Clinico-epidemiological presentations and management of Nipah virus infection during the outbreak in Kozhikode district, Kerala state, India 2023
Anoop Kumar AS, Rima R. Sahay, Chandni Radhakrishnan, and Shihabudheen P are equal first authors.
Sathishkumar Kandath, Deepak Y. Patil, Anita M. Shete, Shamsudheen M, Gayathri Ramakrishnan, Anitha Puduvail Moorkoth, Nivedita Gupta, Pragya D. Yadav, Sheela Godbole, and Lathika Velichapat Ramakrishnan are equal second authors.
Shameer Vadekkandiyil, Danish Ekkalayil, Nithasha V, Anukumar Balakrishnan, Niyas K. Pullor, Neelakandhan Asokan, Reena Kalathil Joseph, Priyanka R. Nair, Sreejayan Meethale Purayil, Thomas Mathew, and Rajaram Kizhakkekandiyil are equal third authors.
Jayesh Kumar Poovullathil, Kannan Sabarinath PS, Ullas PT, Kalpana George, Asma Rahim, Surendra Kumar, Siba S, Sreelekshmy Mohandas, and Lekshmi S. Rajan are equal fourth authors.
Shamin Punnath Ramachandran, Seethu Ponnu Thampi, Ashadevi, Thekkumkara Surendran Anish, Priya Chandran, Anuja Mohan, Bindu Vadakkayil, Shaji Cheriya Koroth, Nimin Hafeez, Rajasi Ranjini Sasi, and Minu Abraham are equal fifth authors.
Abstract
India experienced its sixth Nipah virus (NiV) outbreak in September 2023 in the Kozhikode district of Kerala state. The NiV is primarily transmitted by spillover events from infected bats followed by human-to-human transmission. The clinical specimens were screened using real-time RT-PCR, and positive specimens were further characterized using next-generation sequencing. We describe here an in-depth clinical presentation and management of NiV-confirmed cases and outbreak containment activities. The current outbreak reported a total of six cases with two deaths, with a case fatality ratio of 33.33%. The cases had a mixed presentation of acute respiratory distress syndrome and encephalitis syndrome. Fever was a persistent presentation in all the cases. The Nipah viral RNA was detected in clinical specimens until the post-onset day of illness (POD) 14, with viral load in the range of 1.7–3.3 × 104 viral RNA copies/mL. The genomic analysis showed that the sequences from the current outbreak clustered into the Indian clade similar to the 2018 and 2019 outbreaks. This study highlights the vigilance of the health system to detect and effectively manage the clustering of cases with clinical presentations similar to NiV, which led to early detection and containment activities.
1 INTRODUCTION
Nipah virus (NiV) is a zoonotic paramyxovirus with a pandemic potential transmitted from bats to humans. It is a highly contagious virus with the fatality rate of 40%–75%.1 There is no specific treatment for NiV infection and supportive care is the only option. The virus was first identified in 1999 in Malaysia.2 Since then, there have been outbreaks in several other countries, including Bangladesh, India, and Philippines.3-13
India's first outbreak of NiV was reported from the Siliguri district of West Bengal in January–February 2001, with 45 deaths among 66 cases. The cases primarily presented with fever, headache, myalgia, vomiting, altered sensorium, acute respiratory distress syndrome (ARDS), and convulsions.4 A second outbreak of NiV was reported from Nadia district of West Bengal in April 2007. This was the intra-familial outbreak with the presentation of fever and acute encephalitis-like symptoms, affecting all five family members, who succumbed to infection.5
Kerala, a state in southern India, has experienced four NiV outbreaks since 2018. The first NiV outbreak in the Kozhikode district of Kerala occurred in May 2018. A total of 18 cases were reported, including 16 deaths and the outbreak was contained by June 2018.8, 9 Further, the second outbreak was detected in Ernakulam district, Kerala in June 2019 with a single case who recovered from the infection, and the outbreak was contained by July 2019.10, 11 The Kozhikode district in Kerala reported NiV outbreak again in September 2021, which was the third outbreak with a single fatal case and was contained by October 2021.12
The recent NiV outbreak was confirmed again in Kozhikode, Kerala, India in September 2023, which was the fourth episode and a total of six NiV-positive cases (cases 1–6) were detected. The state, and national authorities coordinated to enhance active, passive, and stimulated passive surveillance of the suspected cases, high and low-risk contacts in order contain the spread of infection. The preparedness of hospitals for infection control practices and case management protocols was enhanced. There was an emphasis on risk communication strategies and community engagement activities for the active support and involvement of the community.
Here, we describe the clinico-epidemiological details, viral RNA kinetics, and management of NiV cases during the current outbreak. We also describe the phylogenetic analysis of the retrieved NiV genomes.
2 MATERIALS AND METHODS
2.1 Study design and cases
The NiV cases were classified as 1D63 according to the International Classification of Diseases, 11th Revision (ICD-11).14 The NiV cases were identified as confirmed cases as per the case definitions of NiV disease given by the National Centre for Disease Control (NCDC), New Delhi, India, and the Directorate of Health Services, Government of Kerala State, India.15-17 The demographical (age and gender), epidemiological (travel history, contact with an ill individual, exposure to bats or history of eating or handling half-eaten fruits), and clinical data (signs, symptoms, past medical history, visits to emergency departments, admissions, duration of illness before diagnosis, length of hospitalization, and recovery status) were collected for all the cases from the medical case history records and also from the history given by cases (n = 6) and relatives between August 29 and September 14, 2023.
The data related to treatment, hematological, biochemical and radiographic findings were also collected. The written informed consent was obtained from the cases or the next of kin. All data analyses were carried out using GraphPad Prism version 9.5.1 (GraphPad Software).
2.2 Laboratory diagnosis using real-time RT-PCR and anti-Nipah IgM and IgG ELISA
A 200 µL volume of the clinical specimens (oropharyngeal swab [OPS], nasopharyngeal swab [NPS], cerebrospinal fluid [CSF], serum, and urine) was used for RNA extraction using the MagMAX™ Viral/Pathogen Nucleic Acid Isolation Kit as per the manufacturer's instructions. Further, the real-time RT-PCR (rRT-PCR) was performed by targeting the nucleoprotein (N) gene of NiV as described earlier.18 All positive clinical specimens were quantified and expressed as number of RNA copies per mL. Besides this, sequential samples of the confirmed nonfatal Nipah cases (n = 4) were tested until it showed the presence of viral RNA by rRT-PCR to understand Nipah viral RNA kinetics. The anti-Nipah IgM and IgG assays were performed as described earlier.11 After two negative rRT-PCR tests on OPS/NPS and urine samples obtained during follow-up, a clinically recovered case was allowed to be discharged.
2.3 Whole-Genome Sequencing
All rRT-PCR-positive specimens were further characterized using whole-genome sequencing (WGS) to identify the NiV genotype. The extracted RNA from the clinical specimens was used to perform the WGS using the Truseq library described earlier.19 Briefly, the steps used for RNA NGS library preparation using the Illumina Truseq stranded mRNA library preparation kit include rRNA depletion, fragmentation, amplification, and Qubit quantification. Whereas the Nanopore approach uses the sequencing of native RNA molecules.20 The complete genome retrieved using both methods was mapped with the reference-based genomes, and a maximum likelihood analysis was performed. A phylogenetic tree was created using IQ-TREE 2 phylogenetic software, as described earlier.21, 22
3 RESULTS
3.1 Detection of NiV outbreak in Kozhikode, Kerala, India
Considering the clustering of cases with similar clinical presentations, NiV infection was suspected by health care officials, and clinical specimens were sent to the regional virus research and diagnostic laboratory (RVRDL) at Government Medical College (GMC), Kozhikode, on September 11, 2023. The samples were tested using rRT-PCR at RVRDL, and two cases (cases 2 and 3) tested positive for NiV, which were further confirmed by the apex laboratory at the Indian Council of Medical Research-National Institute of Virology (ICMR-NIV), Pune. The NiV outbreak in Kozhikode, Kerala was declared on September 12, 2023 after the confirmation of three NiV-positive cases of five suspected cases at ICMR-NIV, Pune. The three cases who were positive include a 9-year male (case 2; family contact of primary case); a 25-year male (case 3; family contact of primary case), and a 40-year male (case 4; hospital contact of primary case).
3.2 Deployment of Mobile Bio-safety level-3 laboratory at outbreak site for onsite diagnosis
After the confirmation of NiV outbreak, ICMR deployed team from ICMR-NIV, Pune along with the Mobile Bio-safety level-3 (MBSL-3) laboratory for onsite NiV diagnosis. The MBSL-3 laboratory was stationed at GMC Kozhikode and functionalized by September 14, 2023.
Subsequently, all the clinical specimens of Nipah suspected cases were categorized into (i) epidemiologically linked symptomatic cases; (ii) epidemiologically linked asymptomatic cases; and (iii) epidemiologically not linked but suspected NiV cases. The MBSL-3 facility was utilized for testing of category (i) cases, while for categories (ii and iii), RVRDL facility was utilized from September 14 to October 14, 2023.
3.3 Case series
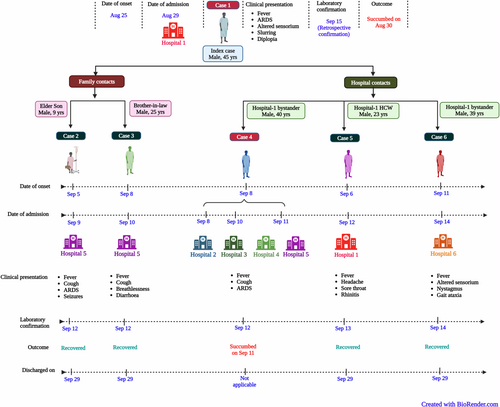
3.3.1 Case 1 (Primary case)
A 45-year male from Kozhikode, Kerala presented with respiratory failure and high-grade fever (103.1°F [39.5°C]) to emergency department of tertiary care hospital in Kozhikode (hospital 1) on August 29, 2023 (Figure 1). He had experienced a low-grade fever, dry cough, headache, and nausea since August 25 for which he has taken anti-pyretics and antacids. He began to experience mental confusion, fatigue, and high-grade fever with chills on August 28 and he presented to nearby hospital for the same. And that time, his pulse was 100/min with high blood pressure (190/100 mmHg) and saturation of peripheral oxygen (SPO2) of 89% with room air. He was initiated on oxygen inhalation 4 L/min and received paracetamol, inj. hydrocortisone, nebulization, and inj. deriphylline. The complete blood count (CBC) revealed mild thrombocytopenia (123 000/µL), leukopenia (3430/µL), and x-ray chest showed right-sided consolidation with bilateral diffuse infiltrates suggestive of ARDS. He was referred to hospital 1, where he had an SPO2 of 72% with room air. With declining SPO2, he was given bilevel positive airway pressure (BiPAP), a noninvasive ventilation support and was started on inj. ceftriaxone, tab. azithromycin and tab. oseltamivir. He was conscious, but with mild mental confusion at the time of admission. With the worsening hypoxia, the case started showing altered sensorium and invasive mechanical ventilation was initiated. The case tested negative for influenza A, B, and SARS-CoV-2 viruses and was tested negative for dengue by serological tests. The inflammatory markers including C-reactive protein (CRP) was raised and Troponin-T was mildly elevated (Table 1). The case developed urinary retention and fluctuation in the blood pressure with two-dimensional echocardiography (2D ECHO) showing hypercontractile left ventricle and collapsing inferior vena cava suggesting a possible capillary leak. Considering the worsening shock, inj. noradrenaline, vasopressin, and hydrocortisone were added in the treatment. On August 30, the condition of the case further deteriorated and he succumbed to the illness.
| Variables | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|
| Primary case | Secondary case | Secondary case | Secondary case | Secondary case | Secondary case | |
|
||||||
| Age | 45 | 9 | 25 | 40 | 23 | 39 |
| Gender | Male | Male | Male | Male | Male | Male |
| Travel history in last 21 days before onset of symptoms | No | No | No | No | No | No |
| Contact with bat | Unknown | No | No | No | No | No |
| Contact type | Not applicable | Family contact | Family contact | Hospital contact | Hospital contact | Hospital contact |
|
||||||
|
August 25, 2023 | September 5, 2023 | September 8, 2023 | September 8, 2023 | September 6, 2023 | September 11, 2023 |
| Date of admission | August 29, 2023 | September 9, 2023 | September 10, 2023 | Multiple hospitalizations on September 8, 10, 11, 2023 | September 12, 2023 | September 14, 2023 |
|
5 | 5 | 3 | 4 | 7 | 4 |
|
September 15, 2023 [retrospective confirmation- Index case] | September 12, 2023 | September 12, 2023 | September 12, 2023 | September 13, 2023 | September 14, 2023 |
|
Fever, ARDS, altered sensorium, slurring, diplopia | Fever, cough, ARDS, seizures | Fever, cough, breathlessness, diarrhea | Fever, cough, ARDS | Fever, headache, sore throat, rhinitis | Fever, altered sensorium, nystagmus, gait ataxia |
| Co-morbidities | Psoriasis | No | No | No | No | No |
|
||||||
| OPS | MCL-23-H-3874a | MCL-23-H-3764a | MCL-23-H-3768 | MCL-23-H-3762a | MCL-23-H-3774 | MCL-23-H-3878 |
| POD 5 (7.2 × 106) | POD 5 (3.7 × 106), POD 14 (3.3 × 104), POD 18 (Negative) |
POD 3 (2.5 × 105), POD 11 (6.4 × 104), POD 16 (Negative) |
POD 4 (1.4 × 107) | POD 7 (1.3 × 105), POD 11 (Negative), POD 18 (Negative) |
POD 4 (Negative), POD 9 (Negative), POD 14 (Negative) |
|
| NPS | MCL-23-H-3879 | |||||
| Not available | Not collected | Not available | Not collected | Not collected | POD 4 (7.2 × 106), POD 9 (1.7 × 104), POD 14 (Negative) |
|
| MCL-23-H-3765 | MCL-23-H-3769 | MCL-23-H-3773 | MCL-23-H-3776 | MCL-23-H-3880 | ||
| Serum | Not available | Volume low; hence not tested | Volume low; hence not tested | Volume low; hence not tested | Volume low; hence not tested | POD 4 (Negative), POD 9 (not tested) POD 14 (not tested) |
| Urine | MCL-23-H-3767 | MCL-23-H-3771 | MCL-23-H-3777 | MCL-23-H-3883 | ||
| Not available | POD 5 (3.3 × 104), POD 14 (1.7 × 104), POD 18 (Negative) |
POD 3 (Negative), POD 11 (6.4 × 104), POD 16 (Negative) |
Not available | POD 7 (3.3 × 104), POD 11 (1.3 × 105), POD 18 (Negative) |
POD 4 (Not collected), POD 9 (6.4 × 104), POD 14 (Negative) |
|
| CSF | 23/N2/NIV05/5 | |||||
| Not available | POD 5 (Negative) | Not collected | Not collected | Not collected | Not collected | |
|
||||||
| Anti-Nipah IgM | Not available | POD 5 (0.37) | POD 3 (Negative) | POD 4 (0.38) | POD 7 (0.58) | POD 4 (0.5) |
| Anti-Nipah IgG | Not available | POD 5 (Negative) | POD 3 (Negative) | POD 4 (Negative) | POD 7 (Negative) | POD 4 (Negative) |
|
||||||
| Hemoglobin (11.6–15.5 g/dL) | 14.7 | 11.1 | 14.7 | Not done | 14.3 | |
| Total leukocyte count (4000–10 000/µL) | 3430 | 6100 | 2940 | Not done | 5650 | 4400 |
| Differential count (neutrophils/lymphocytes/monocytes/eosinophils) | 80/14/5/0 | 40/35/8/16 | 80/15/0.3/0 | Not done | 72/21/7/0 | |
| Platelet count (150 000–450 000/µL) | 123 000 | 468 000 | 169 000 | Not done | 103 000 | |
| Serum urea (17–49 mg/dL) | 20 | Not done | 23 | |||
| Serum creatinine (0.6–1.2 mg/dL) | 0.9 | 0.4 | 0.9 | Not done | 0.9 | |
| Serum sodium (136–146 mmol/L) | 130 | 135.3 | Not done | |||
| Serum potassium (3.5–4.5 mEq/L) | 5.25 | 4.3 | Not done | |||
| Serum bilirubin | 0.35 | Not done | ||||
| Aspartate aminotransferase (24–40 U/L) | 38 | 38 | 44 | Not done | ||
| Alanine transaminase (44–80 U/L) | 38 | 70 | 56 | Not done | ||
| Alkaline phosphatase (50–130 U/L] | 60 | 145 | Not done | 65 | ||
| Total bilirubin (0.1–1.2 mg/dL) | 0.35 | 0.5 | Not done | 0.8 | ||
| Direct bilirubin (up to 0.5 mg/dL) | 0.12 | 0.2 | Not done | 0.2 | ||
| Total protein | 6.5 | 7.8 | Not done | 6.7 | ||
| Serum albumin | 3.7 | 4.6 | Not done | 3.4 | ||
| Serum ferritin | 681.9 | Not done | ||||
| Lactate dehydrogenase | 283 | Not done | ||||
| C-Reactive protein | 126 | 52.7 | Not done | 41.4 | ||
| Troponin-T | 62.52 | Not done | ||||
| Random blood sugar (<200 mg/dL) | 105 | 121 | ||||
| Urine routine microscopy | Albumin++ | Not done | ||||
|
||||||
| Chest X-ray | Bilateral consolidation with diffused infiltrates suggestive of acute respiratory distress syndrome | Bilateral consolidation with diffused infiltrates suggestive of acute respiratory distress syndrome | Left lower zone infiltrates suggestive of pneumonia | Not done | Normal | Normal |
|
Succumbed | Recovered | Recovered | Succumbed | Recovered | Recovered |
|
August 30, 2023 | September 29, 2023 | September 29, 2023 | September 11, 2023 | September 29, 2023 | September 29, 2023 |
- a Nipah virus sequences obtained from the clinical specimens.
The primary case remained undiagnosed and created symptomatic family contacts (cases 2 and 3) and hospital contacts (cases 4, 5 and 6). Cases 2 and 3 were admitted to hospital 5 with the presentation of acute respiratory distress with encephalitis and hence provided a high index suspicion for NiV infection (a familiar cluster having contact with an unknown death case with ARDS [case 1]).
3.3.2 Case 2 (Secondary case-family contact)
A 9-year-old boy (son of case 1) was admitted to a tertiary care hospital in Kozhikode (hospital 5) on September 9, 2023, with a history of high-grade fever and cough since September 5 and breathlessness since September 8. He was hypoxic and his chest x-ray showed diffuse bilateral infiltrates suggestive of ARDS. He was initiated on BiPAP and considering the similar complaints and death of his father (case 1) a week back with a similar presentation, NiV infection was suspected. His initial CBC, liver and renal functions were normal. He improved with BiPAP, but on September 11, 2023, he developed seizures and also showed high-grade fever for which he was started on intravenous lorazepam and levetiracetam. He was intubated and mechanically ventilated. His electroencephalogram (EEG) showed mild diffuse cerebral dysfunction with no evidence of non-convulsive status. On September 12, with the confirmation of NiV infection, the child was started on ribavirin therapy (15 mg/kg body weight) every 6 hourly which was continued for 10 days along with broad-spectrum antibiotics. Slowly ARDS improved and ventilator settings were reduced. He reported no further seizures and could move his limbs intermittently. The pupils were equal and reacting. The child also showed evidence of autonomic dysfunction with episodes of hypertension during the hospitalization period. The magnetic resonance imaging (MRI) of the brain showed multiple non-enhancing T2/FLAIR hyperintensities in bilateral cerebral white matter and pons with diffusion restriction. The CSF was negative for Nipah viral RNA but showed lymphocytic pleocytosis with mild elevation of proteins. The child recovered with no neurological deficit and was discharged on September 29 after confirmation of NiV negative in his clinical specimens.
3.3.3 Case 3 (Secondary case-family contact)
A 25-year-old male presented to tertiary care hospital in Kozhikode (hospital 5) on September 10, 2023, with complaints of high-grade fever since September 8, cough with breathlessness, and loose stools with vomiting since September 9. Initially, he took treatment for fever from a local physician and was advised to take a chest x-ray which showed left lower zone infiltrates suggestive of left lobar pneumonia. On admission to hospital 5, he was conscious, oriented, and was maintaining SPO2 of 95%. In view of viral pneumonia and secondary bacterial infection, he was initiated on tab. oseltamivir, broad-spectrum antibiotics, antipyretics, along with nebulization with bronchodilator. His CBC was normal, but his CRP was raised (52.7 mg/L) (Table 1). On September 11, 2023, he confirmed the contacts with case 1 (brother-in-law) and case 2 (nephew), who also had similar presentation. Case 2 was already admitted to the same hospital 5 and was on treatment. The high suspicion of NiV was raised considering the family clustering with similar presentations. On September 12, 2023, NiV infection was confirmed and case 2 was initiated on inj remdesivir with loading dose of 200 mg intravenously and followed with 100 mg maintenance dose for subsequent 12 days. Considering the fever spikes with elevated serum ferritin level (681.9 ng/mL) for the next 2 days, the antibiotic and oseltamivir regimen was also continued. On September 14, he was afebrile but showed minimal elevation of liver enzymes. He was asymptomatic since September 14, and subsequently, all the inflammatory markers also declined. He recovered completely without complications and was discharged on September 29, 2023, with the NiV negative test reports in the clinical specimens.
3.3.4 Case 4 (Secondary case-hospital contact)
A 40-year-old male presented to emergency department of the hospital 5 on September 11, 2023, with acute onset breathlessness and decreased sensorium since the evening of the same day. His relatives gave history of high-grade fever and cough since September 8, 2023. He took initial treatment from peripheral hospitals 2, 3, and 4 on September 8, 10, and 11, respectively. His tropical fever panel was negative and considering the worsening of symptoms, with elevated CRP and liver enzymes and thrombocytopenia/leukopenia, he was referred to hospital 5 for further management (Table 1). On examination, he was febrile, respiratory rate was high (30/min) with SPO2 of 50% and reduced air entry on auscultation. Immediately he was initiated on BiPAP. The pulse was very feeble, and he was disoriented with the Glasgow Coma Scale of 3/25. With persistent hypoxia and features of severe ARDS, he was intubated and mechanically ventilated. With the initiation of intubation, he went into cardiac arrest, and aggressive resuscitation was initiated as per the Advanced Cardiovascular Life Support protocol. He had initial rhythm asystole, and hence crash intubation was done. In spite of all resuscitative efforts, the patient could not be revived and he succumbed to the infection on September 11. Considering severe ARDS and death, the relatives were enquired in depth, and they confirmed exposure (as bystanders) to a similar case (case 1) in hospital 1, 10 days before onset of his symptoms. Later his clinical specimens were confirmed to be NiV positive.
3.3.5 Case 5 (Secondary case-hospital contact)
A 23-year-old male, nursing staff in hospital 1, came in contact with case 1 (primary case) on August 29, 2023. He started developing fever associated with headache, retro-orbital pain, sore throat, and rhinitis on September 6. He was given outpatient consultation and his throat was congested on examination. With the persistent symptoms, the chest x-ray and CBC were done which were found within normal limits, but the CRP (41.4) was raised (Table 1). He was started on tab. oseltamivir in view of viral upper respiratory tract infection. With the confirmation of NiV outbreak on September 12 and being the contact case, he was immediately isolated on the same day in hospital 1, but he was afebrile and symptomatically better. His OPS was found to be positive for NiV and hence he was started on inj remdesivir with loading dose of 200 mg intravenously and followed with a 100 mg maintenance dose for subsequent 12 days. He continued to be asymptomatic from September 13, 2023, but he continued on the remdesivir management. His CRP declined progressively as well and he was discharged with negative Nipah test reports on September 29. He recovered completely with no focal neurological deficit or no bronchopneumonia-like features.
3.3.6 Case 6 (Secondary case-hospital contact)
A 39-year-old male, with no known comorbidities, presented to a tertiary care hospital in Kozhikode (hospital 6) on September 14, 2023, with altered behavior. He had moderate-grade fever, and vomiting since September 11 and on examination, he was conscious, and showed signs of nystagmus and gait ataxia. He had no respiratory distress or seizures with normal vitals and no autonomic fluctuations. Considering the ongoing NiV outbreak, his specimens were screened for NiV and were found positive on September 14. He was initiated on inj remdesivir with loading dose of 200 mg intravenously and followed with a 100 mg maintenance dose for subsequent 12 days. In detailed history, he gave history of being in the emergency department of hospital 1 on August 29, 2023, considering some medical emergency of the family member. He was bystander when case 1 visited the hospital 1 on August 29. His condition progressively improved and he became asymptomatic on September 21 and after negative NiV results in his clinical specimens, he was discharged on September 29.
3.4 Nipah viral RNA kinetics
The clinical specimens of case 2 (OPS, urine, CSF, and serum), case 3 (OPS, urine, and serum), and case 4 (OPS and serum) were collected on September 11, 2023, and tested at Maximum Containment Facility, ICMR-NIV, Pune on September 12, 2023. The OPS samples of case 2 (3.7 × 106 viral RNA copies/mL), case 3 (2.5 × 105 viral RNA copies/mL), and case 4 (1.4 × 107 viral RNA copies/mL) as well as urine sample of case 2 (3.3 × 104 viral RNA copies/mL) were found to be positive for Nipah viral RNA. Whereas the CSF sample of case 2 and urine sample of case 3 were negative for Nipah viral RNA (Table 1; Figure 2). The serum samples were not tested considering the lower volume available and were utilized for ELISA.
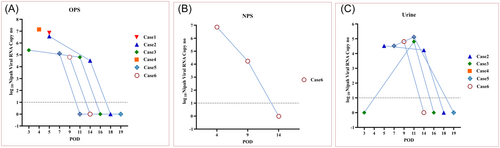
Subsequently, the clinical specimens of case 5 (OPS, urine, and serum) and case 6 (OPS, NPS, and serum) were tested onsite at the MBSL-3 facility on September 13 and September 14, respectively. The OPS (1.3 × 105 viral RNA copies/mL), urine samples (3.3 × 104 viral RNA copies/mL) of case 5 and NPS (7.2 × 106 viral RNA copies/mL) sample of case 6 showed the presence of viral RNA, while OPS and serum samples of case 6 tested negative (Table 1; Figure 2). On September 15, the OPS sample of case 1 (primary case) was retrospectively tested which demonstrated high viral load of 7.2 × 106 viral RNA copies/mL which confirmed the primary case as the index case in this outbreak.
The clinical specimens of four survivors (case 2, case 3, case 5, and case 6) were followed at sequential intervals until it found to be negative for NiV using rRT-PCR. During the first follow-up, the clinical specimens of case 2 (POD 14; OPS [3.3 × 104 viral RNA copies/mL], urine [1.7 × 104 viral RNA copies/mL]); case 3 (POD 11; OPS [6.4 × 104 viral RNA copies/mL], urine [6.4 × 104 viral RNA copies/mL]);case 5 (POD 11; urine [1.3 × 105 viral RNA copies/mL]); and case 6 (POD 9; NPS [1.7 × 104 viral RNA copies/mL], urine [6.4 × 104 viral RNA copies/mL]) showed the presence of Nipah viral RNA (Table 1; Figure 2). While OPS samples of cases 5 and 6 were negative for Nipah. The consequent follow-up specimens of case 2 (OPS, urine), case 3 (OPS, urine), case 5 (OPS, urine), and case 6 (OPS, NPS, urine) were collected on POD 18, 16, 19, and 14, respectively, and found to be negative. The clinical specimens of the Nipah cases showed the presence of Nipah viral RNA until POD 14 with viral load in the range of 1.7 × 104 to 3.3 × 104 viral RNA copies/mL. The OPS samples (cases 2, 3, and 5), urine samples (case 2), and NPS samples (case 6) demonstrated declining viral load at the first follow-up (Table 1; Figure 2). Apparently, case 3 didn't show any viral RNA in the initial screening of the urine sample and had a viral load of 6.4 × 104 viral RNA copies/mL at POD 11 (Table 1; Figure 2). Case 5 showed an increasing viral load in the follow-up urine samples than initial screening. The variation in the viral load in the urine samples at different sampling intervals probably suggests NiV shedding at different time points.
3.5 Anti-Nipah IgM and IgG ELISA
The anti-Nipah IgM antibodies were detected in four out of five eligible cases with optical density (OD) value of 0.37–0.58 between 3 and 7 POD. Anti-Nipah IgG could not be detected until POD 7 (Table 1).
3.6 Genomic characterization and phylogenetic analysis
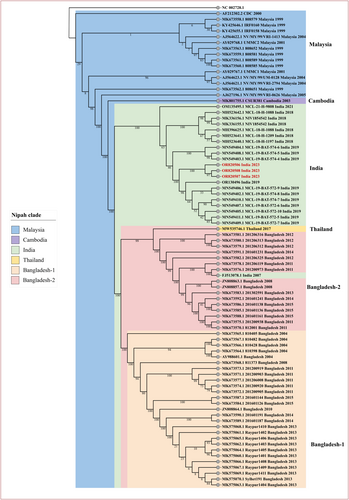
All the positive clinical specimens of the six cases were processed for WGS, but only three OPS specimens from case 1 (MCL-23-H-3874; accession ID: OR820506), case 2 (MCL-23-H-3764; accession ID: OR820507), and case 4 (MCL-23-H-3762; accession ID: OR820508), retrieved the NiV genomes ranging from 96% to 99.8% (17 539–18 210 base pairs). The three retrieved genome sequences from the recent NiV outbreak 2023 (highlighted in red in Figure 3), along with 18 other genome sequences from India (n = 21) were used for phylogenetic analysis. The phylogenetic tree also included the genomes from Bangladesh Clade I (n = 27), Bangladesh Clade II (n = 17), Thailand (n = 1), Cambodia (n = 1), Malaysia (n = 15) and mapped with the reference sequence (NC 002728.1) (Figure 3). The current outbreak sequences under this study belonged to the Indian Clade and the sequences were placed between the 2019 outbreak Indian sequences and matched with the sequences from the 2018 to 2019 Indian NiV outbreak (Figure 3). Percentage nucleotide identity (PNI) and percentage amino acid identity (PAI) were calculated using the aligned mega file as detailed in Supporting Information: Table 1. The sequences from the current outbreak exhibit PNI values of 97.25%, 99.35%, 99.29%, and 99.12% when compared to 2007, 2018, 2019, and 2021 previously retrieved Indian sequences, respectively. Additionally, the current sequences also demonstrate PAI values of 99.45%, 99.45%, 99.48%, and 99.36% when compared to the 2007, 2018, 2019, and 2021 previously retrieved Indian sequences, respectively.
3.7 Enhanced surveillance, contact tracing, and testing
After the declaration of NiV outbreak on September 12, 2023, the Kerala state government imposed lockdown and containment zones in neighbouring nine wards along with the epicentre ward in Kozhikode district. The other neighbouring districts were also set on high alert. The close contacts of the NiV confirmed cases were grouped into primary and secondary contacts and further into high-risk and low-risk contacts.10, 12, 15, 16 All the symptomatic contacts (n = 35; high risk-30, low risk-05) were isolated in the isolation centre and asymptomatic contacts (n = 218; high risk-123, low risk-95) were home quarantined. The non-epidemiologically linked suspected cases (n = 130) were also isolated and screened for NiV infection. The clinical specimens (OPS, NPS, serum, and urine) of the suspected cases along with the high/low risk symptomatic and asymptomatic contacts were screened and found negative for NiV by rRT-PCR and anti-Nipah IgM and IgG antibodies. Of all tested, the positivity was observed for respiratory syncytial virus (n = 12), Influenza B (n = 1), Influenza A H3N2 (n = 1), and human metapneumovirus (n = 1). While cases with acute encephalitis syndrome were tested negative for Japanese encephalitis, West Nile, and Chandipura viruses.
Besides human surveillance, ICMR–NIV, Pune has also conducted surveillance in the bat population within the 60-km range from epicenter of the outbreak. In the current outbreak, we could not detect the Nipah viral RNA from the secretions of bat specimens (0/111), but we could detect the viral RNA in the visceral organs of 3 bats (25% [3/12]) from Wayanad district. While the seropositivity in bat was 28.18% (31/110) from Kozhikode and Wayanad districts.23 The roosting colonies of these bats near the human population could be the probable reason for the spillover and current Nipah outbreak.
4 DISCUSSION
A highly pathogenic nature and rapid spread of NiV through spillover followed by human-to-human transmission pose a potential public health threat. Fruit bats are known to carry NiV without showing any symptoms. Many people in Kerala live in close proximity to fruit bat habitats, such as forests and agricultural areas.
This study highlights the vigilance of the health system to detect the clustering of the cases with clinical presentations similar to NiV. This led to early detection and containment activities with lesser Nipah-confirmed cases than observed in the 2018 outbreak.8, 9 The clinical management was very intensive and started during the early phase of infection (between POD 3 and 7). Also, for the first time, remdesevir treatment was given to the Nipah-confirmed patients. The Nipah viral RNA at the time of detection of infection in fatal cases 1 and 4 were 7.2 × 106 and 1.4 × 107, respectively, which were higher than the viral RNA load in the recovered cases 2, 3, 5, and 6. The CFR was significantly lower this time (two deaths of six cases; 33.33%) than observed in 2001 (CFR 68%), 2007 (CFR 100%), and 2018 (CFR 89%) NiV outbreaks in India.4, 5, 8, 9 The early initiation of enhanced supportive care could be the major reason for higher survival of the cases.
It was also noteworthy that most of the cases were detected during early phases of onset of symptoms (POD 3–7) in all secondary cases as against previous outbreaks in 2018, 2019, and 2021, where NiV could be confirmed after 10 POD.8, 10, 12, 24, 25 Most importantly, this emphasizes that Kerala has a well-developed public health system, which has been effective in containing NiV outbreaks. However, this also means that Kerala is more likely to detect and report NiV cases than other states in India.
In this outbreak, three cases (cases 1, 2, and 4) landed up into ARDS and cases 1 and 4 succumbed to infection. Case 2 also had seizures, while case 6 had acute encephalitis syndrome (AES)-like features including altered sensorium, and gait ataxia. Fever was a persistent finding in all cases. Cases 3 and 5 showed less severe symptoms with early clinical recovery. None of the recovered cases (n = 4) had any residual respiratory or neurological deficit. The mixed clinical presentations of AES and ARDS were similar to the outbreaks reported in 2001 and 2018 in India.4, 8, 22 It becomes challenging for clinicians to differentiate Nipah cases on clinical examination unless other symptoms of autonomous system, myoclonus, myocarditis, and typical T2 flair hyperintensities on brain computed tomography (CT) and MRI were also observed.10, 24-27
Interestingly, CSF specimen of case 2 and serum specimen of case 6 were negative for Nipah viral RNA and we have observed that OPS/NPS and urine were the better clinical specimens for the diagnosis of NiV infection with higher viral load. These specimens also showed the presence of viral RNA for longer duration until the 14th POD. This emphasizes that chances of missing the cases if they present late to the health care system are reduced significantly if specimens (OPS/NPS and urine) are tested. Similarly, the Nipah viral RNA was also detected until the 15th POD in urine specimen of the survived index case of the 2019 outbreak.10
It was noteworthy that all the Indian NiV sequences, except for the 2007, exhibit a higher degree of similarity with each other ranging from 99.12% to 99.55% PNI and are clustered in the India clade.9, 11, 12
Despite the measures taken by the Kerala State government, there is still the risk of future NiV outbreaks. Virus is present in its natural reservoir (bats) and may be maintained in an epizootic cycle in parts of Kerala with the risk of spill-overs. Anthropological exploration could identify the potential risk factors of NiV spillover. Community engagement is essential, involving an in-depth study of local cultural practices, beliefs, food consumption practices, and behaviors relating to possible contact with bats. It is also imperative to understand the role of livelihood, agricultural, and animal husbandry practices, that can contribute to the spillover event.
The best way to prevent NiV infection is to avoid contact with infected bats and to reduce the activities that could lead to the potential spillover. The community should also be aware of the symptoms of NiV infection and seek medical attention immediately if they develop any of these symptoms. Early detection, isolation of cases, and public education are keys to preventing and controlling outbreaks. In conclusion, it is crucial for government, healthcare systems, and international organizations to work together to implement these measures effectively in regions where NiV is a concern. Effective prevention and control strategies, including reducing human-bat interactions and early case identification, are crucial in curbing the spread of NiV.
AUTHOR CONTRIBUTIONS
PDY, CR, NG, and SG supervised and coordinated the outbreak response. AKAS, CR, SP, SK, SM, GR, SV, DE, NV, and JKP were involved in patient care and management. RRS, DYP, AB, KSPS, UPT, Sk, and SS established the field MBSL-3 laboratory and testing. PDY, AMS, RRS, DYP, SM, and LSR supervised the laboratory investigations. LVR, RKJ, TM, SPR, SPT, A, TSA, RK, AR, MA, SCK, PC, BV, and RrS were involved in contact tracing and coordination of outbreak response. APM, NKP, KG, PRN, AM, and NH provided laboratory support during the outbreak. NA, SMP, and TM provided administrative support for outbreak responses. PDY, AKAS, RRS, CR, SP, DYP, AMS, AB, KSPS, UPT, SK, SS, SM, and LR contributed to data collection, interpretation, writing, and critical review. PDY, AKAS, RRS, CR, SP, DYP, and AMS contributed to the critical review and finalization of the paper. All the authors have contributed equally in reviewing and editing the final manuscript.
ACKNOWLEDGMENTS
The authors take this opportunity to convey thanks and appreciation to all individuals who have contributed immensely toward the Nipah virus outbreak response and containment measures in Kozhikode district, Kerala state, India during September 2023. The authors extend sincere gratitude to Smt. Veena George (Hon'ble Minister for Health and Family Welfare, Kerala), for her leadership and coordination of the Nipah Virus disease control activities, and Shri. Mohammed Hanish, Principal Secretary for Health (Kerala) in streamlining the public health responses. The authors are thankful to Dr. Rajiv Bahl, Secretary of the Department of Health Research and the Director General, Indian Council of Medical Research (ICMR), New Delhi for his constant support, guidance, and motivation. The authors would like to thank the members of the district rapid response team, including the team from the State Mission Director of National Health Mission (Shri. Jeevan Babu); Dr. KV Nandakumar, Additional Director of Health Services (Kerala) and Smt. A Geetha, District Collector Kozhikode district for their support in logistics and contact tracing. The authors are also thankful to the District Program Manager—Dr. Sameesha Saidalavi [from Wayanad] for the coordination and support. The authors also would like to thank family members of the Nipah survival and deceased cases for cooperating with the investigation. The sincere and diligent work put in by all members of the technical staff deputed in the field for laboratory testing at Mobile BSL-3 facility from ICMR-NIV, Pune and ICMR-NIV, Kerala unit including Mr. Ratnadeep More, Mr. Rameshwar Khedekar, Mr. Deepak Mali, Mr. Jijo Koshy, Mr. Dinesh Kumar Singh, Mr. Sunil Shelkande, Mr. Hitesh Dighe, and Mrs. Najiya KV were key for the smooth laboratory testing during the outbreak responses. We also acknowledge the excellent technical support from Dr. Rajlaxmi Jain, Mrs. Triparna Majumdar, Mrs. Savita Yadav, Mr. Yash Joshi, Ms. Pranita Gawande, Mrs. Pratiksha Vedpathak, Ms. Vaishnavi Kumari, Ms. Priya Wadhwaniya, Mr. Ganesh Chopade, Mr. Sanjay Thorat, and Mr. Madhav Acharya for the laboratory diagnosis and data management. We extend sincere thanks to the support for the laboratory diagnosis and sample logistic support from the technical team Mr. Akash NP, Mrs. Saritha Sivadas, Ms. Sindhumol U, Ms. Ayisha Jabin P, Ms. Jobisha Merlin, Ms. Fida Gafoor C, Ms. Hiba Said M, Ms. Arya Raveendran Y, Ms. Ayisha Namra Rameez, Mr. Sumesh P, Mr. Athul A, Mr. Navas Shareef KK, and Ms. Suriya A from Microbiology Department and Regional Virus Research and Diagnostic Laboratory at GMC Kozhikode. Authors extend gratitude to the laboratory diagnosis support provided by the heads of departments: Dr. Varsha Potdar, National Influenza Center; Dr. Vijay Bondre, Encephalitis; and Dr. Mallika Lavania, Enteric viruses, from ICMR-NIV, Pune. The grant was provided by the Indian Council of Medical Research, New Delhi, India under the extramural project “Sustainable laboratory network for monitoring of Viral Hemorrhagic Fever viruses in India and enhancing bio-risk mitigation for High-risk group pathogen” with the grant number: VIR/28/2020/ECD-1 dated 10.05.2023.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Institutional Human Ethics Committee of ICMR-NIV, Pune, India under the project “Sustainable laboratory network for monitoring of Viral Hemorrhagic Fever viruses in India and enhancing bio-risk mitigation for High risk group pathogens” (NIV/IEC/March/2021/D-9 dated April 9, 2021).
Open Research
DATA AVAILABILITY STATEMENT
All the data related to this study have been included in the manuscript and available on the public repository GenBank NCBI.




