The kinetics of Torque Teno virus plasma load following calcineurin inhibitor dose change in kidney transplant recipients
Abstract
Torque Teno virus (TTV) is nonpathogenic, highly prevalent, and reflects the immune status of its host. Thus, TTV plasma load was suggested for the guidance of immunosuppression post solid organ transplantation. The present study was designed to determine the kinetics of TTV following changes in calcineurin inhibitor (CNI) dose. A total of 48 adult recipients of a kidney graft transplanted at the Medical University of Vienna between 2018 and 2019 with isolated changes in CNI dose were selected from the prospective TTV-POET trial. TTV plasma load was quantified by in-house PCR. At Day 30 following CNI dose adaptation (median 33% of daily dose) no changes in TTV load were noted. However, at Day 60, following CNI dose reduction a lower TTV load of 6.4 log10 c/mL (median; interquartile range [IQR] 4.9–8.1) compared with the baseline of 7.1 log10 c/mL (IQR 5.3–8.9) was noted (p = 0.001); there was also a trend toward a higher TTV load following CNI increase (6.6 log10 c/mL, IQR 4.1–9.7 vs. 5.2 log10 c/mL, IQR 4.5–6.8; p = 0.09). The data suggested that TTV load changes become noticeable only 2 months after CNI dose adaptation, which might be the ideal time point for TTV load monitoring.
Abbreviations
-
- c/ml
-
- copies per milliliter
-
- CMV
-
- cytomegalovirus
-
- CNI
-
- calcineurin inhibitor
-
- IQR
-
- interquartile range
-
- MPA
-
- mycophenolic acid
-
- TTV
-
- Torque Teno virus
1 INTRODUCTION
Kidney transplantation is the treatment of choice for end-stage renal disease. However, life-long immunosuppression is needed to reduce the risk of organ rejection. Despite this benefit, the compromised immune competence of the graft recipient leads to an increased risk for infectious and oncologic disease. Moreover, current immunosuppressive regimens are unable to sufficiently control the allorecognition of the graft in some patients, which may lead to chronic rejection. Thus, the optimal management of immunosuppressive drug dosing requires a delicate balance between inadequate and excessive immunosuppression.
Monitoring the Torque Teno virus (TTV) is a promising new strategy for quantifying immune function. TTV can be detected in up to 90% of healthy individuals and has not been linked to any human disease. The prevalence of TTV in immunocompromised patients after transplantation is up to 100% and the virus is unaffected by conventional antiviral drug therapy used in the posttransplantation setting.1 The TTV load is directly associated with the amount and type of immunosuppressive drugs administered to the transplant recipient and is thus indirectly associated with graft rejection and infectious disease.2-5
Currently, three randomized controlled trials are recruiting over 500 kidney and lung transplant recipients in seven European countries to test the value of TTV-guided immunosuppression, with results expected from 2024 onwards.6-8 Until then, TTV load cut-off values for the risk stratification of graft rejection and infections based on observational studies might be applied for routine post-transplant care.9-11
To utilize TTV for immune monitoring following solid organ transplantation in clinical practice, it is essential to gain knowledge about the kinetics of TTV load following changes in immunosuppression. In the post-transplant phase immunosuppression is mainly adapted via calcineurin inhibitors (CNI) dose changes. However, no study has analyzed the kinetics of TTV following isolated dose changes in CNI thus far. The present study was designed to fill this knowledge gap.
2 MATERIALS AND METHODS
2.1 Patient cohort and study design
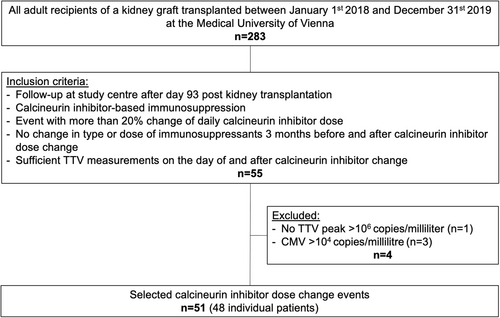
The present study is a retrospective analysis of the prospective TTV-POET trial (approval number of the local institutional review board: 1785/2016; trial registration number of the German Clinical Trials Registry: DRKS00012335). For the present study, all consecutive adult (≥18 years of age) recipients of a kidney allograft transplanted between January 1, 2018, and December 31, 2019, at the Medical University of Vienna, Austria, were screened. Follow-up was performed for up to 3.5 years posttransplant. The predefined inclusion criteria were follow-up at the outpatient clinic of the Medical University of Vienna after Day 93 posttransplantation (rationale: TTV kinetics stabilize in Month 4 posttransplant), CNI-based immunosuppression (tacrolimus or cyclosporine), isolated dose adaptation of the CNI of more than 20% of the total daily dose, no change in type or dose of immunosuppression for a minimum of 3 months before and after the CNI dose adaptation (i.e., unchanged dose of prednisolone and mycophenolate (MPA), no CNI dose change apart from analyzed CNI change), and at least one plasma TTV quantification at a minimum of 15 days and a maximum of 75 days after the CNI dose adaptation. The predefined exclusion criteria were scenarios in which TTV load might not reliably reflect immunosuppression. These were a plasma cytomegalovirus (CMV) load above 104 copies/milliliter (c/mL) or a leukocyte count below 1 G/L at the time of TTV assessment, and patients who never showed a TTV load above 106 c/mL since transplantation. Changes in CNI were performed to reach the CNI through level target of 5–7 ng/mL for tacrolimus and 50–100 ng/mL for cyclosporine A.
2.2 TTV quantification
TTV PCR results at the day of CNI dose adaptation (range −10 to +1 days), 30 days after the dose adaptation (±15 days) and at Day 60 after the dose adaptation (±15 days) were analyzed. TTV loads were measured in 200 µL of plasma by TaqMan real-time PCR as described previously.12 TTV DNA level was quantified in the linear range from 103 to 1011 c/mL. The limit of detection was 103 c/mL. A detailed description of the nucleic acid extraction method and real-time PCR is provided in the Supporting Information.
2.3 Statistical analysis
For binary data, absolute numbers and frequencies in percentages were calculated. Parametric continuous data were summarized by mean and standard deviation and nonparametric continuous data by median and first and third quartiles. Differences of baseline characteristics between groups were calculated using Pearson's χ2 test for categorical variables and Mann–Whitney U Test for continuous variables. The predefined primary outcome of the study was the change in TTV load following CNI dose adaptation at Day 60. The Wilcoxon matched-pairs signed-rank test was used to compare TTV load between Day 0 and Days 30 and 60 following CNI dose adaptation. A p value of 0.05 was the predefined limit of significance. STATA 15 (STAT Cooperation) and SPSS version 23 were applied for data analyses.
3 RESULTS
3.1 Study cohort
A total of 283 patients received a kidney transplant at the Medical University of Vienna between January 1, 2018 and December 31, 2019. Applying the predefined inclusion and exclusion criteria a total of 48 patients with 51 isolated CNI dose adaptations were included in the present analysis. The study flow is detailed in Figure 1. Baseline characteristics for the total cohort of patients transplanted during the screening phase and the study cohort are detailed in Table 1. Within the study cohort (n = 48), the median recipient age at transplantation was 57 years, 29% were female, 19% had a history of prior kidney transplantation, 92% received a kidney from a deceased donor, and 6% had preformed donor-specific antibodies (antibodies directed against donor human leukocyte antigens). There was no major difference in baseline characteristics between the total cohort and the study cohort.
| Total cohorta (n = 235) | Study cohort (n = 48) | p Value | |||
|---|---|---|---|---|---|
| Recipient characteristics | |||||
| Age; years, median (IQR) | 57 | (47–64) | 57 | (44–64) | 0.986 |
| Female sex; n (%) | 83 | (34) | 14 | (29) | 0.505 |
| Cause of end-stage renal disease | 0.700 | ||||
| Immunologic; n (%) | 47 | (20) | 7 | (15) | |
| Cystic kidney disease; n (%) | 35 | (15) | 8 | (17) | |
| Diabetes; n (%) | 23 | (10) | 6 | (13) | |
| Hypertension; n (%) | 23 | (10) | 3 | (6) | |
| Hereditary; n (%) | 10 | (4) | 1 | (2) | |
| Other; n (%) | 37 | (16) | 7 | (15) | |
| Undefined cause; n (%) | 60 | (26) | 16 | (33) | |
| Total time of renal replacement therapy; years, median (IQR) | 3.6 | (1.9–5.5) | 4.0 | (2.7–12.3) | 0.033 |
| Donor characteristics | |||||
| Deceased donor; n (%) | 201 | (86) | 44 | (92) | 0.504 |
| Donation after circulatory death; n (%) | 32 | (14) | 8 | (17) | 0.579 |
| Donor age; years, median (IQR) | 55 | (44–67) | 54 | (45–64) | 0.775 |
| Female donor; n (%) | 107 | (46) | 22 | (46) | 0.847 |
| Transplant characteristics | |||||
| Pre-emptive transplantation; n (%) | 29 | (12) | 5 | (10) | 0.812 |
| Retransplantation; n (%) | 35 | (15) | 9 | (19) | 0.463 |
| ABO-incompatible transplantation; n (%) | 7 | (3) | 0 | (0) | 0.387 |
| HLA-A/B/DR mismatch; N, median (IQR) | 3 | (2–4) | 3 | (2–4) | 0.363 |
| Donor-specific antibody; n (%) | 24 | (10) | 3 | (6) | 0.739 |
| Induction with ATG | 22 | (9) | 3 | (4) | 0.822 |
| Induction with IL 2 receptor blocker | 203 | (86) | 44 | (92) | 0.579 |
| Peri-transplant immunoapheresis | 24 | (10) | 3 | (6) | 0.123 |
| Cold ischemia time; hours, median (IQR) | 14 | (9–19) | 14 | (9–18) | 0.650 |
| Delayed graft functionb; n (%) | 59 | (25) | 16 | (33) | 0.460 |
- Abbreviations: ATG, anti-thymocyte globulin; HLA, human leukocyte antigen; IQR, interquartile range; IL 2, interleukin 2; N/n, number, TTV, Torque Teno virus.
- a Study cohort is excluded.
- b Delayed graft function was defined by the necessity for >1 renal replacement therapy posttransplantation.
3.2 CNI dose adaptation
Adjustments of CNI dose were performed at a median of 365 days (interquartile range, IQR 256-649) posttransplant. Clinical data from the day of dose adjustment are presented in Table 2. Almost all patients (96%) received triple immunosuppression based on CNI, MPA and corticosteroids (one patient received Azathioprine and one patient received no antimetabolite). Most participants (n = 47) received tacrolimus-based immunosuppression with a daily median dose of 3 mg (IQR 2–4; trough level 8.4 ng/mL, IQR 4.3–10.1), and only one patient received cyclosporine A. A total of 17 of the analyzed changes in CNI dose were dose increases, with a median change of 1 mg tacrolimus per day (IQR 1–1; 50% of the daily dose), and 34 of the analyzed changes were decreases, with a median change of 1 mg tacrolimus per day (IQR 1–1; 33% of the daily dose). Following CNI dose increase, the median tacrolimus trough level changed from 4.2 ng/mL (IQR 3.2–4.4) at baseline (Day 0) to 6.5 ng/mL (IQR 5.8–6.9) at Day 30 and 6.7 ng/mL (IQR 5.5–7.2) at Day 60 (Figure 2, Supporting Information: Table 1). Median tacrolimus changes from 9.7 ng/mL at baseline (Day 0; IQR 8.3–10.7) to 5.8 ng/mL (IQR 4.8–7.1) at Day 30 and 6.2 ng/mL (IQR 5.5–7.8) at Day 60 were noted following CNI dose decrease.
| Clinical variable | Median | IQR |
|---|---|---|
| Time after TX; days | 365 | (256–649) |
| Creatinine; mg/dL | 1.5 | (1.2–1.8) |
| eGFRa; mL/min | 43 | (36–50) |
| Leukocytes; G/L | 6.2 | (5–7.8) |
| Lymphocytes; G/L | 1.5 | (1.2–2) |
| Daily MPA dose; mg | 2000 | (1000–2000) |
| Daily tacrolimus dose; mg | 3 | (2–4) |
| Tacrolimus level; ng/mL | 8.4 | (4.3–10.1) |
| Prednisolone dose; mg/day | 5 | (5–5) |
| CMV plasma load,b c/mL | 124 | (53–244) |
- Abbreviations: CMV, cytomegalovirus; eGFR, estimated glomerular filtration rate; MPA, mycophenolic acid; TX, transplantation.
- a Calculated by the abbreviated modification of Diet in Renal Disease equation.
- b The CMV plasma load at events with a positive CMV-PCR (8 of 51) is reported.
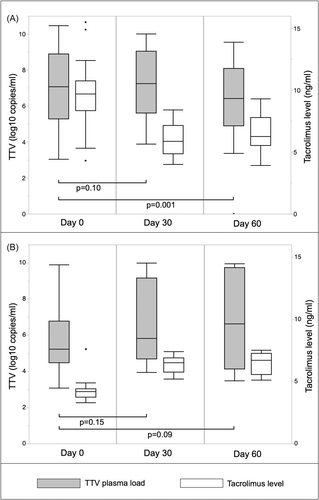
3.3 Torque Teno virus kinetics
The median baseline TTV load was 6.4 log10 c/mL (IQR 5.0–8.7) in the total study cohort, 7.1 log10 c/mL (IQR 5.3–8.9) in patients with a CNI decrease (n = 34) and 5.2 log10 c/mL (IQR 4.5–6.8) in patients with a CNI increase (n = 17) (Figure 2; Supporting Information: Table 1). At Day 30 (median 30; IQR 20–40), TTV load was unchanged, being 7.2 log10 c/m; (IQR 5.6–9.0; p = 0.1, n = 23) following drug decrease and 5.8 log10 c/mL (IQR 4.7–9.2; p = 0.15, n = 14) following increase. However, at Day 60 (median 57; IQR 54–68), patients with a CNI dose reduction showed a lower TTV load of 6.4 log10 c/mL (IQR 4.9–8.1; p = 0.001, n = 21), while there was a trend toward higher TTV load in patients with a drug dose increase 6.6 log10 c/mL (IQR 4.1–9.7; p = 0.09, n = 12). No significant CMV viremia was observed during the follow-up (Supporting Information: Table 1).
4 DISCUSSION
This is the first report to describe the magnitude and kinetics of plasma TTV load following isolated CNI dose modifications in kidney transplant recipients. Its main finding was, that the TTV c/mL changed by one log level following a 33% adaptation in daily CNI dose with a delay of 2 months. Given the increasing interest in the TTV-guided adaptation of immunosuppression in solid organ transplantation, this finding has significant clinical implications. This work suggests that a CNI adaptation of one-third of the daily dose is detectable by TTV monitoring and that TTV load should be monitored for at least 2 months to capture the full effect of CNI adaptation on TTV load. Our work might also have impact on planned interventional clinical trials on TTV-guided immunosuppression.6-8
A significant reduction in TTV load following a CNI dose decrease was observed, whereas the rise in TTV load following a CNI dose increase did not reach the predefined level of statistical significance. Of note, tacrolimus levels in both groups exhibited anticipated kinetics, with a significant increase or decrease by Day 30 and a stable trajectory at Day 60 and both groups showed a similar effect size of about one log level in TTV c/mL change following CNI adaptation. Therefore, the small sample size of the group of patients with CNI increase and the consequent low power might have led to a false negative statistical test result (beta error). Alternatively, the lower absolute CNI level change noted in the group with CNI increase compared with the group with a CNI decrease (2.3 vs. 3.9 ng/mL at Day 30) might account for the lack of significance.
So far, no studies have been published on plasma TTV kinetics following isolated CNI changes in solid organ transplantation. However, two studies described TTV load changes in kidney transplant patients following a pause in MPA administration.13, 14 Both, Benning et al. and Regele et al., reported a decrease of about half a log level in TTV load following a pause in MPA administration lasting 2–4 weeks. The state of equilibrium of the TTV load was achieved 6–8 weeks after modifications of the MPA dosage. TTV kinetics following CNI changes in the current study also showed its full effect only after 2 months and published literature has consistently described the TTV load to peak 3 months after the initiation of immunosuppression in solid organ transplantation.11 In this respect, it is interesting to note, that clearance of BK polyomavirus is seen only after 2–6 months postadaption in immunosuppression.15, 16 Given the high replication rate of TTV, further studies addressing the immunologic control of the TTV infection are needed to understand this delay.17
The major limitations of this study is its single-center design and the small sample size. Furthermore, the lack of TTV measurements at later time points following CNI adaptation prevented an analysis of the extended trajectory of TTV kinetics. The analysis was restricted to a minimum of 20% change in daily CNI dose. Adjustment for confounders of the association between TTV load and CNI dose adaption has not been performed. However, the application of the Wilcoxon matched-pairs signed-rank test to compare TTV load in individuals at Day 0, 30, and 60 eliminates the possibility of confounding due to these variables, because they do not change over time (host sex and age), change in a similar way in both groups (time since transplantation) or do not change (immunosuppressive therapy, except CNI). The present findings might not be applicable to scenarios excluded from the analysis.
Taken together, this study demonstrates that changes in TTV load are expected to peak not earlier than 2 months following CNI dose adaptations. This finding, in conjunction with previously published data on MPA pause, recommends the interpretation of TTV load not earlier than 2 months after changes in immunosuppression. Ongoing interventional trials will provide further insight into the dynamics of TTV load following adaptation of immunosuppression.6-8
AUTHOR CONTRIBUTIONS
Florina Regele: Conceptualization; investigation; data curation; methodology; visualization; writing—original draft. Frederik Haupenthal: Conceptualization; data curation; investigation; project administration; writing—review and editing. Konstantin Doberer: Conceptualization; data curation; writing—review and editing. Irene Görzer: Investigation; writing—review and editing. Sebastian Kapps: Data curation; writing—review and editing. Robert Strassl: Data curation; writing—review and editing. Gregor Bond: Conceptualization; data curation; formal analysis; funding acquisition; investigation; project administration; resources; supervision; validation; visualization; writing—original draft.
ACKNOWLEDGMENTS
This work was funded by the European Union's Horizon 2020 research and innovation program under grant agreement number 896932 (TTVguideTX project consortium; consortium lead: Medical University of Vienna, Gregor Bond).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




