining medical records to exclude the records of participants with other diseases. Medical records were reviewed by certified ophthalmologists, and patients were included based on findings, such as the presence of cells (leukocytes) or increased protein (flare) in the anterior chamber, keratic precipitates, vitreous opacities, and retinal lesions.1, 9
2.4 Statistical analyses
To describe the baseline characteristics of the participants according to HTLV-1 infection status, we calculated the mean age and proportions of each sex. We computed crude and age- and sex-adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of uveitis according to HTLV-1 infection status using a logistic regression model. Age was considered a continuous variable. For the sensitivity analysis, we excluded newly diagnosed carriers and indeterminate carriers to avoid misclassification. Additionally, we examined the interaction effect by including the product term of HTLV-1 infection and age (continuous), or HTLV-1 infection and sex.
Furthermore, we described the possible etiologies of the included uveitis cases according to HTLV-1 infection.
All statistical tests were two-tailed, and the statistical significance threshold was set at p < 0.05. All analyses were performed using R version 4.3.3 (R Foundation).
3 RESULTS
The participant characteristics are presented in Table 1. There were 1671 (39.2%) men. The participants' ages ranged from 20 to 99 years on July 1, 2019, with a mean age of 69.9 (standard deviation [SD] = 11.8) years. A total of 685 (16.1%) participants were definitive HTLV-1 carriers, and one (0.0%) was an indeterminate carrier (considered a noncarrier in this study) (Figure 3). None of the participants were newly diagnosed carriers. Women and older participants were more likely to be HTLV-1 carriers.
| Characteristics | HTLV-1 noncarrier (n = 3580) | HTLV-1 carrier (n = 685) |
|---|---|---|
| Age (years) | 68.9 (12.1) | 74.9 (8.8) |
| <40 (n) | 110 (3.1%) | 1 (0.1%) |
| 40–49 (n) | 151 (4.2%) | 8 (1.2%) |
| 50–59 (n) | 358 (10.0%) | 17 (2.5%) |
| 60–69 (n) | 1098 (30.7%) | 157 (22.9%) |
| 70–79 (n) | 1171 (32.7%) | 278 (40.6%) |
| 80–89 (n) | 643 (18.0%) | 213 (31.1%) |
| ≥90 (n) | 49 (1.4%) | 11 (1.6%) |
| Men (n) | 1457 (40.7%) | 214 (31.2%) |
- Note: The figures represent the means (standard deviations) or numbers (percentages).
The results of the univariable and multivariable logistic regression analyses are presented in Table 2. During the study period, 32 (0.8%) participants exhibited uveitis (age ranging from 62 to 88 years on July 1, 2019, and eight participants were men). HTLV-1 carriers exhibited a high 6.5-year prevalence of uveitis. Compared with the noncarriers, the crude and the age- and sex-adjusted ORs (95% CIs) of uveitis were 4.13 (2.01–8.32) and 3.27 (1.57–6.72) for HTLV-1 carriers, respectively. The results of the sensitivity analysis were consistent with the primary results (Table S1). There was no significant interaction effect on the association by age (p for interaction = 0.95) and sex (p for interaction = 0.92).
| HTLV-1 noncarrier (n = 3580) | HTLV-1 carrier (n = 685) | |
|---|---|---|
| Case (n, %) | 18 (0.5%) | 14 (2.0%) |
| Crude OR | Reference | 4.13 (2.01–8.32)*** |
| Age- and sex-adjusted OR | Reference | 3.27 (1.57–6.72)** |
- ** p < 0.01
- *** p < 0.001.
The possible etiologies for each case are presented in Table 3. In one HTLV-1 carrier with uveitis, the disease was possibly caused by cytomegalovirus.
| HTLV-1 noncarrier (n = 18) | HTLV-1 carrier (n = 14) | |
|---|---|---|
| Sarcoidosis | 1 (5.6%) | 0 |
| Vogt–Koyanagi–Harada disease | 0 | 0 |
| HLA-B27-associated uveitis | 0 | 0 |
| Herpetic iritis | 6 (33.3%) | 0 |
| Behçet's disease | 0 | 0 |
| Posner–Schlossman syndrome | 1 (5.6%) | 0 |
| Diabetic iritis | 2 (11.1%) | 0 |
| Cytomegalovirus retinitis | 0 | 1 (7.1%) |
| Others | 8 (44.4%) | 13 (92.9%) |
- Note: The figures represent the numbers (percentages).
- Abbreviation: HLA, human leukocyte antigen.
4 DISCUSSION
In this cross-sectional study, we found that HTLV-1 infection was associated with a higher risk of uveitis among middle-aged and older Japanese individuals living in a highly endemic area of HTLV-1. We found that the 6.5-year prevalence of uveitis was almost three-fold higher in HTLV-1 carriers than in noncarriers. This study highlights two important clinical issues. Physicians who treat HTLV-1 carriers should be aware of the risk of uveitis and should assess ocular symptoms, and those who diagnose patients with uveitis should consider the possibility of HTLV-1 infection.
The novelty of our study is that we screened all participants from the same cohort for anti-HTLV-1 antibodies and compared the period prevalence of uveitis between HTLV-1 carriers and noncarriers. To the best of our knowledge, this is the first study to estimate the risk of uveitis in HTLV-1 carriers compared with noncarriers, including screening all participants from the same cohort for anti-HTLV-1 antibodies and registering their diagnoses of uveitis. A cross-sectional study in Brazil screened all participants for ophthalmologic findings and reported that the prevalence of uveitis was 0% (0 of 55) in HTLV-1 noncarriers and 1.9% (4 of 207) in asymptomatic HTLV-1 carriers, among blood donors.11 However, in that study, due to the small number of cases, the association between HTLV-1 infection and uveitis could not be proven. Another hospital-based cross-sectional study in Brazil indicated that the prevalence ratio of patients with uveitis in HTLV-1 carriers to that in noncarriers was 9.76 (95% CI, 2.79–34.15), based on ophthalmologic examination of all participants.12 However, in that study, HTLV-1 carriers and noncarriers were sampled separately without matching methods; this could lead to biased estimates. Four cross-sectional studies that included patients of all ages and compared the prevalence of HTLV-1 carriers in patients with uveitis with other eye diseases were conducted in Japan during 1989–1995.13-16 A multicenter study conducted at various ophthalmic departments of university hospitals reported that HTLV-1 carriers accounted for 14.2% (55 of 386) among patients with uveitis and 5.8% (20 of 347) among those with other eye diseases, at seven university hospitals in the Kyushu and Okinawa districts, which are highly endemic areas of HTLV-1, while the proportions of HTLV-1 carriers were 7.5% (3 of 40) among patients with uveitis and 0.0% (0 of 41) with other eye diseases at two university hospitals in the Kanto district, a nonhighly endemic area.13 Another study conducted in the Kyushu district reported that the proportion of HTLV-1 carriers was 27.7% (70 of 253) among patients with uveitis and 16.1% (42 of 261) with other eye diseases, at one ophthalmic institution, and 8.5% (11 of 130) and 4.2% (9 of 215), respectively, at one university hospital.14 In the Kanto district, the proportions (HTLV-1 carriers in patients with uveitis and those with other eye diseases) were 3.1% (9 of 287) and 2.0% (9 of 454), respectively, at one university and one community hospital,15 and 4.0% (9 of 226) and 2.1% (29 of 1353), respectively, at one community hospital.16 In our study, the HTLV-1 carriers comprised 43.8% (14 of 32) of patients with uveitis diagnosed at two community hospitals and two clinics. The accumulation of HTLV-1 carriers due to the remoteness of the islands may have led to a higher prevalence in participants with uveitis included in our study than that reported in other studies. Additionally, our inclusion criterion was residents who underwent our health checkups, which affects regional representativeness in terms of age and sex distribution (Table 1). The proportion of participants aged 60–79 years was higher than that of residents of Goto City, and the proportion of men was lower than that of women. In a prior study conducted at one ophthalmic institution between 2015 and 2020, the peak age at HTLV-1 uveitis onset was in the 60s, and 73.1% of the patients were women.1 Hence, external validation of our findings in other regions is necessary.
A strength of our study is that we were able to thoroughly follow the participants and register the development of uveitis because of the limited number of medical facilities available on the remote islands. This study had several limitations. First, we have to consider the influence of participants' death or loss to follow-up from our cohort study (NaIS) during the study period because the period for uveitis identification was 6.5 years (Figure 1). A meta-analysis indicated that HTLV-1 infection increased all-cause mortality with an estimated relative risk of 1.57.4 We could not consider the follow-up period because of the difficulty in determining the onset due to the clinical course of uveitis, which frequently recurs.1, 9, 10 Second, the period prevalence of uveitis may have been underestimated because it was impossible to identify participants with uveitis, such as (1) those who did not visit medical facilities due to minor symptoms, (2) those who visited medical facilities only before the study period, or (3) those who visited medical facilities outside the islands. If HTLV-1-positive participants were more likely to visit medical facilities outside the islands because of their comorbidities, this could have affected our findings. Unlike two prior studies that compared HTLV-1 carriers and noncarriers,11, 12 we could not perform ophthalmologic examinations on all study participants to assess uveitis. Third, possible confounders such as family history and hometown were not examined. Fourth, we could not calculate the cumulative incidence of uveitis because some cases were prevalent at the time of exposure measurement and could not be distinguished from new cases. If all study participants could undergo ophthalmologic examinations to assess uveitis in the annual surveys, we could calculate the cumulative incidence. Fifth, due to the cross-sectional design, we could not prove the causality between HTLV-1 infection and uveitis because we could not determine the before/after relationship between exposure and outcome. However, as uveitis is unlikely to cause HTLV-1 infection, it is clear that HTLV-1 infection increases the risk of uveitis.
5 CONCLUSIONS
HTLV-1 infection was associated with a higher risk of uveitis among mostly middle-aged and older Japanese individuals living in areas highly endemic for HTLV-1. This study revealed that the 6.5-year prevalence of uveitis was almost three-fold higher in HTLV-1 carriers. Our findings suggest that physicians who treat HTLV-1 carriers should assess ocular symptoms to avoid not detecting uveitis, and that those who diagnose patients with uveitis should consider HTLV-1 infection.
AUTHOR CONTRIBUTIONS
Jun Miyata: Conceptualization; methodology; formal analysis; resources; investigation; writing—original draft; funding acquisition. Hirotomo Yamanashi: Conceptualization; methodology; investigation; writing—review & editing; funding acquisition. Yoshinori Dake: Conceptualization; investigation; writing—review & editing. Kenichi Nobusue: Conceptualization; investigation; writing—review & editing; funding acquisition. Yusuke Doi: Investigation; writing—review & editing. Yukiko Honda: Methodology; investigation; writing—review & editing. Fumiaki Nonaka: Investigation; writing—review & editing. Kazuhiko Arima: Resources; investigation; writing—review & Editing. Mami Tamai: Resources; investigation; writing—review & Editing. Daisuke Sasaki: Resources; writing—review & editing. Yuji Shimizu: Investigation; writing—review & editing; funding acquisition. Hiroo Hasegawa: Resources; writing—review & editing. Takashi Kitaoka: Investigation; writing—review & editing. Katsunori Yanagihara: Resources; writing—review & editing. Kiyoshi Aoyagi: Investigation; writing—review & editing; project administration. Atsushi Kawakami: Investigation; writing—review & editing; project administration. Takahiro Maeda: Conceptualization; methodology; investigation; writing—review & editing; supervision; project administration; funding acquisition.
ACKNOWLEDGMENTS
This study was conducted with the cooperation of the Goto City Municipal Office, Nagasaki Prefecture Medical Health Operation Group, Nagasaki Goto Chuoh (Central) Hospital and Naru Satellite Clinic, Nagasaki Tomie Hospital, and Dake Eye Clinic. The authors thank all staff members for their efforts in conducting the baseline and follow-up surveys and Wenbo Song, a Joint PhD student at London School of Hygiene and Tropical Medicine and Nagasaki University, for data entry of uveitis registry. This research was supported by JSPS KAKENHI grant numbers 17H03740, 18K06448, 19K07915, 19K10664, 21H02575, and 22K20859 from the Japan Society for the Promotion of Science and Nagasaki University WISE Programme. The funding source had no role in the study design, conduct, data analysis, or decision to submit the manuscript for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study protocol was developed by the research team and approved by the Ethics Committee of the Nagasaki University Graduate School of Biomedical Sciences (project registration number: 14051404 [latest version: 17th edition]). This study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its subsequent amendments. All the participants provided written informed consent.
Open Research
DATA AVAILABILITY STATEMENT
An anonymous data set supporting the findings of this study is available from the corresponding author upon reasonable request.




