Letermovir use may impact on the Cytomegalovirus DNA fragmentation profile in plasma from allogeneic hematopoietic stem cell transplant recipients
Estela Giménez and Roberto Gozalbo-Rovira are contributed equally to this study.
Abstract
Cytomegalovirus (CMV) DNA in plasma is mainly unprotected and highly fragmented. The size of the amplicon largely explains the variation in CMV DNA loads quantified across PCR platforms. In this proof-of-concept study, we assessed whether the CMV DNA fragmentation profile may vary across allogeneic hematopoietic stem cell transplant recipients (allo-SCT), within the same patient over time, or is affected by letermovir (LMV) use. A total of 52 plasma specimens from 14 nonconsecutive allo-SCT recipients were included. The RealTime CMV PCR (Abbott Molecular), was used to monitor CMV DNA load in plasma, and fragmentation was assessed with a laboratory-designed PCR generating overlapping amplicons (around 90−110 bp) within the CMV UL34, UL80.5, and UL54 genes. Intrapatient, inter-patient, and LMV-associated qualitative and quantitative variations in seven amplicons were observed. These variations were seemingly unrelated to the CMV DNA loads measured by the Abbott PCR assay. CMV DNA loads quantified by UL34_4, UL54.5, and UL80.5_1 PCR assays discriminate between LMV and non-LMV patients. Our observations may have relevant implications in the management of active CMV infection in allo-SCT recipients, either treated or not with LMV, although the data need further validation.
1 INTRODUCTION
Monitoring of Cytomegalovirus (CMV) DNA in blood is a cornerstone in the management of CMV infection in the allogeneic hematopoietic stem cell transplant setting (allo-SCT) as it allows timely administration of anti-CMV drugs (preemptive antiviral therapy [PET]) to prevent the occurrence of CMV end-organ disease.1, 2 Commercially available Real-time PCR assays, either performed on whole blood or plasma, are recommended for CMV DNA quantification in the systemic compartment.3 CMV DNA loads quantified by these assays may substantially vary across PCR platforms,4-6 despite their normalization to the whole-virus-based 1st WHO international standard.7 Several reasons may account for this phenomenon (e.g., differences in starting and elution volumes for DNA extraction and efficiency of viral DNA extraction),8 however, it has been robustly shown that the size of the amplicon generated during PCR amplification largely explains such variability; in fact, the amplicon size inversely correlates with the magnitude of CMV DNA loads measured across the assays.9-13 This is due to the unprotected (free) and highly fragmented nature of CMV DNA in blood,9, 11-13 particularly plasma, where viral DNA fragments shorter than 200 bp account for most of the total viral DNA content.13 Fragmentation of CMV DNA that accesses the blood compartment is thought to originate from CMV-infected cells in organs, tissues, or even blood undergoing apoptosis or necroptosis,14 although viral DNA may also be targeted by blood nucleases. It is uncertain whether the CMV DNA fragmentation pattern may differ between patients, or if it may vary over time in a given patient.
Due to its efficacy in the pivotal clinical trial and real-life experience, prophylaxis with letermovir (LMV) is widely used as the first choice for the prevention of CMV end-organ disease in CMV-seropositive allo-SCT recipients.15-17 LMV targets the terminase complex, which excises concatemeric viral DNA to generate full-length monomeric genomes that can be encapsidated, but does not prevent CMV DNA replication.17 Breakthrough CMV DNAemia may occur in patients under LMV prophylaxis and may either truly reflect active CMV infection potentially tributary of PET or, more commonly, an abortive self-resolving infection episode that would not require LMV cessation and subsequent inception of PET.18-20 Discrimination between these two possibilities is unfeasible when low CMV DNA thresholds are used for triggering PET.18 We hypothesized that, due to differences in their spatial configuration, concatemeric and monomeric CMV DNA may be fragmented differently, thus resulting in a dissimilar pattern of CMV DNA fragmentation in plasma. Here, by using a selected set of primer pairs amplifying conserved sequences within the CMV UL34, UL54, and UL80.5 genes, as these are frequently targeted by commercially available real-time PCR assays, we assessed whether the CMV DNA fragmentation pattern varies across patients, within the same patient over time, or is affected by LMV use. The data presented herein may have implications in the management of CMV DNAemia in allo-SCT recipients, particularly that occurring in patients under LMV prophylaxis, to design more commutable CMV DNA quantification standards.5
2 MATERIAL AND METHODS
2.1 Clinical specimens and patients
In this retrospective observational study, a total of 52 plasma specimens with detectable CMV DNAemia from 14 nonconsecutive randomly selected allo-SCT recipients, collected between May 2020 and May 2022, were included (Table S1). We collected 16 plasma samples (median, four specimens/patient; range, 1−7) from patients on LMV that developed breakthrough CMV DNAemia (median time after LMV inception, 23 days; IQR, 18−29) (LMV group). All episodes of LMV-breakthrough CMV DNAemia cleared spontaneously. A total of 23 plasma specimens were available from 10 LMV-untreated patients with CMV DNAemia who were not receiving PET at the time of sampling (non-PET group). Finally, a total of 13 specimens from five LMV-untreated patients who developed clinically significant CMV infection (CsCMVi), that is, requiring PET as per local guidelines, were also available (median three specimens/patient; range 3−4). These 13 specimens were collected while patients were under (val)ganciclovir treatment (Under-PET group). The current study was approved by the institutional ethical review board of the INCLIVA Biomedical Research Institute (January 2020). The request for informed consent was waived by the institution due to its retrospective nature.
2.2 Monitoring and management of CMV infection
The RealTime CMV PCR (Abbott Molecular), a dual target (UL34 and UL80.5) PCR, with a limit of detection and quantification of 31.4 IU/mL (95% confidence interval) was employed to monitor CMV DNA load in plasma. Amplification reactions were run on the m2000rt platform (Abbott). Viral DNA extraction was performed using the Abbott m2000sp platform (Abbott). LMV prophylaxis was administered to high-risk CMV seropositive patients at conventional doses within the week after cell infusion until day +100, following local guidelines. PET with valganciclovir (900 mg/12 h) or intravenous ganciclovir (5 mg/kg/12 h) was initiated, regardless of whether the patients were under LMV prophylaxis, when the plasma CMV DNA load reached ≥ 1500 IU/mL, or when the CMV DNA doubling time was ≤2.0 days, whichever occurred first.21, 22
2.3 Assessment of CMV DNA fragmentation
Experiments assessing CMV DNA fragmentation in plasma were carried out in two phases. In the first phase, which took place before the implementation of LMV at our center, we investigated whether the CMV DNA fragmentation profile may differ across or within five randomly selected allo-SCT recipients. A total of 45 end-point PCR assays targeting the CMV UL34 (n = 20), UL80.5 (n = 19), and UL54 (n = 6) genes were used in preliminary experiments, as described below. The primer pairs (Table S2) were designed to overlap with the neighboring ones and to amplify fragments of 90−110 bp to cover the corresponding full-length gene sequences of UL34 and UL80.5 (1224 and 1122 bp, respectively), and a selected region of 368 bp of UL54, UL34, UL80.5, and UL54 sequences were cloned into pGEMTeasy vectors to generate plasmids incorporating the corresponding sequence. These plasmids were used as templates for the amplification reactions. All PCR reactions generated amplicons with the expected size (data not shown). In the second phase of the study, after the implementation of LMV at our center, quantitative real-time PCR assays generating amplicons that were differentially detected in study phase one (primer pairs; UL34_4, UL34_12, UL34_17, UL34_18, UL54_3, UL54_5, UL80.5_2, UL80.5_5, UL80.5_6, UL80.5_12, and UL80.5_19), were developed using an Exicycler RT-PCR thermal cycler (Bioneer), RT2 SYBR green Rox qPCR Mastermix (Qiagen), following the manufacturer's instructions. Each 10 μL reaction contained 5 μL of x2 Master Mix (Qiagen), 1.5 μL of each primer (0.25 μM final concentration each), and 2 μL of eluate. A positive CMV control and a negative control (nuclease-free water) were included in every PCR run. Serial dilutions of the plasmid containing the respective CMV gene (from 102 to 108 genome equivalent/mL) were used to generate a calibration curve (genome copies number/μL) and to estimate primer pair efficiencies by using the calibration dilution curve and slope calculation method (Efficiency (%) = (10(−1/slope)-1) x 100).23 The cycling conditions were the following: initial denaturation at 95°C for 5 min, 45 cycles of 95°C for 15 s, and 60°C for 1 min, followed by a melting curve ranging from 60°C to 95°C (acquiring fluorescence data every 0.3°C).
2.4 Statistical analysis
A comparison of categorical variables was carried out with the Chi-Square test or Fisher's test, as appropriate. Comparison of continuous variables (medians and IQRs) between two or more groups was carried out with the non-parametric Mann−Whitney U test or the Kruskal−Wallis test, respectively. Correlation between continuous variables was assessed with the Spearman Rank test. Based upon the quantification of specific PCR amplicons, a logistic regression analysis was performed to generate a model that would categorize plasma specimens as belonging to LMV or non-LMV-treated patients. All amplicons (in genomes/µL) were introduced as quantitative variables into the model and discarded one by one by the backward-elimination rule until the remaining variables reached statistical significance. Performance metrics included accuracy, area under a curve (AUC), sensitivity, specificity, precision, and F-measure. The diagnostic performance was assessed by a confusion matrix. p ≤ 0.05 (two-tailed) were considered statistically significant. Graphs and statistical analyses were performed using GraphPad Prism software version 9.0.
3 RESULTS
In the first phase of the study, we carried out a set of experiments to assess whether the CMV DNA fragmentation pattern in plasma varied across or within five allo-SCT patients developing CsCMVi. To this end, we designed a set of overlapping primer pairs amplifying short sequences within the CMV UL34, UL80.5, and UL54 genes, as described in the methods section. The choice of these genes was based upon the facts that CMV UL34 and UL80.5 are targeted by the RealTime CMV PCR, currently in use at our laboratory for CMV DNA quantification in allo-SCT recipients, and that UL54 is targeted by several commercially available PCR assays (e.g., COBAS® AmpliPrep/COBAS® TaqMan® CMV Test from Roche Diagnostics). A total of 20 specimens from five patients with CsCMVi collected either before (n = 8) or after (n = 12) the administration of (val)ganciclovir were randomly selected for these experiments. Eleven of the 45 amplicons generated by the corresponding PCR reactions (primer pairs: UL34_4, UL34_12, UL34_17, UL34_18, UL80.5_2, UL80.5_5, UL80.5_6, UL80.5_12, UL80.5_19, UL54_3, and UL54_5) were differentially present either across patients, in a given patient over time, or both. Figure 1 shows a representative example illustrating differences involving the UL54_3 and UL54_5 PCR amplicons in two patients.
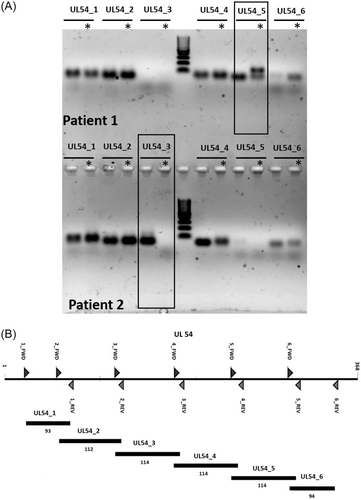
3.1 Intra- and inter-patient qualitative differences in the CMV DNA fragmentation pattern in plasma
In the second phase of the study, we optimized quantitative real-time PCR reactions using the aforementioned selected primer pairs for further experiments. We investigated whether the CMV DNA fragmentation profile as inferred from the number of detected amplicons differed within a given patient over time, between patients belonging to the same study group, or across patient groups (Table 1). Overall, one or more PCR amplicons were not detected in 29/52 samples (56%). Specimens with missing PCR amplicons were unevenly distributed across study groups: 14/16 (88%) in samples from LMV patients, 11/16 (69%) in samples from non-PET patients, and 4/20 (20%) in samples from under-PET patients (p < 0.01). Nevertheless, the mean number of missing PCR amplicons was not significantly different across groups (2, 1, and 1 in LMV, non-PET, and under-PET patients, respectively; p > 0.05). PCR amplicons that were differentially detected included those generated by primer pairs UL34_17, UL54_3, UL54_5, UL80.5_2, UL80.5_5, UL80.5_6, and most notably UL80.5_19. Moreover, as shown in Figure 2, intra-patient variations in the number of detected PCR amplicons over time were observed (in 3/3 LMV patients sampled more than once, 5/5 non-PET patients, and 3/5 Under-PET patients).
| Patient groupa | Patient number | Sample number | Number of PCR amplicons detected | Missing PCR amplicon/PCR amplification efficiency | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CMV DNA load (IU/mL) in plasma | UL34_17/70% | UL54_3/97% | UL54_5/67% | UL80.5_2/109% | UL80.5_5/72% | UL80.5_6/61% | UL80.5_19/79% | ||||
| Under-PET | 1 | 1 (Pre-PET) | 11 | 890 | |||||||
| 2 | 11 | 3201 | |||||||||
| 3 | 11 | 4408 | |||||||||
| 4 | 11 | 2995 | |||||||||
| Under-PET | 2 | 1 (Pre-PET) | 11 | 984 | |||||||
| 2 (Pre-PET) | 11 | 20 133 | |||||||||
| 3 | 10 | 24 100 | X | ||||||||
| 4 | 10 | 19 602 | X | ||||||||
| Under-PET | 3 | 1 (Pre-PET) | 11 | 2388 | |||||||
| 2 | 10 | 1549 | X | ||||||||
| 3 | 11 | 1243 | |||||||||
| 4 | 11 | 1200 | |||||||||
| Under-PET | 4 | 1 (Pre-PET) | 11 | 6920 | |||||||
| 2 | 11 | 4968 | |||||||||
| 3 | 11 | 1694 | |||||||||
| 4 | 11 | 8094 | |||||||||
| Under-PET | 5 | 1 (Pre-PET) | 11 | 6966 | |||||||
| 2 (Pre-PET) | 11 | 7817 | |||||||||
| 3 | 11 | 10962 | |||||||||
| 4 | 9 | 5352 | X | X | |||||||
| Non-PET | 6 | 1 | 10 | 343 | X | ||||||
| 2 | 9 | 464 | X | X | |||||||
| 3 | 11 | 461 | |||||||||
| Non-PET | 7 | 1 | 10 | 999 | X | ||||||
| 2 | 10 | 469 | X | ||||||||
| 3 | 11 | 1355 | |||||||||
| Non-PET | 8 | 1 | 9 | 400 | X | X | |||||
| 2 | 11 | 530 | |||||||||
| 3 | 11 | 1829 | |||||||||
| Non-PET | 9 | 1 | 9 | 511 | X | X | |||||
| 2 | 9 | 695 | X | X | |||||||
| 3 | 11 | 2553 | |||||||||
| Non-PET | 10 | 1 | 8 | 308 | X | X | X | ||||
| 2 | 9 | 447 | X | X | |||||||
| 3 | 9 | 541 | X | X | |||||||
| 4 | 7 | 414 | X | X | X | X | |||||
| LMV | 11 | 1 | 7 | 60 | X | X | X | X | |||
| LMV | 12 | 1 | 10 | 80 | X | ||||||
| 2 | 10 | 104 | X | ||||||||
| 3 | 10 | 260 | X | ||||||||
| 4 | 10 | 150 | X | ||||||||
| 5 | 10 | 220 | X | ||||||||
| 6 | 9 | 144 | X | X | |||||||
| 7 | 8 | 57 | X | X | X | ||||||
| LMV | 13 | 1 | 8 | 60 | X | X | X | ||||
| 2 | 11 | 76 | |||||||||
| 3 | 10 | 114 | X | ||||||||
| 4 | 10 | 98 | X | ||||||||
| LMV | 14 | 1 | 9 | 139 | X | X | |||||
| 2 | 8 | 265 | X | X | X | ||||||
| 3 | 11 | 397 | |||||||||
| 4 | 9 | 165 | X | X | |||||||
- Abbreviations: CMV, Cytomegalovirus; LMV, Letermovir; PET, Preemptive antiviral therapy; X, missing PCR amplicon.
- a LMV, patients under LMV prophylaxis at the time of sampling; Non-PET, patients not under LMV prophylaxis, and untreated with (val)ganciclovir at the time of sampling; Pre-PET, plasma specimens from patients who developed clinically significant CMV infection collected before the administration of PET; Under-PET, patients not undergoing LMV prophylaxis, and treated with (val)ganciclovir at certain sampling times.
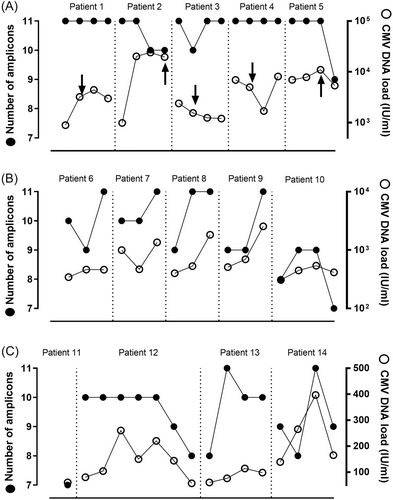
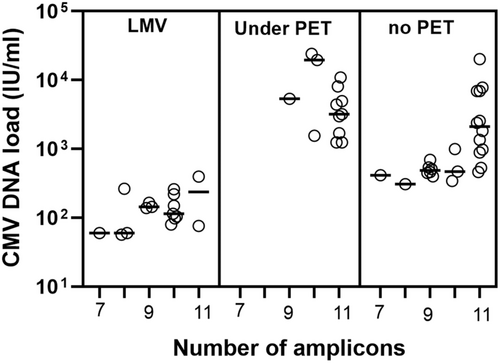
As stated above, specimens with missing PCR amplicons were substantially less represented among under-PET plasma specimens compared with non-PET and LMV samples. Since CMV DNA loads measured by the Abbott assay were overall higher in under-PET specimens, we next investigated whether the number of detected amplicons was directly related to the magnitude of the CMV DNA load. No such association was evident (overall data in Table 1 and Figure 3). We conducted a series of subanalyses to further assess this issue. First, we compared the number and profile of PCR amplicons generated from specimens from LMV and non-PET patients displaying CMV DNA loads < 500 IU/mL (median, 127 IU/mL [IQR, 78−193] and 431 IU/mL [IQR, 372−463], in LMV-patients and non-PET patients, respectively). As shown in Figure 4A, the frequency of specimens with 10 or 11 PCR amplicons detected was higher (p = 0.04) among LMV patients (57%) compared with that in non-PET patients (38%). Moreover, the PCR amplicon profile was substantially different between LMV samples and non-PET specimens (Figure 4B); notably, UL54 PCR amplicons were more frequently missing in specimens from LMV patients than in those from under-PET patients, whereas the opposite held true for UL80.5 PCR amplicons, especially UL80.5_19 (Figure 4B). Likewise, evident differences in both the number and pattern of PCR amplicons detected were noticed across non-PET and under-PET specimens, irrespective of the CMV DNA load, graded as between 500 and 5000 IU/mL (median 984 IU/mL [IQR 541−1829] in non-PET specimens and median, 2,345 IU/mL [IQR 1396−3805], in Under-PET specimens), or >5000 IU/mL (median, 7392 IU/mL [IQR 6943−13 975] in non-PET samples and median, 10 962 IU/mL [IQR 8094−19 602], in Under-PET specimens) (Figures 4C,D). In this sense, strikingly, among specimens with high viral loads (>5000 IU/mL), those from under-PET patients had fewer detected PCR amplicons (40% of specimens with 11 detectable amplicons) than those from non-PET patients (100% of specimens with 11 amplicons), despite higher CMV DNA loads. Importantly, no PCR amplicon profile could consistently discriminate between patient groups.
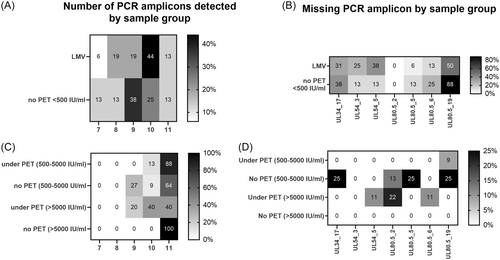
We also assessed whether the efficiency of the different real-time PCR reactions had an impact on the pattern of PCR amplicons observed across specimens. The efficiency of real-time PCR assays generating amplicons differentially detected across specimens varied between 61% (UL80.5.6) and 109% (UL80.5.2) (data shown in Table 1). We observed no obvious association between the above two parameters; for example, in specimens from LMV and non-PET (CMV DNA load, <500 IU/mL), the UL80.5.19 amplicon was substantially more frequently undetected than UL80.5.5, despite PCR reactions displaying a comparable efficiency (79% vs. 72%) (see Figure 4B).
3.2 Differences in CMV DNA loads quantified by amplicon-specific PCR assays across patients
We next aimed to determine whether there were differences in CMV DNA loads measured by UL34, UL80.5, and UL54 real-time PCR assays (n = 11) across specimens from patients in the different study groups. We first noticed that the correlation between CMV DNA loads quantified by the Abbott RealTime CMV PCR assay (in IU/mL) and those measured by the different “in-house” real-time PCR assays (in genome copies/µL) varied widely: the correlation was strong (rho, 0.61−0.80; p < 0.001) for UL54_3, UL34_18, and UL80.5_12 real-time PCR assays, moderate (rho, 0.41−0.60; p < 0.001) for UL34_12, UL34_17, UL80.5_5, UL80.5_6, and UL80.5_19, and absent for UL34_4, UL54_5, and UL80.5_2. The level of correlation was seemingly unrelated to the respective efficiency of the real-time PCR assays (not shown).
Relevant differences in CMV DNA loads quantified by laboratory-developed real-time PCR assays were as follows (Table 2): (i) CMV DNA loads measured by the UL34_4 and UL80.5_2 real-time PCRs were significantly higher in LMV specimens than in non-PET specimens displaying CMV DNA loads <500 IU/mL in the Abbott assay (p < 0.001 and p = 0.007, respectively). In contrast, CMV DNA load quantified by the UL34_18 real-time PCR assay was significantly lower (p = 0.04) in specimens from the LMV patients as compared with those from non-PET specimens; (ii) Only CMV DNA load as measured by the UL54_3 real-time PCR assay differed significantly between non-PET and Under-PET specimens (p = 0.04 for CMV DNA loads between 500 and 5000 IU/mL, as measured by the Abbott assay) (Table 2).
| PCR Amplicon | CMV DNA load measured by a given PCR in genome copies/µL (IQR) | ||
|---|---|---|---|
| LMV versus Non-PET (CMV DNA load < 500 IU/mL)/p Value | Non-PET versus Under PET (CMV DNA loads between 500−5000) IU/mL/p Value | Non-PET versus Under PET (CMV DNA loads > 5000 IU/mL)/p Value | |
| UL34_4 | 17 (6-36)/0.6 (0.5−0.9)/ < 0.001 | 5 (0.7−7)/3 (2−6)/1.0 | 10 (8−46)/17 (15−51)/0.46 |
| UL34_12 | 3 (1−7)/2 (1−3)/0.43 | 7 (2−13)/7 (5−14)/0.68 | 12 (8−37/35 (28−51)/0.46 |
| UL34_17 | 2 (0−19)/15 (0−55)/0.69 | 23 (14-60)/24 (22-97)/0.32 | 120 (70−279)/120 (41−204)/1.0 |
| UL34_18 | 37 (23−63)/64 (47−77)/0.04 | 76 (67−99)/80 (54−89)/0.74 | 84 (62−140)/87 (77−129)/0.81 |
| UL54_3 | 0.08 (0.03−0.89)/0.2 (0.02−0.8)/0.50 | 2 (1−4)/7 (3−27)/0.04 | 8 (5−26)/29 (20−31)/0.62 |
| UL54_5 | 23 (0−575)/6 (3−7)/0.29 | 11 (8 16)/16 (10−23)/0.19 | 13 (11−48)/30 (13−66)/0.46 |
| UL80.5_2 | 1 (0.5−35)/0.3 (0.1−0.5)/0.007 | 1 (0.3−2)/2 (1−3)/0.25 | 4 (3−8)/1.3 (0−7)/0.33 |
| UL80.5_5 | 2 (0.9−16)/1 (0.8−2)/0.20 | 11 (0.7−19)/6 (3−9)/0.32 | 9 (3−90)/17 (7−55)/0.46 |
| UL80.5_6 | 6 (2−28)/18 (8−36)/0.54 | 71 (51−104)/59 (43−61)/0.14 | 41 (25−432)/42 (39−938)/0.62 |
| UL80.5_12 | 0.7 (0.4−2)/2 (0.4−2)/0.46 | 4 (0.7−7)/4 (2−15)/0.41 | 6 (4−32)/15 (13−32)/0.46 |
| UL80.5_19 | 0.8 (0−10)/0 (0−0)/0.08 | 1 (0.6−4)/4 (1−24)/0.16 | 4 (3−16)/12 (8−19)/0.62 |
- Note: Bold values indicate statistically significant differences across comparison subgroups.
- Abbreviations: CMV, Cytomegalovirus; LMV, patients under LMV prophylaxis at the time of sampling; Non-PET, patients not under LMV prophylaxis, and untreated with (val)ganciclovir at the time of sampling; Under-PET, patients not undergoing LMV prophylaxis, and treated with (val)ganciclovir at the time of sampling PET.
3.3 CMV DNA loads quantified by UL34_4, UL54.5, and UL80.5_1 PCRs discriminate between plasma from LMV and non-LMV patients
A stepwise logistic regression was performed using quantitative data from all 11 UL34, UL80.5, and UL54 real-time PCR assays to build a model that was tested for its ability to discriminate between specimens from patients undergoing LMV prophylaxis and those from LMV-untreated patients (including non-PET and Under-PET). The data are shown in Figure S1. While quantitative data from any individual real-time PCR assay did not allow discrimination, the combination of specific cut-offs selected by the model (>20 genome copies/µL of UL34_4, >500 genome copies/µL of UL54.5, and <5 genome copies/µL of UL80.5_12) afforded perfect discrimination (accuracy, AUC, sensitivity, specificity, and precision of 100%).
4 DISCUSSION
It has been established that non-encapsidated and highly fragmented DNA is the major biological form of the CMV genome in plasma from transplant patients developing active CMV infection.9, 11-13 Nevertheless, to our knowledge, whether the CMV DNA fragmentation pattern varies across patients or even within the same patient over time or is affected by the use of anti-CMV drugs, in particular LMV, are potentially relevant questions that, to our knowledge, have not been investigated. Regarding the potential impact of the use of LMV on the CMV DNA fragmentation profile in plasma, we hypothesized that concatemeric unexcised CMV DNA, which accumulates in CMV-infected cells under the effect of LMV, and monomeric CMV DNA may be fragmented differently, potentially resulting in dissimilar profiles of CMV DNA fragmentation in plasma. Preliminary experiments using 45 end-point PCR assays generating small-size amplicons within the CMV UL34, UL80.5, and UL54 genes and plasma from five allo-SCT recipients, revealed that the CMV DNA fragmentation profile in plasma, as reflected by the number of amplicons detected, could in fact vary both in a given patient over time and between patients. There were 11 PCR amplicons differentially detected in these specimens. We next developed quantitative real-time PCR assays generating these amplicons for further investigations. Serial plasma specimens from LMV, non-PET, and under-PET patients were tested with these “in-house” real-time PCR assays. Based upon the number of present or missing PCR amplicons, our results further suggested that the CMV DNA fragmentation pattern in plasma could, in effect, vary within a given patient over time and across patients or may be influenced by the use of (val)ganciclovir and, most notably, LMV. Overall, one or more missing PCR amplicons were more frequently noticed in LMV specimens (particularly involving UL54 PCR amplicons) compared with non-PET and under-PET samples, although this was not the case when only specimens with low CMV DNA loads, as measured by the Abbott assay, were taken for the analyses. Importantly, the number of amplicons detected in plasma specimens was apparently unrelated, at least in part, either to the magnitude of the CMV DNA loads quantified by the Abbott assay, or the efficiency of the laboratory-developed PCR reactions.
From a quantitative standpoint, differences in CMV DNA loads measured by “in-house” real-time PCR assays were observed across patient groups. Notably, CMV DNA loads measured by the UL34_4 and UL80.5_2 real-time PCRs were significantly higher in specimens from LMV patients than in non-PET specimens displaying comparable CMV DNA loads, as determined by the Abbott assay, whereas the reverse was true for CMV DNA loads quantified by the UL34_18 real-time PCR assay. A particularly relevant finding of our investigations was that a model incorporating the combination of the following quantitative estimates > 20 genome copies/µL of UL34_4, >500 genome copies/µL of UL54.5, and <5 genome copies/µL of UL80.5_12 allowed a reliable categorization of plasma specimens belonging to LMV patients. Although speculative, the most likely explanation for this phenomenon is that concatemeric CMV DNA is fragmented differently than monomeric CMV DNA, resulting in certain amplicons being amplified to a greater extent, thus being more represented than others in plasma from LMV patients compared with non-PET and Under-PET specimens. The data presented may have relevant implications in the management of active CMV infection in allo-SCT patients. First, we provide evidence suggesting that our experimental approach may reliably identify self-resolving (abortive) breakthrough CMV DNAemia episodes occurring in LMV patients; this, when low CMV DNA thresholds are used for triggering PET, would allow discrimination between bona fide and abortive CMV infection, thus avoiding unnecessary LMV interruption.18-20 Further investigations are needed to confirm our findings and determine whether this strategy may crystallize in the design of a commercial PCR assay for the above purpose. Second, variations in the CMV DNA fragmentation profile may impact on the magnitude of CMV DNA loads returned by commercial real-time PCR assays. In this context, the correlation between CMV DNA loads measured by the Abbott assay or the “in-house” real-time PCR assays varied. For example, the correlation between CMV DNA loads provided by the Abbott assay and our “in-house” PCR assays was strong for UL34_18 and UL80.5_12 but absent for UL34_4, and UL80.5_2. Likewise, viral loads returned by UL34_4 real-time PCR were significantly higher in plasma from LMV patients compared with that from non-PET patients displaying low CMV DNA loads by the Abbott assay, whereas the opposite was true when samples were run in the Abbott assay.
It is uncertain whether this phenomenon may have relevant consequences in the management of CMV infection in the allo-SCT setting, however, it is conceivable (i.e., underestimation of CMV DNA load leading to ill-timed administration or interruption of PET). Third, it has been recently shown that the commutability of the primary WHO international standard and secondary standards normalized to the former was markedly increased following the fragmentation of both CMV standards by ultrasonication (average fragment size of 166 bp).6 Nevertheless, our findings somehow obscure the possibility of generating a fragmented standard that drastically minimizes interassay quantitative variability.
In addition to the relatively small sample size, the current study has two major limitations; first, by only targeting three CMV genes, our experimental strategy only provides a narrow view of a seemingly complex phenomenon, such as CMV DNA fragmentation in plasma. Definitely, high-resolution profiling of CMV cell-free DNA by next-generation sequencing should provide a more comprehensive outlook. Second, we cannot rule out that the efficiency of CMV DNA extraction could have varied across plasma specimens belonging to the different study groups.
In summary, our work provides proof-of-concept evidence highlighting the heterogeneity of the CMV DNA fragmentation profile in plasma from allo-SCT, which was shown to vary widely across patients and on an individual basis over time and to be influenced by the use of LMV. Further studies are needed to validate our findings and elucidate whether this phenomenon may have any relevance in the management of active CMV infection in this setting.
AUTHOR CONTRIBUTIONS
Estela Giménez, Roberto Gozalbo-Rovira, and Eliseo Albert: Methodology and analysis of the data. José Luis Piñana and Carlos Solano: Patient management and data curation. David Navarro: Conceptualization, data analysis, and manuscript drafting. All authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
Eliseo Albert holds a Juan Rodés Contract (JR20/00011) funded by the Carlos III Health Institute (co-financed by the European Regional Development Fund, ERDF/FEDER).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




