Omicron breakthrough infected individuals show enhanced nasal antibody responses and preserved T cell responses against the EG.5.1 and BA.2.86
Zheng Zhu, Shixiong Li, Junhao Fan, Shihao Shang, Yao Zhang, and Qiong Zi are contributed equally to this study.
Coronavirus disease 2019 remains a threat to the global public health with the continual emerging variants, and vaccination has helped to mitigate the impact of the global pandemic. China effectively sustained local containment of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection before the omicron BA.5 wave, primarily ascribing to the high vaccination rates and massive public health interventions. However, immediately after easing the dynamic zero-COVID policy, the first wave of infection surged in winter of 2022, leading to an estimated over 90% infection rate mostly by BA.5.1 A second wave shaped approximately 6 months thereafter, likely resulting from the waning immunity and eased public health management, and prevailing with a mixture of XBB variants.2 By October 2023, EG.5.1 dominated globally and accounted for greater than 90% of prevalence in China. In addition, a most phylogenetically divergent variant BA.2.86 emerged, harboring 58 spike substitutions compared with the ancestral strain. Both variants have been shown to be strongly neutralizing antibody (NAb) invasive,3-6 potentially straining our previous vaccination efforts. However, the immune status particularly the T cell responses and mucosal immune responses, thought to be essential for the prevention of severe illness and viral transmission,7, 8 respectively, remains less clear in the Chinese population.
We enrolled a total of 58 individuals from the Fourth Military Medical University, Xi'an on November 2023 for this immunologic study, including 6 vaccinated individuals who tested negative for SARS-CoV-2 and further confirmed by serologic assays (Vac only), 29 vaccinated individuals who tested positive for SARS-CoV-2 during the first wave of infection between November 2022 and January 2023 (Vac+ BA.5), and 23 vaccinated individuals who tested positive for SARS-CoV-2 during the first wave and subsequently the second wave between May 2023 and July 2023 (Vac+ BA.5 + XBB) (Supporting Information: Table S1 and Supporting Information: Figure 1). Participants were either vaccinated with Ad5-nCoV (CanSino) or inactivated vaccines (SinoVac or SinoPharm), which were validated by Ad5-specific neutralization assay (Supporting Information: Figure S2D). And two platforms elicited comparable immune responses (see results below and Supporting Information: Figure 2A-C).
SARS-CoV-2 antibody responses in plasma were assessed by nucleocapsid (N)- and receptor binding domain (RBD)-specific IgG, IgA, and IgM enzyme-linked immunosorbent assays (ELISAs), and pseudovirus NAb assays. Median N-specific IgG titers in the inactivated vaccines group were threefold greater than that in the Ad5-nCoV group, consistent with the presence of N protein in the inactivated vaccines (Supporting Information: Figure 3A). When breakthrough infections were considered, median N IgG antibodies in Vac+ BA.5 and Vac+ BA.5 + XBB individuals were 2.7- and 3.6-fold greater than the Vac only individuals (Supporting Information: Figure 3B), while N IgM titers were equivalent, implicating relative long-term (5−10 months) from last SARS-CoV-2 exposure (Supporting Information: Table S1 and Supporting Information: Figure 3C). Median RBD-specific IgG ELISA titers against the SARS-CoV-2 WA1/2020, BF.7, XBB, EG.5.1, and BA.2.86 variants were comparable in Vac+ BA.5 compared with the Vac only individuals (Figure 1A; p = 0.1276 for all variants, unpaired Mann−Whitney test), and median IgG ELISA titers against the variants were less than twofold higher in the Vac+ BA.5 + XBB individuals compared with Vac only individuals (Figure 1A; p = 0.0002 for all variants, unpaired Mann−Whitney test). Notably, median IgG titer against BA.2.86 decreased by twofold compared with that against ancestral strain (Figure 1A). Median RBD-specific IgA and IgM ELISA titers against the WA1/2020, BF.7, XBB, EG.5.1, and BA.2.86 variants followed a similar trend, with BA.2.86 showing the lowest IgA binding antibody while BF.7 and XBB the lowest IgM titers (Supporting Information: Figure 4; p = 0.1875 and p = 0.0496 for all variants, respectively, unpaired Mann-Whitney test). Median NAb titers against the WA1/2020, BF.7, XBB, EG.5.1, and BA.2.86 variants in Vac+ BA.5 individuals were 1.4-, 2.3-, 2.5-, 1.4- and 2.3-fold of NAb titers in uninfected individuals (Figure 1B; p = 0.0024 for all variants, unpaired Mann−Whitney test), and NAb titer against the variants were 3.0-, 3.6-, 2.8-, 1.9- and 4.6-fold higher in Vac+ BA.5 + XBB individuals compared with vaccinated uninfected individuals (Figure 1B; p < 0.0001 for all variants, unpaired Mann-Whitney test). The antigenic distances among variants were calculated by using the plasma neutralization data, showing that the BA.2.86 was antigenically less divergent than the EG.5.1 from the ancestral strain (Supporting Information: Figure 5A). Strong correlations were observed between the binding IgG and neutralizing antibodies for both the ancestral and EG.5.1 variants in this cohort (Supporting Information: Figure 6A, r = 0.5063, p < 0.0001 and Supporting Information: Figure 6B r = 0.4597, p = 0.0071, respectively, two-sided Spearman rank-correlation tests), but not for IgM or IgA (Supporting Information: Figure 6C and 6D). Interestingly, the phylogenetically more divergent BA.2.86 was noted to be less neutralizing evasive than the EG.5.1 (Figure 1B and Supporting Information: Figure 5B), while in contrast, the RBD-specific binding antibody titer against BA.2.86 was shown to be lower than EG.5.1 (Figure 1A). Regardless, the RBD-specific binding antibody for either IgG, IgA, or IgM were well-maintained and additional omicron exposures failed to elevate the overall binding titers.
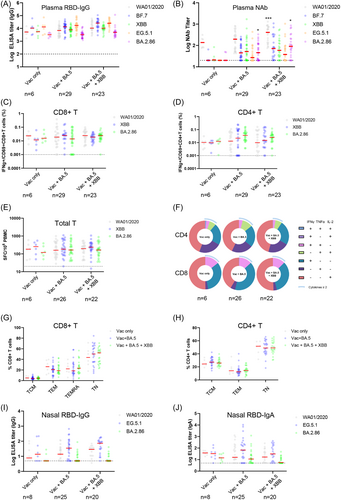
SARS-CoV-2 cellular immune responses were assessed by intracellular cytokine staining assays (gating strategy shown in Supporting Information: Figure S7) and ELISPOT assays using pooled spike peptides. Median Spike-specific CD8+ T cell responses to SARS-CoV-2 WA1/2020, XBB, and BA.2.86 in Vac+ BA.5 individuals were 0.8-, 2.1 and 0.9-fold respectively, of vaccinated uninfected individuals (Figure 1C; p = 0.6470 for all variants, unpaired Mann−Whitney test), and in Vac+ BA.5 + XBB individuals were 1.0-, 1.6- and 1.5-fold, respectively, of vaccinated uninfected individuals (Figure 1C; p = 0.2930 for all variants, unpaired Mann−Whitney test). Spike-specific CD4+ T cell responses to WA1/2020, XBB, and BA.2.86 in Vac+ BA.5 individuals were 1.3-, 2.3-, and 2.9-fold of Vac only individuals, respectively (Figure 1D; p = 0.0123, unpaired Mann-Whitney test), and Vac+ BA.5 + XBB individuals showed 1.0-, 1.5-, and 2.0-fold of CD4 responses in uninfected individuals (Figure 1D; p = 0.025, unpaired Mann-Whitney test). Comparable T cell responses among variants were confirmed by ELISPOT assay (Figure 1E; p = 0.0101 and p = 0.0089, respectively, unpaired Mann−Whitney test) and by correlations analysis of variant-specific and WA1/2020-specific cellular immune responses (Supporting Information: Figure 8). Average polyfunctional T cell responses against variants were not statistically different among groups, however, the three-cytokine secreting CD8+ T cells appeared to decrease after omicron exposure (Vac only 0.76%, Vac+ BA.5 0%, and Vac+ BA.5+ XBB 0.24%, respectively; Figure 1F). Spike-specific memory T cell subsets were further analyzed, and no substantial differences were noted for naïve T (TN), central memory T (TCM), effector memory T (TEM) or terminally differentiated effector memory (TEMRA) CD8+ subsets (Figure 1G), neither for TN, TCM, TEM CD4+ subsets (Figure 1H).
We assessed the RBD-specific IgA and IgG responses in the mucosal secretions extracted from the nasal swabs. Nasal RBD-specific IgG responses to WA1/2020, EG.5.1 and BA.2.86 were 2.6-, 7.0-, and 1.0-fold higher, respectively, in vaccinated single-infected individuals compared with vaccinated uninfected individuals, and were 5.3-, 14.7-, and 1.0-fold higher, respectively, in vaccinated double-infected individuals compared with vaccinated uninfected individuals (Figure 1I; p = 0.0041 and p = 0.0007, respectively for all variants, unpaired Mann-Whitney tests). Nasal RBD-specific IgA responses to SARS-CoV-2 WA1/2020 and EG.5.1, and BA.2.86 in vaccinated single-infected individuals were 2.3-, 11.5-, and 2.4-fold, respectively, of vaccinated uninfected individuals, and 2.2-, 6.1-, and 2.0-fold, respectively, in vaccinated double-infected individuals of vaccinated uninfected individuals (Figure 1J; p = 0.0001 and p = 0.001, respectively for all variants, unpaired Mann-Whitney tests). The neutralizing capacity of mucosal secretions against EG.5.1 and BA.2.86 were further determined and only basal level of neutralizing activity were noted (Supporting Information: Figure 9). The correlation analyses between the nasal EG.5.1 NAb and the nasal IgA, IgG, or plasma NAb showed no significant correlation (Supporting Information: Figure 10), indicating that the nasal neutralizing activity may not be conferred by one Ig subtype, nor elicited through the same immune mechanism as the plasma NAb is.
Overall, this small cohort study snapshots the current immune landscape of Chinese population who followed the same vaccination and breakthrough trajectory. The well-reserved binding antibody titer as well as the T cell responses in this population suggest that the protection against EG.5.1 and BA.2.86-mediated severe disease likely still holds. In addition, the higher mucosal IgG and IgA binding antibody level in breakthrough infected individuals indicates that multiple exposures may contribute to the prevention of EG.5.1 or BA.2.86 infection, which may be also achievable by mucosal vaccinations. Larger cohort studies are warranted for a confirmative answer.
AUTHOR CONTRIBUTIONS
Jingyou Yu and Zheng Zhu conceived and supervised the project. Shixiong Li, Junhao Fan, Yao Zhang, Qiong Zi, Jihao Zheng, Dongfang Wang, and Xiaoli Mou. performed the immunological and virological assays. Zheng Zhu, Shihao Shang, Kepu Liu, Maoxin Lv, and Jianlin Yuan led the cohort enrollment and clinical sample processing. Jingyou Yu, Zhongfang Wang, and Zheng Zhu analyzed the data. Jingyou Yu wrote the paper with all co-authors.
ACKNOWLEDGMENTS
The authors thank the study participants, and Drs. Jincun Zhao and Airu Zhu for advice, assistance, and reagents. This work was supported by the National Natural Science Foundation of China (NSFC 82371831 to J. Y.), the Young Talent Program of China (HJJH22-004 to J. Y.), the Guangzhou Laboratory Start-up funding (J. Y.), and the Major Project of Guangzhou National Laboratory Grants (GZNL2023A01005 and GZNL2023A01009 to J. Y.).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, [[email protected]], upon reasonable request.




