Clinical characteristics and immunogenicity after Omicron breakthrough infection in patients with chronic hepatitis B infection: A longitudinal observational study
Guanhua Zha and Zhiwei Chen contributed equally to this work as co-first authors.
Abstract
The clinical and immunological features after breakthrough infection (BTI) during Omicron wave in patients with chronic hepatitis B virus infection (CHB) are still unclear. A total of 101 patients with CHB from our previous coronavirus disease 2019 (COVID-19) vaccination cohort (NCT05007665), were continued to be followed up at the Second Affiliated Hospital of Chongqing Medical University after BTI, while an additional 39 healthcare workers after BTI were recruited as healthy controls (HCs). Clinical data were collected using questionnaire survey and electronic medical record. Blood samples were used to determine the antibody responses, as well as B and T cell responses. After BTI, the clinical symptoms of COVID-19 were mild to moderate in patients with CHB, with a median duration of 5 days. Compared with HCs, patients with CHB were more susceptible to develop moderate COVID-19. The liver function was not significantly damaged, and HBV-DNA was not activated in patients with CHB after BTI. Patients with CHB could elicit robust antibody responses after BTI (NAbs 13.0-fold, BA.5 IgG: 24.2-fold, respectively), which was also significantly higher than that in every period after vaccination (all p < 0.001), and compared to that in HCs after BTI. The CD4+, cTfh, and CD8+ T cell responses were also augmented in patients with CHB after BTI, while exhibiting comparability to those observed in HCs. In patients with CHB after BTI, the immune imprint was observed in B cell responses, rather than in T cell responses. In conclusion, Omicron breakthrough infection induced mild to moderate COVID-19 symptoms in patients with CHB, without exacerbating the progress of liver diseases. Meanwhile, BTI demonstrated the ability to induce robust antibody and T cell responses in patients with CHB, which was comparable to those observed in HCs.
1 INTRODUCTION
Since the first case of Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported in South Africa in December 2021, Omicron variant has rapidly become the predominate sub-lineage of coronavirus disease 2019 (COVID-19) globally.1, 2 Omicron contains a large number of previously confirmed mutations in other variants (such as Alpha, Beta, Gamma, or Delta). Compared to the 16 mutations present in the already highly infectious Delta version, the Omicron variant includes at least 32 mutations in the spike protein alone, as well as 15 mutations in the receptor binding domain (RBD), which leads to its strong immune escape and infectivity.3-5 As of November 16, 2023, the cumulative number of confirmed COVID-19 cases worldwide had exceeded 770 million, resulting in more than 6.9 million deaths.6 Although vaccination can prevent infection, hospitalization, and severe illness of COVID-19, its protection against Omicron variant transmission is poor,7, 8 this is termed as Omicron breakthrough infection (BTI). Robust immune responses against Omicron after BTI have been observed in the general population9; however, the immune responses in patients with underlying diseases are still unclear.
Patients with chronic liver disease have a higher risk of infection and worse outcomes from COVID-19 than do individuals without liver disease.10, 11 However, clinical studies have demonstrated that chronic hepatitis B virus infection (CHB) does not necessarily predispose patients with COVID-19 to more severe outcomes.12, 13 These controversial conclusions suggest the need for further study of the interaction between HBV and SARS-CoV-2 co-infection. Our previous studies revealed that antibody response to primary inactivated vaccines was lower in patients with CHB than in healthy controls (HCs); booster vaccination could improve the situation.14-16 In addition, the booster vaccination to patients with CHB was well-tolerated. However, whether Omicron BTI induces a stronger antibody response and exacerbates liver disease progression in patients with CHB is currently unknown.
Since China lifted the epidemic isolation policy in December 2022, a nationally widespread Omicron infection occurred.17, 18 This provided an opportunity for us to study the impact of Omicron BTI on patients with CHB and the immune response in these patients after BTI. Based on our previous prospective, observational cohort (NCT05007665), we continued follow-up of 101 patients with CHB, vaccinated previously with inactivated vaccines (CoronaVac and BBIBP-CorV) or recombinant SARS-CoV-2 protein vaccines (ZF2001, uses a dimeric receptor-binding domain [RBD] as the antigen). Dynamic changes of antibodies against two different strains of SARS-CoV-2 (Wild strain [WT] and BA.5 strain) were assessed at multiple time points after vaccination and/or after BTI. Moreover, B and T cells responses against these two different SARS-CoV-2 strains in patients with CHB before and after BTI were also determined.
2 MATERIALS AND METHODS
2.1 Study cohort and design
All patients with CHB were derived from our previous prospective observational cohort (NCT05007665) and continued to be followed up. In brief, the cohort was recruited in the Second Affiliated Hospital of Chongqing Medical University in May 2021, with the aim to evaluate the safety and immunogenicity after COVID-19 vaccination in patients with CHB.14, 16 Overall, 101 patients with CHB underwent BTI since December 1, 2022, which was confirmed using positive nucleic acid and/or antigen detection. Subsequently, these patients were followed up at 3 and 6 months after BTI. At each visit, serum samples were obtained for laboratory testing and assessment of antibody responses. Peripheral blood mononuclear cells were used for the evaluation of SARS-CoV-2-specific B cell and T cell responses. Meanwhile, 39 healthcare workers from the Second Affiliated Hospital of Chongqing Medical University, after undergoing BTI during the same period were recruited as HCs. The symptoms of COVID-19 and clinical characteristics were collected from all the participants using a questionnaire. Type assessment of COVID-19 was defined according to the guidelines for diagnosis and treatment of COVID-19 (10th edition) in China.19 All participants were considered as having no SARS-CoV-2 infection before the BTI, in accordance with regular negative results of nucleic acid and/or antigen detection tests and very few COVID-19 cases in Chongqing. The phase of CHB natural history was defined according to the “EASL 2017 Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection.”20 This study was approved by the ethics committee of The Second Affiliated Hospital of Chongqing Medical University and conformed to the ethical guidelines of the Declaration of Helsinki. All individuals provided written informed consent before participating in the study.
2.2 Measurement of neutralizing antibodies and Omicron spike-specific IgG titer
Capture chemiluminescence immunoassays were used to detect neutralizing antibodies (NAbs) in the serum samples using MAGLUMI X8 (Snibe). The sensitivity and specificity of the kit (45421121401; Snibe) for NAbs are 100% and 100%, respectively. IgG against Omicron BA.5 variant was detected using ELISA (Sino Biological Inc) following the manufacturer's instructions.
2.3 Detection of B cells and T cells using flow cytometry
The peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood using Histopaque density gradient centrifugation (10771; Sigma-Aldrich), according to the manufacturer's instructions, resuspended in cryoprotectant mixed with 10% fetal calf serum (FCS) and dimethylsulfoxide (DMSO) at a 9:1 volume ratio, and stored at liquid nitrogen until used.
B cells and their memory B cell (MBC) subsets specific to WT/BA.5 variants, CD4+ T cells specific to WT/BA.5 variants, circulating Tfh (cTfh) cells specific to WT/BA.5 variants, and CD8+ T cells specific to WT/BA.5 variants were analyzed. Approximately 1.0 × 106 events were collected within a lymphocyte gate on a flow cytometer, and analyzed for cell populations. The detailed steps are provided in Supporting Information. The full gating strategy is illustrated in Supporting Information S1: Figure 1.
2.4 Statistical analysis
For normal and nonnormally distributed data, continuous variables were expressed as mean and standard deviation (SD) or median and interquartile range (IQR), respectively. The categorical variables were presented as percentage (%). Intergroup difference analysis was conducted using analysis of chi-square tests, Student t-test tests, Wilcoxon test, or Mann–Whitney U test as appropriate. We used multivariate logistic regression analysis to investigate the factors affecting the antibody response after BTI. All statistical analysis was performed using R 4.0 (http://www.R-project.org, the R Foundation), SPSS (version 22.0) and GraphPad Prism (8.0.2; GraphPad Software Inc.), and visualized using GraphPad Prism. A two-sided p value < 0.05 was considered statistically significant.
3 RESULTS
3.1 Clinical characteristics of participants after BTI
In general, 101 patients with CHB with BTI were included in this analysis. As shown in Table 1, the median age was 46 years, and most of them were male (58.4%). Around 23.8% of the patients were HBeAg positive, 68.3% of the patients received antiviral therapy, and the mean viral load of HBV-DNA was 50 IU/ml. Moreover, most of the patients received primary and booster doses of the inactivated vaccination (77.2%), and the rest of them received three doses of ZF2001 vaccination. The median interval days between BTI and the last dose of vaccination was 357 days. The clinical characteristics of the HCs group after BTI are shown in Supporting Information S1: Table 1.
| Variables | CHB (n = 101) | Variables | CHB (n = 101) |
|---|---|---|---|
| Age (years) | 46 (37–51) | M (109/L) | 0.31 (0.26–0.39) |
| Gender | PLT (109/L) | 215 (166–248) | |
| Male (%), (n/n) | 58.4% (59/101) | ALB (g/L) | 50.1 (49.0–51.2) |
| Female (%), (n/n) | 41.6% (42/101) | ALT (U/L) | 23 (16–36) |
| BMI (kg/m2) | 23.28 (21.43–25.14) | AST (U/L) | 23.5 (19.0–30.0) |
| Antiviral therapy | γ-GT (U/L) | 20 (14–28) | |
| Yes (%), (n/n) | 68.3% (69/101) | TB (μmol/L) | 11.65 (9.28–15.88) |
| No (%), (n/n) | 31.7% (32/101) | DB (μmol/L) | 3.50 (2.90–4.48) |
| HBeAg | HBV-DNA (IU/mL) | 50 (10–772) | |
| Positive (%), (n/n) | 23.8% (24/101) | Vaccine | |
| Negative (%), (n/n) | 76.2% (77/101) | Inactivated (%), (n/n) | 77.2% (78/101) |
| RBC (1012/L) | 4.87 (4.50–5.31) | ZF2001 (%), (n/n) | 22.8 (23/101) |
| HB (g/L) | 146.5 (134.3–157.0) | Interval days between | |
| WBC (109/L) | 5.36 (4.54–6.64) | Inf and last dose of | 357 (305–397) |
| Lym (109/L) | 1.76 (1.47–2.06) | vaccination |
- Note: Data are displayed as median (interquartile range) and percentage (n/N).
- Abbreviations: ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CHB, chronic hepatitis B virus; DB, direct bilirubin; HB, hemoglobin; γ-GT, γ-glutamyltranspeptidase; Lym, lymphocyte; M, monocyte; PLT, platelet; RBC, red blood cell; TB, total bilirubin; WBC, white blood cell.
After BTI, all the 101 patients with CHB were symptomatic (Table 2). The most common symptom was fever (72.3%), followed by cough, pain, and fatigue (40.6%-66.3%). Similar results were also observed in the HCs. According to the classification of COVID-19, 51.5% and 48.5% of patients with CHB had mild and moderate COVID-19, respectively. However, none of them were judged as having a severe type of COVID-19 or were hospitalized (Table 2). Compared with that in HCs, the clinical manifestation after BTI in patients with CHB appeared to be relatively severe, implying that patients with CHB should prioritize COVID-19 protection measures to prevent reinfection.
| Variables | HCs (39) | CHB (101) | p-Value |
|---|---|---|---|
| Symptoms | |||
| Fever, n (%) | 28 (71.8%) | 73 (72.3%) | 0.954 |
| Tmax (°C), median (IQR) | 38.5 (38.3-38.8) | 38.4 (38.3-38.7) | 0.296 |
| Fever duration (days), median (IQR) | 3 (2–4) | 3 (2–4) | 0.254 |
| Cough, n (%) | 23 (59.0%) | 67 (66.3%) | 0.415 |
| Pain, n (%) | 21 (53.8%) | 50 (49.5%) | 0.645 |
| Fatigue, n (%) | 18 (46.2%) | 41 (40.6%) | 0.550 |
| Runny nose, n (%) | 9 (23.1%) | 13 (12.9%) | 0.137 |
| Insomnia, n (%) | 3 (7.7%) | 6 (5.9%) | 0.709 |
| Diarrhea, n (%) | 3 (7.7%) | 5 (5.0%) | 0.685 |
| Breathlessness, n (%) | 1 (2.6%) | 4 (4.0%) | 1.0 |
| Rash, n (%) | 0 (0%) | 2 (2.0%) | 1.0 |
| Duration of symptoms | 5 (2-7) | 5 (3-9) | 0.085 |
| Severity of Symptoms | <0.001 | ||
| Asymptomatic | 20% (7/39) | 0 | |
| Mild | 43.3% (18/39) | 51.5% (52/101) | |
| Moderate | 36.7% (14/39) | 48.5% (49/101) | |
- Note: Data are displayed as median (interquartile range), percentage (n/N), and number (%). p < 0.05 was considered statistically significant.
- Abbreviations: CHB, chronic hepatitis B virus; COVID-19, coronavirus disease 2019; HCs, healthy controls; Tmax, Temperature maximum.
Meanwhile, we also assessed the effect of COVID-19 on patients with CHB. Compared to laboratory indices before BTI, the levels of platelet (PLT), albumin (ALB), alanine aminotransferase (ALT), and globulin (GLB) slightly fluctuated in patients with CHB after BTI. The HBeAg status and HBV-DNA viral load showed no changes (Table 3). These results suggested that SARS-CoV-2 infection did not cause significant aggravation of liver diseases in patients with CHB.
| Variables | Before Inf (n = 45) | After Inf (n = 45) | p-Value |
|---|---|---|---|
| RBC (1012/L) | 4.71 (4.36–5.30) | 4.77 (4.42–5.23) | 0.417 |
| HB (g/L) | 143.0 (129.5–151.5) | 144.0 (132.5–155.5) | 0.065 |
| WBC (109/L) | 5.80 (4.62–6.42) | 5.77 (4.42–6.64) | 0.717 |
| Lym (109/L) | 1.72 (1.59–2.08) | 1.75 (1.46–1.99) | 0.278 |
| M (109/L) | 0.31 (0.26–0.36) | 0.31 (0.26–0.37) | 0.964 |
| PLT (109/L) | 204 (152–232) | 228 (177–251) | <0.001 |
| ALB (g/L) | 47.6 (46.0–49.6) | 50.3 (48.7–51.2) | <0.001 |
| GLB (g/L) | 27.9 (25.7–30.6) | 24.8 (22.5–27.1) | 0.001 |
| ALT (U/L) | 22.0 (16.0–30.0) | 24.0 (16.0–34.0) | 0.031 |
| AST (U/L) | 23.0 (19.0–25.0) | 24.0 (18.5–32.0) | 0.254 |
| γ-GT (U/L) | 18.0 (13.0–27.5) | 18.5 (13.3–35.0) | 0.716 |
| TB (μmol/L) | 15.4 (9.7–19.3) | 13.3 (9.7–16.9) | 0.119 |
| DB (μmol/L) | 3.9 (3.2–5.8) | 3.9 (2.9–4.6) | 0.001 |
| HBeAg | 1 | ||
| Positive (%), (n/n) | 21.7% (5/23) | 21.7% (5/23) | |
| Negative (%), (n/n) | 78.3% (18/23) | 78.3% (18/23) | |
| HBV-DNA (IU/mL) | 50 (10–586) | 50 (10–7246) | 0.28 |
- Note: Data are displayed as median (interquartile range) and percentage (n/N). p < 0.05 was considered statistically significant.
- Abbreviations: ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CHB, chronic hepatitis B virus; DB, direct bilirubin; γ-GT, γ-glutamyltranspeptidase; GLB, globulin; HB, hemoglobin; Inf, Infection; Lym, lymphocyte; M, monocyte; PLT, platelet; RBC, red blood cell; TB, total bilirubin; WBC, white blood cell.
3.2 Antibody responses of participants after BTI
Benefited from the previous cohort maintenance and follow-up, we elaborately depicted the dynamic changes of antibody response from vaccination to BTI, that is, the antibody titers at 1-month (T1), 3-month (T2), and 6-month (T3) after primary vaccination (PV); 3-month (T4) and 6-month (T5) after booster vaccination (BV); and 3-month (T6) and 6-month (T7) after BTI were evaluated for inactivated vaccination. As shown in Figure 1A, the titers of BA.5 IgG stepped up after primary inactivated vaccination, BV, and BTI, but gradually decreased over time (Titer: 75.40 at T1 vs. 36.13 at T2 vs. 17.71 at T3 vs. 224.76 at T4 vs. 86.66 at T5 vs. 2098.96 at T6 vs. 1232.90 at T7, all p < 0.001). Compared with that at T1 and T4, the titer of BA.5 IgG at T6 increased 27.84-fold and 9.34-fold, respectively. Even though the BA.5 IgG titer after BTI decreased with time, it was still higher than that after BV (1232.90 at T7 vs. 224.76 at T4, p < 0.001). Interestingly, similar results were observed in NAbs titers (Figure 1B), and the titer of NAbs at T6 increased 18.88-fold and 5.66-fold, respectively (Titer: 7.93 μg/mL at T6 vs. 0.42 μg/mL at T1 vs. 1.40 μg/mL at T4, all p < 0.001). As the homologous booster dose of ZF2001 vaccine was not implemented in China, the antibody responses at T1, T2, T3, and T4 after PV and BTI was assessed for ZF2001 vaccination. As shown in Figure 1C, the BA.5 IgG titer was increased significantly after the Omicron BTI (Titer: 5623.65 at T4 vs. 900.14 at T1 vs. 245.40 at T2 vs. 140.22 at T3, all p < 0.001). NAbs showed the same trend as did BA.5 IgG in this group (Figure 1D). The NAbs and BA.5 IgG were highly correlated in patients with CHB and HCs (CHB: r = 0.892, p < 0.001; HCs: r = 0.741, p < 0.001) (Supporting Information S1: Figure 2).
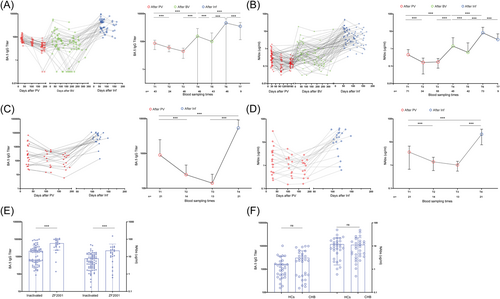
Notably, patients who received the ZF2001 vaccine had significantly higher antibody titers after BTI than did patients with CHB who received the inactivated vaccine (Figure 1E, both p < 0.001). Meanwhile, the results of two-variable correlation and multiple linear regression analysis also showed that the antibody response of patients with CHB after BTI was mainly related to the type of vaccination (Table 4). In addition, there was no significant difference in antibody response after BTI among patients with CHB with different phase of CHB natural history (Supplementary Figure 3). Regardless of normal or abnormal ALT, antiviral therapy or not, HBV-DNA/HBeAg positive or negative, each group of patients with CHB elicited a more robust antibody response after BTI (Supplementary Figure 4).
| NAbs (n = 101) | BA.5 IgG (n = 101) | |||||||
|---|---|---|---|---|---|---|---|---|
| r | p | β | p | r | p | β | p | |
| Age (years) | −0.092 | 0.360 | 0.077 | 0.434 | −0.085 | 0.397 | −0.032 | 0.715 |
| Gender (male/female) | 0.036 | 0.659 | 0.184 | 0.06 | 0.001 | 0.989 | −0.058 | 0.524 |
| BMI(kg/m2) | 0.036 | 0.722 | 0.060 | 0.540 | 0.016 | 0.874 | 0.085 | 0.335 |
| ALB (g/L) | 0.127 | 0.207 | 0.054 | 0.580 | 0.194 | 0.053 | 0.129 | 0.145 |
| ALT (U/L) | −0.038 | 0.706 | −0.082 | 0.404 | −0.031 | 0.769 | −0.019 | 0.830 |
| AST (U/L) | −0.009 | 0.931 | −0.044 | 0.658 | −0.048 | 0.633 | −0.076 | 0.390 |
| γ-GT (U/L) | 0.106 | 0.296 | 0.024 | 0.144 | 0.156 | 0.922 | 0.091 | 0.303 |
| TB (μmol/L) | −0.145 | 0.149 | −0.152 | 0.121 | −0.102 | 0.312 | −0.133 | 0.131 |
| DB (μmol/L) | −0.179 | 0.075 | −0.129 | 0.187 | −0.155 | 0.123 | −0.153 | 0.080 |
| HBV DNA (IU/mL) | −0.098 | 0.331 | −0.030 | 0.764 | −0.166 | 0.248 | −0.020 | 0.822 |
| HBeAg (±) | 0.126 | 0.126 | 0.127 | 0.196 | 0.137 | 0.095 | 0.189 | 0.033 |
| Anti-HBV therapy (yes/no) | −0.013 | 0.872 | −0.051 | 0.604 | 0.000 | 1.000 | 0.026 | 0.767 |
| Days between last dose of vaccine and Inf | 0.234 | 0.022 | −0.056 | 0.743 | 0.271 | 0.008 | 0.019 | 0.901 |
| Vaccine type (Inactivated/ZF2001) | 0.243 | 0.003 | 0.354 | <0.001 | 0.330 | <0.001 | 0.517 | <0.001 |
- Note: In two-variable correlation analysis, Spearman's rank correlation was used for two continuous variables, and Kendall's rank correlation was used if one of the variables was discontinuous; the r values represent the correlation coefficients. In multiple linear regression analysis, the stepwise regression was used to determine the variables in the regression equation; the β values represent the standardized regression coefficients.
- Abbreviations: ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DB, direct bilirubin; γ-GT, gamma-glutamyl transpeptidase; HBeAg, hepatitis B e antigen; HBV DNA, hepatitis B virus deoxyribonucleic acid; Inf, infection; NAbs, neutralizing antibodies; TB, total bilirubin.
To determine whether HBV would affect the antibody response after PV, BV, and BTI, we depicted the antibody titers of HCs and patients with CHB after PV, BV, and BTI in a cross-sectional analysis (Supporting Information S1: Figure 5). Similar to that in patients with CHB, antibody response in HCs also showed a significant decline over time after PV. Both BV and BTI significantly improved the antibody response in HCs. The antibody response in HCs was stronger than in patients with CHB after PV and BV, but was similar after BTI. We further matched 30 patients with CHB and 30 HCs through propensity score by sex, age, body mass index (BMI), time interval after BTI, and vaccine type (Supporting Information S1: Table 2). Similarly, there was no significant difference in BA.5 IgG and NAbs responses between patients with CHB and HCs after BTI (Figure 1F, both p > 0.05).
Taken together, our findings suggest that patients with CHB can elicit robust antibody responses against the BA.5 variant after Omicron BTI, comparable to those observed in healthy individuals.
3.3 B cell responses of participants after BTI
After evaluating the antibody responses after BTI in patients with CHB, we wanted to investigate the alterations in B cells after BTI. Flow cytometry was used to detect the WT- and BA.5 strains-specific B cells and MBCs responses in patients with CHB and HCs. As shown in Figure 2A, WT-specific B cells were significantly increased after BTI, while BA.5-specific B cells did not change significantly (WT: 5.69% vs. 6.63%, p = 0.022; BA.5:1.49% vs. 1.23%, p = 0.118). On the contrary, there was no significant change in WT-specific MBCs, but BA.5-specific MBCs showed a significant decrease (WT: 5.37% vs. 6.18%, p = 0.061; BA.5:1.83% vs. 0.76%, p < 0.001). Notably, both the frequencies of WT-specific B cells and MBCs was significantly higher than that of BA.5-specific B cells and MBCs in patients with CHB after BTI (B cell: 6.63% vs. 1.23%; MBCs: 6.18% vs. 0.76%, both p < 0.001; Figure 2A). This indicated that even after the Omicron BTI, the humoral immunity in patients with CHB still favored to against WT strain.

Subgroup analysis showed that there was no significant difference in WT- and BA.5-specific B cells and MBCs responses between the two different vaccine groups in patients with CHB after BTI (Figure 2B). One exception was that the frequency of BA.5-specific B cells was higher in ZF2001 vaccine recipients than in inactivated vaccine recipients (1.13% vs. 1.55%, p = 0.014). We then compared B cell responses after BTI between patients with CHB and HCs. As shown in Figure 2C, the frequencies of BA.5-/WT-specific B cells and WT-specific MBCs in the HCs group were significantly higher than those in the CHB group after BTI (BA.5-specific B cells: 1.08% vs. 2.09%; WT-specific B cells: 6.26% vs. 11.15%; WT-specific MBCs: 5.91% vs. 9.70%, all p < 0.05).
In brief, our results showed that B cell responses primarily target the wild-type strain in patients with CHB after Omicron BTI, while exhibiting a lower magnitude compared with that in HCs.
3.4 T cell responses of participants after BTI
In addition to humoral immunity, T cell-associated cellular immunity also protects against virus exposure.21, 22 Activation-induced marker (AIM) assay was used to measure CD4+ T and CD8+ T cell responses with the overlapping peptide pools. As shown in Figure 3A, both WT- and BA.5-specific CD4+ T cells frequencies increased significantly in patients with CHB after BTI (WT: 0.063% vs. 0.225%, BA.5: 0.045% vs. 0.363%; both p < 0.001). In contrast to the results of B cell responses, the frequency of BA.5-specific CD4+ T cells was significantly elevated in patients with CHB after Omicron BTI than before. Further focusing on the cTfh cell, a subtype of CD4+ T cells, similar results were observed. That is, the BA.5- and WT-specific cTfh cells had 6.35-fold and 3.29-fold increase after BTI, respectively (BA.5: 0.037% vs. 0.235%, WT:0.042% vs. 0.138%, both p < 0.001). Moreover, the results of CD8+ T cells responses were also in accordance with that of the CD4+ T cells (WT: 0.095% vs. 0.502%, BA.5: 0.151% vs. 0.876%; both p < 0.001). Noticeably, both BA.5-specific CD4+ T cells and BA.5-specific cTfh were correlated significantly with ALT and AST in patients with CHB; however, WT-specific CD4+ T cells and WT-specific cTfh were not significantly correlated with ALT and AST (Supporting Information S1: Figure 6).

Subgroup analysis showed that the frequency of BA.5-specific CD8+ T cells was higher in the inactivated vaccine group than in the ZF2001 vaccine group, while no significant difference for other T cells was observed (Figure 3B). In addition, compared to that in HCs, we found that patients with CHB showed higher BA.5-specific CD4+ T cell and cTfh responses after Omicron BTI (CD4+T cell: 0.37% vs 0.17%; cTfh: 0.28% vs 0.08%, both p < 0.05; Figure 3C).
Altogether, in patients with CHB with Omicron BTI, the T cell response specific to BA.5 was significantly enhanced compared to WT-specific responses, while remaining comparable to that in HCs.
4 DISCUSSION
To date, Omicron variants has become the predominant lineage in the world. The clinical characteristics and immunogenicity of patients with CHB with Omicron BTI is still unclear. The main findings of our study were as follows: (1) The severity of COVID-19 was relatively higher in patients with CHB compared to that in HCs following Omicron BTI, whereas, BTI in patients with CHB did not exacerbate the progress of liver disease; (2) Patients with CHB exhibited robust antibody responses against WT and BA.5 after BTI, comparable to those observed in HCs; and (3) The BA.5-specific T cell responses, rather than B cell responses, were significantly augmented in patients with CHB after BTI, while exhibiting comparability to that in HCs.
Owing to multiple mutations in the RBD region, Omicron (B.1.1.529) exhibits strong immune escape from most vaccines and monoclonal antibodies, which target the ancestral strain of SARS-CoV-2.23 Even though our previous cohort study showed that booster dose of vaccination can elicit robust antibody responses against the WT strain,16 all patients with CHB with ongoing follow-up in this cohort were infected with COVID-19 during the first wave of Omicron. This is similar to that reported in previous studies on the general population,24, 25 and further confirms the weak protection of WT vaccine against Omicron infection. After BTI, the clinical symptoms of COVID-19 were mild to moderate, which was similar to previous observations.13, 25, 26 This may be attributed to the low virulence of Omicron or the protection against severe illness of vaccines. No exacerbation of liver disease occurred in patients with CHB after BTI; however, compared to that in HCs, our results showed a higher proportion of moderate COVID-19 in patients with CHB. Hence, more attention should be paid to this special population.
As expected, BTI further enhanced the antibody response induced by vaccination in patients with CHB, which was similar to that reported in previous studies in healthy people.27, 28 This suggested that the mixed immunity formed by vaccination and BTI has a higher protective effect against variants of concern.29-31 Similar to that in our previous study,16 the antibody titers of patients with CHB were as high as those of HCs after BTI. This indicated that BTI could still elicit robust antibody responses, like it did in HCs, in patients with CHB. Notably, not only BA.5 IgG titer but also WT NAbs titer were also significantly increased after Omicron BTI. Combined with the results of B cell responses, that is, WT-specific B cells were higher than BA.5-specific B cells after BTI, our results indicated a strong immune imprint of humoral immunity after COVID-19 infection in patients with CHB. This was also observed in a previous study.32 However, WT/BA.5-specific B cell response and WT-specific memory B cell response was higher in HCs than in CHB; this may be related to the disruption of B cell homeostasis by HBV infection, leading to global B cell dysfunction.33, 34
Beyond humoral immunity, cellular immune responses also play an important role in the clearance of viral infection.21, 22 In this study, the CD4+ T cell responses were significantly enhanced in patients with CHB after BTI. Remarkably, one of the subtypes of CD4+ T cells, cTfh cells was also increased after BTI. Tfh cells can secrete cytokines, such as IL-21 and IL-4, that promote the growth and differentiation of B cells and result in antibody secretion.35-37 Thus, this result can at least partly explain the elevated antibody titers in patients with CHB after BTI. In addition, the CD8+ T cell responses were also augmented in patients with CHB after BTI, which indicated that the direct killing of virus-infected cells was strengthened by BTI.38 These results in T cell responses were similar to that in a previous study.39 Interestingly, we found that the T cell responses to BA.5 were significantly higher than those to WT strain in patients with CHB after BTI. This suggests that the immune imprint may mainly be presented in B-cell responses, rather than in T-cell responses. Further study is needed to assess and validate our findings. Furthermore, BA.5-specific CD8+T cell response was higher in the inactivated vaccine group than in the ZF-2001 group, which may be because ZF2001 targets the highly immunogenic region of RBD instead of the full-length S glycoprotein. One study showed that responses directly related to antiviral signaling after vaccination with ZF2001 vaccine, such as activation of pattern recognition receptors, expression of genes involved in RNA degradation, and transcription inhibition, were weaker than those of mRNA vaccines, which may affect antiviral-associated CD8+T cell response after BTI.40 Further studies are needed to assess the differences with inactivated vaccines. Noticeably, our findings suggest that patients with CHB exhibit a higher BA.5-specific CD4+T cells response and BA.5-specific cTfh cells response after BTI. This may be due to a significant increase in Tfh cells in CHB compared with that in HCs; also, the frequency of cTfh cells was correlated with the serum levels of ALT and AST in CHB.34, 41 which is similar to our findings.
To the best of our knowledge, our study is the first longitudinal, observational study to describe the full course of immune dynamic changes after vaccination and Omicron BTI in patients with CHB. However, our study also has some limitations. First, the sample size was relatively small; because the clinical symptoms of COVID-19 were slight, most patients were unwilling to participate and be followed up in this study. Nonetheless, benefited from the coherent follow-up in our previous cohort since May 2021, we elaborately depicted the dynamics of immune responses from vaccination to infection in patients with CHB. Second, the Omicron variant infection was not proved using sequencing as no nucleic acid samples were obtained from most of the participants. However, according to the survey report of the Chinese Center for Disease Control and Prevention (CCDC), the BA.5.2 strain accounted for 95.7% of the infected patients in Chongqing during the first wave of COVID-19 infection.42 Thus, we assumed that all the participants were infected with the Omicron variant in this study. Third, as the description of symptoms is subjective, bias seems inevitable among patients. All of our questionnaires were completed by the same physician in accordance with the diagnosis and treatment Guidelines for COVID-19. This serves to minimize bias and draw definitive conclusions. Fourth, limited by the available blood sampling volume, the B cell and T cell responses were not portrayed in detail, such as plasmacyte, T memory cells, and cytokines secreted by these immune cells. Thus, a well-designed study with a larger sample size to further clarify this is highly warranted.
In conclusion, Omicron BTI could elicit robust antibody and T cell responses in patients with CHB, while exhibiting comparability to that in HCs. However, the B cell responses were weakened in patients with CHB. BTI did not aggravate the progress of liver disease in patients with CHB, however, the severity of COVID-19 was relatively higher in patients with CHB compared to that in HCs. Therefore, more attention should be paid to this special population.
AUTHOR CONTRIBUTIONS
Hong Ren, Peng Hu, and Mingli Peng designed this study. Hong Ren acquired the funding. Guanhua Zha, Zhiwei Chen, Na Wu, Tianquan Huang, Zhiling Deng, and Dachuan Cai recruited participants and collected the data. Guanhua Zha, Zhiwei Chen, Na Wu, Tianquan Huang, and Zhiling Deng performed the experiments. Guanhua Zha, Zhiwei Chen, and Na Wu analyzed and interpreted the data. Guanhua Zha, Zhiwei Chen, and Na Wu visualized the results. Guanhua Zha and Zhiwei Chen drafted the manuscript. Hong Ren critically revised the manuscript. Hong Ren, Peng Hu, and Mingli Peng provided administrative support. Hong Ren supervised this study. All authors approved the final manuscript version.
ACKNOWLEDGMENTS
We thank Tianquan Huang and Zhiling Deng from Institute for Viral Hepatitis, Chongqing Medical University for their assistance in blood sampling. We also thank the Department of Laboratory Medicine, and The Second Affiliated Hospital of Chongqing Medical University for their support in the detection of clinical characteristics. The work was partly supported by the Chongqing Postdoctoral Science Foundation Project (CSTB2023NSCQ-BHX0082), the Science and technology research project of Chongqing Education Commission (KJQN202300404), Remarkable Innovation-Clinical Research Project, The Second Affiliated Hospital of Chongqing Medical University, and The First batch of key Disciplines on Public Health in Chongqing, Health Commission of Chongqing, China.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




