UMP-CMP kinase 2 inhibits ZIKV replication through activation of type I IFN signaling pathway
Ya Zhu and Qi Tan contribute equally and share first authorship.
Abstract
Cytidine/uridine monophosphate kinase 2 (UMP-CMP kinase 2, CMPK2) has been reported as an antiviral interferon-stimulated gene (ISG). We previously observed that the expression of CMPK2 was significantly upregulated after Zika Virus (ZIKV) infection in A549 cells. However, the association and the underlying mechanisms between CMPK2 induction and ZIKV replication remain to be determined. We investigated the induction of CMPK2 during ZIKV infection and the effect of CMPK2 on ZIKV replication in A549, U251, Vero, IFNAR-deficient U5A and its parental 2fTGH cells, Huh7 and its RIG-I-deficient derivatives Huh7.5.1 cells. The activation status of Jak-STAT signaling pathway was determined by detecting the phosphorylation level of STAT1, the activity of interferon stimulated response element (ISRE) and the expression of several interferon stimulated genes (ISGs). We found that ZIKV infection induced CMPK2 expression through an IFNAR and RIG-I dependent manner. Overexpression of CMPK2 inhibited while CMPK2 knockdown promoted ZIKV replication in A549 and U251 cells. Mechanically, we found that CMPK2 overexpression increased IFNβ expression and activated Jak/STAT signaling pathway as shown by the increased level of p-STAT1, enhanced activity of ISRE, and the upregulated expression of downstream ISGs. These findings suggest that ZIKV infection induced CMPK2 expression, which inhibited ZIKV replication and serves as a positive feedback regulator for IFN-Jak/STAT pathway.
1 INTRODUCTION
Zika virus (ZIKV) was originally isolated from a sentinel rhesus monkey in Uganda in 1947,1 and firstly detected in human in 1952.2 ZIKV is a positive-single-stranded RNA virus belonging to the Flaviviridae family. The genome of ZIKV encodes three structural proteins (envelope (E), membrane (M), and capsid (C)) and seven nonstructural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5).3 ZIKV infection in pregnant women can lead to congenital defects of newborns such as microcephaly, which has drawn global attention. To date, neither vaccines nor specific antiviral drugs are available for the prevention or treatment of ZIKV infection. Therefore, exploring the detailed pathogenesis and the host antiviral immune response following ZIKV infection may facilitate the development of effective vaccines and antiviral drugs.
Type-I interferon (IFN-I, mainly IFNα/β) is the first line defence against virus infection. After infection, ZIKV can be recognized by the pattern recognition receptors (PRRs) to activate the downstream signaling pathways leading to the production of IFN-I. Retinoic acid-induced gene I (RIG-I) like receptors (RLRs) are RNA sensors that detect viral RNA in the cytosol and induce IFN response. It has been reported that the expression of several IFN-I related molecules including IFNα, IFNβ, and RIG-I were significantly increased in the peripheral blood of ZIKV-infected patients, indicating that the host innate immune system could be activated by ZIKV infection.4 Moreover, RIG-I has been identified as a major PRRs during ZIKV infection.5 After induction, IFNα and IFNβ bind to their respective receptors (IFNARs) and activate the receptor-associated Tyrosine kinase 2 (Tyk2) and Janus kinase (JAK1) leading to phosphorylation and recruitments of signal transducer and activator of transcription (STAT1/2). Then, phosphorylated STAT1 and STAT2 release from the receptor and form a heterotrimeric complexes ISGF3 with IFN regulatory factor 9 (IRF9). ISGF3 translocates to the nucleus, where it binds to the ISRE and induces the expression of a few hundred ISGs to exert their antiviral or pro-viral activity.6-9
The UMP-CMP kinase 2 (CMPK2) has recently been reported to be associated with virus infection and host antiviral response. CMPK2 consists of a N-terminal domain (NTD) which functions as a mitochondrial localization sequence, and a C-terminal domain that is responsible for its kinase activity.10 CMPK2 has been found to be upregulated by several virus including Hepatitis E virus (HEV) and porcine reproductive and respiratory syndrome (PRRS) virus.11, 12 Lai et al demonstrated that CMPK2 mediated anti-Dengue virus (DENV) activity through IFN-dependent and IFN-independent manners, which played a critical role in DENV-induced antiviral innate immunity.13 CMPK2 has also been found to restrict HIV infection and carp spring viremia virus (SVCV) replication in vitro.14, 15 Most recently, CMPK2 has been reported to restrict ZIKV replication by inhibiting ZIKV RNA translation, which depends on its mitochondria localization but independent of its C-terminal kinase domain.10 However, the detailed mechanism by which ZIKV induces CMPK2 expression and CMPK2 regulates ZIKV replication through regulating IFN pathway has not been well characterized.
In this study, we investigated the association between CMPK2 induction and ZIKV replication as well as the underlying mechanisms. We found that ZIKV infection induced CMPK2 expression in an RIG-I and IFNAR-dependent manner. Meanwhile, CMPK2 inhibited ZIKV replication and serves as a positive-feedback regulator for IFN-mediated Jak/STAT signaling pathway.
2 MATERIALS AND METHODS
2.1 Cells and zika virus
Human non-small-cell lung cancer cell line (A549 cells), Vero cells and human glioma cell line (U251 cells) were purchased from West China Hospital, Sichuan University and cultured in Dulbecco's modified Eagle's medium (DMEM) (Hyclone, Logan, Utah, USA) supplemented with 10% fetal bovine serum (FBS) (BIOIND, Israel) and 1% Penicillin-Streptomycin (P/S) (Hyclone, Logan, Utah, USA) in a 5% humidified CO2 incubator at a constant temperature of 37°C. Hepatoma Huh7, Huh7.5.1, human IFNAR-deficient U5A cells and its parental human fibrosarcoma 2fTGH cells were kindly provided by Dr. Raymond Chung (Massachusetts General Hospital, USA) and cultured as described before.16, 17 ZIKV (GZ01) was a generous gift from Dr. Chengfeng Qin (Beijing Institute of Microbiology and Epidemiology, China) for scientific research.
2.2 Zika virus infection
A549, U251, Huh7, Huh7.5.1, U5A and 2fTGH cells were seeded at 1.5 × 105 cells/mL in the 12-well plate (1 mL/well). All the cells were cultured until approximately 80% confluence and infected with ZIKV at a certain MOI as described in each assay for 4 h at 37°C. Then the cells were washed with PBS (Sangon Biotech, China) for three times and refilled with fresh medium.
2.3 Plaque assay
Viral titers in cells supernatant were determined by plaque assay. Briefly, Vero cells were seeded in 12-well plates at 2.5 × 105 cells/well, and after a specific time point postinfection, virus-containing cell supernatants were serially diluted in DMEM and adsorbed on Vero monolayer for 2 h. Afterward, the viral particles in the medium were removed and overlaid with MEM containing 2% FBS and 2% agarose. After 6 days of incubation, cells were fixed with 4% formaldehyde for 4 h and were then stained with crystal violet for 10 min. The visible plaques were counted, and viral loads were measured as a plaque-forming unit (PFU) per mL of supernatant (Figure 1). All data are expressed as the means of triplicate samples.
2.4 siRNA, plasmids and transfection
Human CMPK2 (siCMPK2) (5′-CAGUGGCAGAUUCACUUAAUU-3′) and corresponding negative control siRNA (siNC) (5′-UUCUCCGAACGUGUCACGU-3′) were synthesized by Sangon Biotech, China. SiRNAs were transfected using RNAiMAX (Invitrogen, USA) according to the manufacturer's instructions.
CMPK2 and RIG-I ORFs were respectively amplified and cloned into the p3 × flag-cmv-7.1 vector to generate p3 × flag-cmv-7.1-CMPK2 (pCMPK2) and p3 × flag-cmv-7.1-RIG-I (pRIG-I) recombinant plasmids. The plasmids and their corresponding negative controls (pEmpty) were transfected into cells using polyethyleneimine (PEI) as previous described.16, 18
2.5 RNA isolation, reverse transcription and RT-qPCR
Total RNAs from cells were extracted by TRIzol™ (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. cDNA was synthesized using the Rever Tra Ace qPCR RT Master Mix (Toyobo, Japan). To detect mRNA expression, quantitative real-time PCR (RT-qPCR) was performed with SYBR Green Real-time Master Mix (Toyobo, Japan) in CFX96 Real-time PCR System (Bio-Rad, Hercules, CA, USA). The qPCR primers used in this study are listed in Table 1. The selected gene mRNA levels were calculated using 2-ΔΔCt method and normalized to GAPDH.
| Gene Name | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| GAPDH | GCCTCCTGCACCACCAACTG | ACGCCTGCTTCACCACCTTC |
| GZ01 NS5 | CCTTGGATTCTTGAACGAGGA | AGAGCTTCATTCTCCAGATCAA |
| CMPK2 | GTACCTCCTTTATTCCTGAAGCC | ATGGCAACAACCTGGAACTTT |
| IFNα | TCGCCCTTTGCTTTACTGAT | GGGTCTCAGGGAGATCACAG |
| IFNβ | AAACTC ATAGCAGTCTGCA | AGGAGATCTTCAGTTTCGGAGG |
| IFNλ | CCAGTGATGATTCTCTTGAGAGC | CCCCAAAGCGTAGAGGTCCA |
| RIG-I | AGTGAGCATGCACGAATGAA | GGGATCCCTGGAAACACTTT |
| MDA5 | GACTTGCCCTCTCCATCGTT | CCTCCTCCATGCACTTATCCAA |
| IFIT2 | CTTGACTGTGAGGAAGGG | CAATGGCGTTCTGAGATG |
| MxA | GTGCATTGCAGAAGGTCAGA | CTGGTGATAGGCCATCAGGT |
| IFNAR1 | AAAATGGCAATGATAGG | CAGGCTGAGCAGAAGG |
2.6 Dual luciferase reporter assay
To assess the effect of CMPK2 on ISRE activity, the plasmid pISRE-Luc (1 µg/well) encoding firefly luciferase and pRL-TK (4 ng/well) expressing Renilla luciferase as an internal control were cotransfected with CMPK2 plasmid (1 µg/well) into cells for 48 h. And the luciferase activity was assessed using the dual luciferase assays kit (Promega, Madison, WI, USA). Relative luciferase activity was calculated by dividing the firefly luciferase value by Renilla luciferase value as described previously.7, 16, 19
2.7 Protein sample preparation and Western blot
Cells were washed 3 times with PBS and harvested with radioimmune precipitation assay (RIPA) lysis buffer (Beyotime, China) containing PMSF protease inhibitor (Biosharp, China). Cell lysates were centrifuged at 15,000 g for 20 min at 4°C. Protein concentration was determined with BCA Protein Assay Kit (Beyotime, China). 30 µg total protein was boiled at 99°C for 5 min in SDS-PAGE sample buffer and loaded into each well. Proteins were separated by SDS-PAGE and transferred into PVDF membranes (Millipore, Billerica, MA, USA). After blocking with 5% bovine serum albumin (BSA) (Solarbio, China) at room temperature for 2 h, the membranes were then incubated with specific primary antibodies at 4°C overnight, followed by washing with tris-buffered saline with Tween (TBST) three times. The primary antibodies were used as follows: anti-GADPH (Proteintech, China), anti-CMPK2 (abcam, USA), anti-FLAG (Sigma, St. Louis, MO, USA), anti-p-STAT1 phosphorylated Tyr701 (Cell Signaling Technology, Danvers, MA, USA), anti-STAT1 (Cell Signaling Technology, Danvers, MA, USA), anti-ZIKV NS1 (GeneTex, Irvine, CA, USA). The secondary antibodies used were HRP-labeled goat anti-mouse IgG or anti-rabbit IgG (Beyotime, China). The protein bands were visualized using the ECL Western blot analysis Analysis System (Mllipore, Billerica, MA, USA) on Image Quant LAS 4000 mini system (GE, Milwaukee, WI, USA).
2.8 CCK-8 assay
Cell viability was detected by CCK-8 kit (TransGen Biotech, China) according to the manufacturer's protocols. Briefly, cells were seeded at 8000/100 µL in each well of the 96-well plate, and 10 µL CCK8 detection solution was added into each well at the indicated time for 30 min at 37°C, and the absorbance at OD 450 nm was tested.
2.9 Statistical analysis
Data from all cell culture-based assays were expressed as mean ± standard deviation (SD) of at least three biologic replicates. Data were analyzed using 2-tailed Student's t-test when compared between 2 groups and one-way ANOVA for more than 2 groups. In all analyses, ns represents p > 0.05, * represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001 and **** represents p < 0.0001 for indicated comparisons. p < 0.05 is considered as statistically significant.
3 RESULTS
3.1 ZIKV infection regulated host innate antiviral genes expression including CMPK2
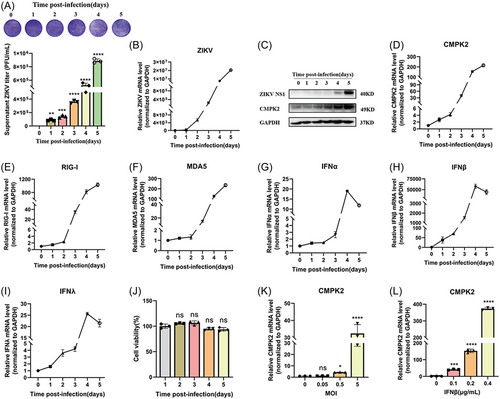
We previously reported that ZIKV infection significantly regulated the host genes expression profiles in A549 cells.16 We infected A549 cells with ZIKV at a multiplicity of infection (MOI) of 0.5 and detected the viral titers in the supernatant, the virus RNA and NS1 expression in cells at 1-5 days postinfection (Figure 2A-D). We confirmed virus replication by observing the significant increase of viral titers in cells supernatant by plaque assay (Figure 2A), as well as ZIKV RNA and NS1 expression in the cells from 0 to 5 days postinfection (dpi) (Figures 2B, 2C). We found that CMPK2 mRNA and protein levels were significantly increased after ZIKV infection (Figures 2C, 2D). In addition, we also observed that a subset of interferon related genes (RIG-I, MDA5, IFNα, IFNβ and IFNλ) were induced by ZIKV infection from day 0 to 5, though IFNα, IFNβ and IFNλ reached its peak on day 4 and then decreased slightly on day 5 (Figure 2E-I). ZIKV infection did not affect cell viability in A549 cells (Figure 2J). We also confirmed the induction of CMPK2 by ZIKV infection at different MOIs (MOI = 0, 0.05, 0.5 and 5) in A549 cells, and found that CMPK2 mRNA was significantly increased with the increase of ZIKV MOI at 48hrs postinfection (Figure 2K). It has been reported that CMPK2 is one of ISGs.11 To confirm CMPK2 can be induced by IFNβ in A549 cells, we treated the cells with increasing dosage of IFNβ at 0.1, 0.2 and 0.4 μg/mL, and found CMPK2 mRNA was remarkably increased with the increase of IFNβ dose (Figure 2L). These results indicated that ZIKV infection activated RIG-I/MDA5/IFN pathway and induced CMPK2 expression.
3.2 ZIKV infection induced CMPK2 expression through a RIG-I/IFN dependent pathway
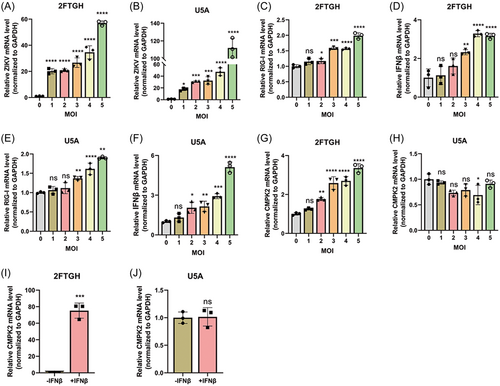
To assess whether ZIKV induced CMPK2 expression through an IFN-dependent pathway, we used an IFNAR-deficient U5A cell and its parental 2fTGH cell. Firstly, we infected both U5A and 2fTGH cells with ZIKV at MOI of 0, 1, 2, 3, 4, and 5 and we confirmed that ZIKV can successfully infect and replicate in both 2fTGH and U5A cells. We observed that ZIKV replicated more successfully in U5A cells compared with 2fTGH cells (Figures 3A, 3B). We found that ZIKV infection increased the expression of RIG-I and IFNβ both in 2fTGH (Figures 3C, 3D) and U5A cells (Figures 3E, 3F) when MOI ≥ 3. However, CMPK2 was only induced significantly in 2fTGH cells but not in U5A after ZIKV infection (Figures 3G, 3H). We verified that IFNβ treatment only induced CMPK2 mRNA expression in 2fTGH cells but not in the IFNAR-deficient U5A cells (Figures 3I, 3J). These indicated that ZIKV induced CMPK2 may need the binding of IFN-I to its receptors. To further confirm our results, we also knocked down IFNAR1 using CRISPR/Cas12a sgRNA in A549 cells and then infected the cells with ZIKV at MOI of 1. We found that knockdown of IFNAR1 significantly suppressed the induction of CMPK2 by ZIKV infection in A549 cells (Figure S1A-B). These results suggested that ZIKV induced CMPK2 dependent on the binding of IFN-I to its receptors (IFNARs) to activate the downstream pathway.
Schilling and colleagues reported that RIG-I is the main sensor for ZIKV infection in A549 cells.20 Esser-Nobis K et al. further identified that RIG-I, but not the other two RIG-I-like receptors (MDA5 and LGP2), plays a major role for the induction of innate immune response during ZIKV infection.5 We therefore focus our study on RIG-I, not other sensor molecules. Huh7 cells and RIG-I deficient Huh7.5.1 cells were used and infected with ZIKV (MOI = 1). The cellular total RNA was collected at different time-points post infection and ZIKV RNA was tested. Our results indicated that although ZIKV infected both Huh7 and Huh7.5.1 cells successfully, it replicated more abundantly in Huh7.5.1 than in Huh7 cells (Figures 4A, 4B). ZIKV infection did not affect the cell viability of Huh7 and Huh7.5.1 cells (Figures 4C, 4D), while increased CMPK2 mRNA expression significantly in Huh7 cells (Figure 4E) but not in Huh7.5.1 cells (Figure 4F). Therefore, we speculated that RIG-I is required for ZIKV-induced CMPK2 expression. Then, we transfected RIG-I plasmid into the RIG-I deficient Huh7.5.1 cells and infected the cells with ZIKV (Figure 4G). We found that ZIKV infection upregulated CMPK2 mRNA level markedly in RIG-I overexpressed Huh7.5.1 compared with the empty vector group (Figure 4H). Moreover, RIG-I overexpression also decreased ZIKV RNA and NS1 protein level (Figures 4I, 4J). Furthermore, we knocked down RIG-I in A549 cells by CRISPR/Cas12a sgRNA and infected the cells with ZIKV at MOI of 1. We found that knockdown of RIG-I significantly decreased the induction of CMPK2 by ZIKV infection in A549 cells (Figure S2A-B).
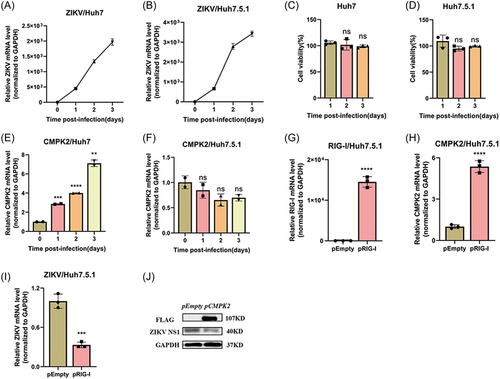
All these findings suggested that ZIKV-induced CMPK2 expression dependent on RIG-I and the integrality of IFN pathway.
3.3 CMPK2 inhibited ZIKV replication
Next, we explored the effect of CMPK2 on ZIKV replication. We constructed CMPK2 plasmid and transfected it into A549 cells followed by ZIKV infection at MOI of 1. We found CMPK2 mRNA and protein levels were gradually increased with the increase dose of pCMPK2 (Figures 5A, 5C). CMPK2 overexpression inhibited ZIKV RNA and NS1 expression significantly (Figures 5B, 5C). Meanwhile, the plaque assay of the supernatant from CMPK2-overexpressed A549 cells demonstrated that overexpression of CMPK2 significantly reduced ZIKV viral titers (Figure 5E). On the contrary, CMPK2 siRNA decreased CMPK2 mRNA and protein significantly, promoted ZIKV RNA replication and NS1 protein expression in cells remarkably (Figure 5F-H). Consistent with this, the viral titer in the supernatant quantified by plaque assay was significantly increased (Figure 5J). CCK8 assay confirmed that both pCMPK2 plasmid and siCMPK2 transfection did not affect the viability of A549 cells (Figures 5D, 5I).
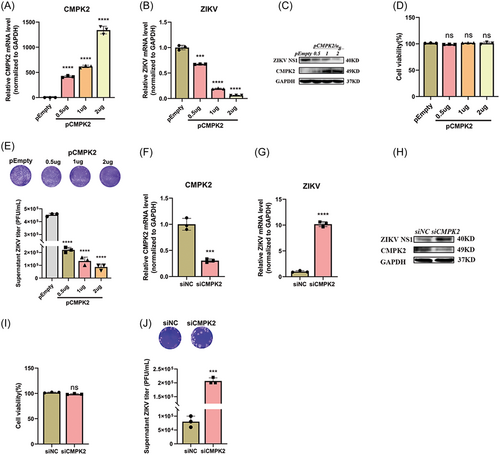
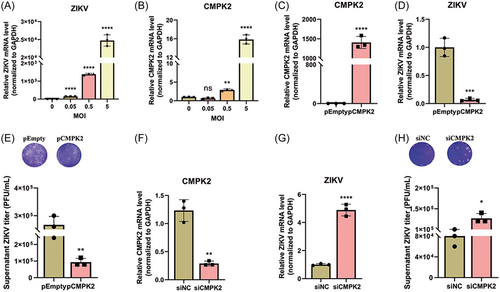
Moreover, we also confirmed the induction of CMPK2 following ZIKV infection in U251 cells. Firstly, we infected U251 cells with ZIKV at MOIs of 0, 0.05, 0.5, and 5, respectively and CMPK2 mRNA expression was detected at 48 h postinfection. Consistent with the findings we observed in A549 cells, ZIKV infection induced CMPK2 expression significantly (Figure 6A-B). Secondly, we overexpressed or knocked down CMPK2 in U251 cells to analyze its effect on ZIKV replication. Overexpression of CMPK2 significantly reduced ZIKV RNA replication in cells and viral titers in the supernatant of U251 cells (Figure 6C-E), while knocking down of CMPK2 promoted (Figure 6F-H). All these findings suggested that CMPK2 inhibits ZIKV replication in both A549 and U251 cells.
3.4 CMPK2 inhibited ZIKV replication through the activation of IFN-Induced Jak/STAT signaling pathway
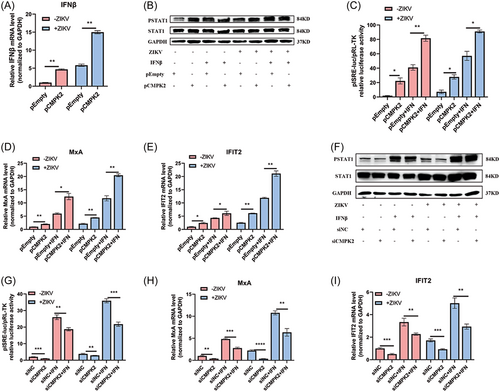
Innate immune response is the first line of defense against virus infection and plays an important role in the antiviral immune response. To investigate whether CMPK2 inhibited ZIKV replication through activating IFN-induced classical Jak/STAT pathway, we overexpressed or knocked down CMPK2 in A549 cells with or without ZIKV infection and treated the cells with or without IFNβ (0.1 µg/mL). We found that both overexpression of CMPK2 and ZIKV infection significantly induced IFNβ mRNA expression in A549 cells compared with empty vector control (Figure 7A). Overexpression of CMPK2 increased p-STAT1 level, ISRE activity, and ISGs including Myxovirus resistance-A (MxA) and interferon-induced protein 2 (IFIT2) expression in both A549 and ZIKV-infected A549 cells with or without IFNβ treatment compared to empty vector (Figure 7B-E). On the contrary, knocked-down of CMPK2 decreased the p-STAT1 level, ISRE activity and mRNA levels of downstream ISGs, such as MxA and IFIT2 (Figure 7F-I). These findings indicated that CMPK2 serves as a positive-feedback regulator for the production of type I IFN and activation of the Jak/STAT pathway.
4 DISCUSSION
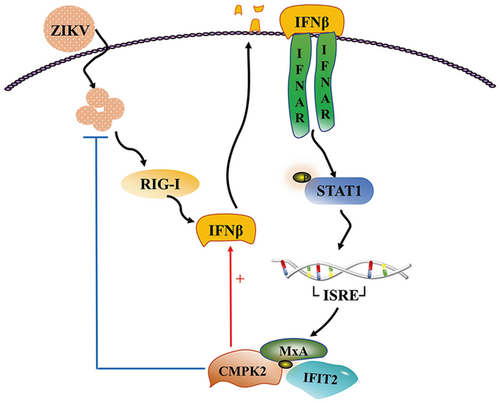
Although ZIKV was first isolated in Uganda in 1947, it did not pose a serious threat until about 66 years later when it caused an epidemic in French Polynesia in 2013.21 ZIKV has spread globally and caused panic, with approximately 440 000–1300 000 cases in Brazil during the 2016 outbreak.22 In July 2019, ZIKV spread to 87 countries and territories globally with autochthonous transmission, distributed across four of the six WHO regions.23 On the other hand, the coronavirus disease 2019 (COVID-19) pandemic has also likely resulted in underreporting of ZIKV infections due to the lockdowns and over-loading the health-care system.24 No therapies were available to treat ZIKV infection at present. Therefore, extensive study on the pathogenesis of ZIKV is essential to facilitate vaccine and antiviral drug development. In this study, we clarified that ZIKV infection induced CMPK2 expression through an RIG-I and IFN-dependent manner; the production of CMPK2 inhibited ZIKV replication and promoted the expression of IFNβ by a positive feedback loop to further activate Jak/STAT signaling pathway (Figure 8). Our findings broadened the understanding of the antiviral function of CMPK2 and provided potential therapeutic targets against ZIKV infection.
The activation of IFN-mediated Jak/STAT signaling pathway is the first-line defence for virus infection. In our previous study, we profiled the differentially expressed genes after ZIKV infection in A549 cells by RNAseq, which showed that multiple ISGs were upregulated after ZIKV infection.16 Here, we again confirmed the significant induction of IFNs and ISGs after ZIKV infection, though the induction occurred 48 h postinfection, which seems a little bit delayed. Previous study has shown that Myxovirus resistance protein A (MxA) is induced 48 h after the Asian ZIKV strain (GZ01) infection at lower MOI (0.1).7 We speculate that the delayed activation of IFNs in this study maybe because of the low MOI (0.5) and the Asian ZIKV strain (GZ01) we used, which has previously been reported to induce much weaker and delayed innate immune signaling in infected cells.5 Next, we clarified one of the ISGs, CMPK2, is significantly induced during ZIKV infection in human non-small-cell lung cancer cell line (A549 cells) and human glioma cell line (U251 cells). CMPK2 has also been found to be significantly upregulated in the leukocytes and nasopharyngeal tissues of patients infected with SARS-CoV-2.25 RIG-I has been found to play a major role for induction of antiviral innate immunity during ZIKV infection.5, 20 Using both RIG-I-deficient or IFNAR-deficient cells and knockdown of RIG-I or IFNAR1 directly, we further investigate the detailed mechanisms of CMPK2 induction. We observed that ZIKV infection induced a significant increase of CMPK2 in 2fTGH but not IFNAR-deficient U5A, though RIG-I and IFNβ were significantly upregulated in both 2fTGH and U5A cells, indicating that IFNAR is necessary for CMPK2 induction. Similarly, the induction of CMPK2 by ZIKV was only observed in Huh7 but not its RIG-I-deficient derivatives Huh7.5.1. Meanwhile, complementation of Huh7.5.1 with RIG-I plasmid recovered CMPK2 induction after ZIKV infection. Though 2fTGH/U5A and Huh7/Huh7.5.1 were widely used in vitro study for the comparison of disrupted interferon signaling, we confirmed our findings by knockdown RIG-I and IFNAR1 in A549 cell directly. As respect, knockdown of RIG-I and IFNAR1 significantly decreased the induction of CMPK2 after ZIKV infection. And similar to our current study, Kim et al. reported that the induction of CMPK2 in macrophages is dependent on the IRF3-IFNAR signaling axis.26 Taken together, these results suggested that ZIKV induced CMPK2 expression through RIG-I and IFN signaling integrality-dependent pathways.
Many studies have reported that ISGs play critical roles to restrict ZIKV replication.27-33 For example, Viperin has been reported to inhibit ZIKV RNA translation by activating ribosome collision-dependent pathway.27 IFITM1 and IFITM3 inhibited ZIKV infection at the early stage of the viral life cycle and prevented ZIKV-induced cell death.28 ADP-ribosyl transferase PARP11 has been identified as an anti-ZIKV ISG, which interacted with PARP12 to promote PARP12-mediated ZIKV NS1 and NS3 degradation.29 OAS2 inhibits ZIKV replication through type I IFN signaling pathway.16 CMPK2 is an ISG, which has been reported to play multiple roles on anti-viruses, and inflammation response.10, 13, 34 The antiviral effect of CMPK2 has already been reported for several viruses such as DENV, HIV, HEV4, SVCV and multiple coronaviruses.11, 13-15, 35 It has been reported that CMPK2 is crucial for the phosphorylation of dCMP, dUMP and further generating mitochondrial DNA in mitochondria.13 Zhao et al. demonstrate CMPK2 deficiency alters mitochondrial structures and functions, leading to mitochondria dysregulation.36 DENV infection specifically increased cytosolic mtDNA levels and CMPK2 KO significantly abolished DENV-induced mtDNA release into the cytosol.13 Release of mitochondrial DNA into the cytoplasm and out into the extracellular milieu activates a plethora of different pattern recognition receptors and innate immune responses, including cGAS-STING, TLR9 and inflammasome formation leading to, among others, robust type I interferon responses and exert antiviral response eventually.37
Most recently, Pawlak et al also reported that CMPK2 inhibited ZIKV replication although the mechanism is different from what we described here.10 They found CMPK2 directly inhibits the translation of viral proteins, and this restricting effect is dependent on the N-terminal domain (NTD) of CMPK2 which lacks kinase activity. And the mitochondrial localization of CMPK2 is required for its antiviral effects.10 It has been well known that virus-host interaction plays an important role in the host defence mechanisms against many virus infections. Our results indicated that the anti-ZIKV effect of CMPK2 is RIG-I- and IFNAR-dependent and CMPK2 is a positive regulator by forming a regulation loop to stimulate the production of type-I IFN and further activate the Jak/STAT signaling pathway, leading to enhanced production of antiviral ISGs, including CMPK2 (Figure 8). Therefore, our study advances the field of cell signaling during innate immune response against ZIKV infection. However, Pawlak et al demonstrated that CMPK2 acted on ZIKV itself to inhibit viral protein translation. We believe these two independent studies are complementary to each other, providing a more in-depth deciphering of the mechanisms on how CMPK2 restricts ZIKV infection.
In conclusion, ZIKV infection induces CMPK2 expression, which in turn to suppress ZIKV replication and serves as a positive-feedback regulator to promote the expression of IFNβ and activate Jak/STAT signaling pathway. Our findings broadened the understanding of the antiviral function of CMPK2 and provided potential therapeutic targets against ZIKV infection.
ACKNOWLEDGMENTS
This research is supported by the National Key Research and Development Program of China (2018YFE0107500); Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine (CAMS-2021-I2M-1-060); Natural Science Foundation of China (NSFC 82102383); Sichuan Science and Technology Program under Grant (2022JDRC0047); Science and Technology Partnership Program, Ministry of Science and Technology of China (KY201904011); QinChuangyuan recruited high-level innovation and entrepreneurship talents project of Science and Technology Department of Shanxi Province (QCYRCXM-2022-56); Medical Research project of Xi'an Science and Technology Bureau (22YXYJ0120).
CONFLICT OF INTEREST STATEMENT
All authors disclosed no relevant relationships.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




