Development of a virus-based affinity ultrafiltration method for screening virus-surface-protein-targeted compounds from complex matrixes: Herbal medicines as a case study
Abstract
Herbal medicines (HMs) are one of the main sources for the development of lead antiviral compounds. However, due to the complex composition of HMs, the screening of active compounds within these is inefficient and requires a significant time investment. We report a novel and efficient virus-based screening method for antiviral active compounds in HMs. This method involves the centrifugal ultrafiltration of viruses, known as the virus-based affinity ultrafiltration method (VAUM). This method is suitable to identify virus specific active compounds from complex matrices such as HMs. The effectiveness of the VAUM was evaluated using influenza A virus (IAV) H1N1. Using this method, four compounds that bind to the surface protein of H1N1 were identified from dried fruits of Terminalia chebula (TC). Through competitive inhibition assays, the influenza surface protein, neuraminidase (NA), was identified as the target protein of these four TC-derived compounds. Three compounds were identified by high performance liquid chromatography (HPLC) and liquid chromatography/mass spectrometry (LC/MS), and their anti-H1N1 activities were verified by examining the cytopathic effect (CPE) and by performing a virus yield reduction assay. Further mechanistic studies demonstrated that these three compounds directly bind to NA and inhibit its activity. In summary, we describe here a VAUM that we designed, one that can be used to accurately screen antiviral active compounds in HMs and also help improve the efficiency of screening antiviral drugs found in natural products.
1 INTRODUCTION
According to the World Health Organization (WHO), there are approximately 1 billion influenza (flu) virus cases annually, leading to a large disease burden.1, 2 Seasonal influenza A virus (IAV) infections are the most common cause of pneumonia-related deaths in developed countries, with H1N1 in particular having the greatest impact among all types of IAVs.3, 4 The main course of prevention and treatment of H1N1 infections are vaccines and antiviral drugs, respectively.5, 6 Influenza vaccines need to be administered annually due to the prevalent antigenic drift of hemagglutinin (HA), the main entry glycoprotein of influenza viruses. Due to this, influenza vaccine development relies on the prediction of future circulating strains, rendering them less effective due to inaccurate annual predictions.7 At present, the main drugs used to treat H1N1 infections are neuraminidase (NA) inhibitors (oseltamivir, zanamivir, and peramivir),8 M2 ion channel (M2) inhibitors (amantadine),9 and viral RNA-dependent RNA polymerase (RdRP) inhibitors (favipiravir and baloxavir marboxil).10, 11 Unfortunately, the use of these drugs is limited due to viral resistance and side effects.12-14 Therefore, there is an urgent need to develop novel IAV inhibitors, specifically against H1N1.
Herbal medicines (HMs) are a natural treasure trove of rich chemical skeletons and have become one of the main sources of lead compounds for drugs.15, 16 Since 1981–2019, 63.1% of the small molecule drugs in the world market were associated with natural products.17 In recent years, the identification of antiviral compounds derived from HMs has gained popularity as a key starting point for antiviral drug discovery. However, due to the large number of chemical structures contained in HMs, the screening and analysis of active compounds is very difficult.
At present, the methods for discovery of HM pharmacodynamic substances include systematic isolation and screening,18-20 activity tracking and screening,18-20 serum pharmacochemistry,21 cell membrane chromatography (CMC),22 computer-aided virtual screens,23 chemical substance-omics,24 component knockout/knock-in,25 compound library and high throughput screening,26 and affinity chromatography.27 In general, the discovery of active substances in HMs mainly adopts classical phytochemical methods. However, these approaches have their own limitations: (1) Bioactivity-oriented systematic isolation screening, activity tracking screening18-20: this method is inefficient, labor-intensive, expensive, time-consuming, and sample consuming.28 (2) High-throughput screening methods have also been used to efficiently identify virus-targeted compounds,26 however, the purpose of high-throughput screening methods is to screen pure compounds and is not suitable for directly detecting active compounds from complex matrices.28 (3) Screening NA inhibitors through affinity ultrafiltration and verifying their antiviral activity through pharmacological experiments,27 however, producing recombinant proteins is expensive, and the complete exposure of NA to a mixture of compounds increases the probability that the binding site of the compounds is not an active site. (4) Component knockout/knock-in method is more suitable for the validation of active ingredients rather than screening.25 (5) The operation of CMC is complicated, hence it is difficult to commercialize it.22 (6) The computer aided virtual screen method is quick and simple to screen the pharmacodynamic substances in HMs, however, the accuracy of the results is limited and further verification is needed.23 (7) The use of the serum pharmacochemistry method alone is difficult to screen the active compounds in HMs. This method requires the combination of other methods, such as network pharmacology, serum pharmacology, metabolomics, and so forth.21
Centrifugal ultrafiltration utilizes centrifugal force and a semipermeable membrane to retain suspended solids and high molecular weight solutes, while allowing liquid and low molecular weight solutes to penetrate the membrane based on the pore size of the membrane. Due to its simple operation, high speed, and high reliability, centrifugal ultrafiltration has become a useful technology for screening active compounds from natural complex matrices.27, 29-31 However, there are currently no reported screening methods for directly identifying virus-targeted compounds from complex matrices.
Therefore, it is necessary to further develop a simple, rapid and effective screening method to specifically screen active compounds targeting viruses from complex matrices such as HMs.
In this study, specific virus-binding compounds were screened from HMs by combining viral and centrifugal ultrafiltration. The ultrafiltrate was collected and injected into an HPLC or LC/MS system to isolate and identify the active compounds. Finally, an effective VAUM was established to screen the antiviral compounds in HMs. Through this method, potential virus-binding compounds were screened from dried fruits of Terminalia chebula (TC), and the antiviral activity & target of the virus-binding compounds were verified. Our results show that VAUM is an effective method to effectively screen potential active compounds from HMs that bind to viruses, as shown by our studies using H1N1 as a target. VAUM may also contribute to the elucidation of the mechanism of action (MOA) of antivirals and aid drug discovery using HMs as sources of lead compounds.
2 MATERIALS AND METHODS
2.1 Chemicals and reagents
Oseltamivir acid, favipiravir, zanamivir, amantadine, punicalagin, ellagic acid (all purities above were ≥98%) were obtained from Shanghai Yuanye Bio-Technology. Antibodies against the influenza nucleoprotein (NP) were obtained from GeneTex, Inc. Antibodies against GAPDH were obtained from ABclonal Biotech Co., Ltd. The SDS‒PAGE gel kit was obtained from Beijing Solarbio Science & Technology Co., Ltd. Influenza A H1N1 (A/California/04/2009) Neuraminidase/NA (His Tag) was obtained from Sino Biological, Inc. Ammonium acetate buffer (pH 7.4) was obtained from Shanghai Yuanye Bio-Technology. HPLC grade acetonitrile was supplied by Tedia Company, Inc. Centrifugal filter (Amicon Ultra-0.5, 100 kDa) was obtained from Millipore Co., TC was purchased from Beijing Tong Ren Tang Chinese Medicine Co., Ltd. Neuraminidase Inhibitors Screen Kit was obtained from Beyotime Biotech Inc. Co., Ltd. TC is extracted from 50% ethanol aqueous solution, filtered, concentrated, and dried for subsequent experiments.
2.2 Cells and viruses
Madin-Darby canine kidney (MDCK) epithelial cells and Human Epithelioma-2 (Hep2) were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 1000 units/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2 incubator. Influenza viruses A/Puerto Rico/8/1934 (H1N1) and respiratory syncytial virus (RSV) were rescued and preserved in −80°C.
2.3 Screening procedure of VAUM
Pa and Pb are the peak areas in the experiment and control group, respectively.
2.4 HPLC and LC/MS analysis
HPLC analyses were primarily performed by an Agilent 1260 system. Analytes were separated on a reverse phase C18 column (Kromasil 100-5 C18, 250 × 4.6 mm). Samples with a volume of 20 µL were injected into the column for separation at a column temperature of 30°C. At a flow rate of 1.0 mL/min. The mobile phase composed of solvent mixture of acetonitrile (solvent A) and 0.1% aqueous phosphoric acid (solvent B), was programmed with a linear gradient of solvent A as follows, 0–10 min, 8%–8%; 10–11 min, 8%–20%; 11–18 min, 20%–20%; 18–19 min, 20%–30%; 19–27 min, 30%–30%; 27–28 min, 30%–90%; 28–35 min, 90%–90%; 35–36 min, 90%–100% (oseltamivir acid, ING-1466, favipiravir) or 0–10 min, 5%–5%; 10–45 min, 5%–15%; 45–55 min, 15%–19%; 55–60 min, 19%–25%; 60–75 min, 25%–70%; 75–76 min, 70%–100%; 76–85 min, 100%–100% (TC). The detection wavelength of diode array detector (DAD) was set at 250 nm (TC), 234 nm (favipiravir), and 207 nm (ING-1466 and oseltamivir acid).
The LC-MS and MS/MS was obtained on UHPLC Ultimate 3000 instrument coupled with a Q-Exactive Orbitrap-MS spectrometer (Thermo Fisher Scientific) with Halo C18 column (2.1 × 100 mm, 2.7 μm). The mobile phase consisted of acetonitrile (A) and 0.1% formic acid in water (B). The UPLC elution processes were as follows: 0–15 min, 5%−60% A, 15–20 min, 60%–100% A, 20–25 min, 100%–100% A. Analysis was performed at a flow rate of 0.4 mL/min, the column temperature was 40°C, with an injection volume of 10 μL. The ESI-MS/MS parameters were applied as follows: negative full scan dd-MS2 modes, aux gas flow rate was 10 arb, capillary temperature was 350°C, spray voltage was 3.0 kV, sheath gas flow rate was 45 arb, s-lens RF level was 55.0 V, scan range m/z was 80–1000 Da at the resolution of 70 000, the stepped Normalized Collisional Energy was 20, 40, and 60 eV.
2.5 CPE assay
To determine the improvements mediated by oseltamivir acid, favipiravir, ING-1466, punicalagin, ellagic acid, or TC with respect to the cytopathic effects of the virus, a CPE assay was performed. MDCK cells seeded into 96-well plates and were infected with H1N1 at a multiplicity of infection (MOI) of 0.01 in the presence of different concentrations of oseltamivir acid (50, 150 μM), favipiravir (50, 150 μM), ING-1466 (50, 150 μM), punicalagin (12.5, 25, 50 μM), ellagic acid (6.25, 12.5, 25 μM) or TC (25, 50, 100 μg/mL). An MTT assay was performed determine cell viability after 48 h of incubation. Hep2 cells seeded into 96-well plates were infected with RSV (MOI = 0.01) in the presence of different concentrations of oseltamivir acid (50, 150 μM), favipiravir (50, 150 μM), ING-1466 (50, 150 μM) for 2 h. The inoculum was discarded, and Hep2 cells were washed three times with phosphate buffer saline (PBS) to remove unabsorbed viruses. Then, the cells were treated with fresh DMEM with 10% FBS. An MTT assay was performed to determine cell viability after 72 h of incubation.
2.6 Virus yield reduction assay
MDCK cells seeded in 12-well plates were infected with H1N1 at an MOI of 0.01. The cells were cultured with virus isolation serum-free medium (2 μg/mL TPCK–trypsin) in the presence of oseltamivir acid (30 μM), punicalagin (12.5 μM), ellagic acid (12.5 μM), or TC (50 μg/mL). At 36 h postinfection, the supernatants were collected and the virus titers were measured using MDCK cells.
2.7 Western blot analysis
MDCK cells were seeded at a density of 2 × 105 cells/well in 12-well plates. After 12 h, the cells were infected with H1N1 (MOI = 0.2) in the presence of different concentrations of punicalagin (25 μM) or ellagic acid (25 μM). Protein expression was measured by western blot (WB) analysis at 8 and 24 h postinfection. Proteins were extracted with RIPA buffer (high) according to the manufacturer's instructions. Protease inhibitors were included in all buffers to preserve protein integrity. Proteins were separated by SDS‒PAGE and transferred onto an immobilon-NC membrane. The membrane was immersed in Tris-buffered saline-Tween (TBST) blocking solution containing 5% skim milk to block nonspecific binding. After a short wash in TBST, the membranes were incubated with primary antibodies overnight at 4°C. After a brief wash in TBST, the membranes were incubated with the appropriate secondary antibodies for 1.5 h at room temperature. The blots were then developed using an enhanced chemiluminescence assay. The images obtained were quantified using ImageJ 1.8.0 software.
2.8 Immunocytofluorescence
Cell slides were placed into 12-well plates, and MDCK cells were seeded at a density of 1 × 105. After 12 h, the cells were infected with H1N1 at an MOI of 0.2 in the presence of different concentrations of punicalagin (25 μM) or ellagic acid (25 μM). The medium was removed after 8 or 24 h, and the cells were washed twice with chilled PBS at 4°C. The cells were fixed with 500 µL of acetone/methanol (1:1 v/v) that was prechilled at −20°C for 5 min. Then washed with 1 mL of prechilled PBS at 4°C for 5 min. The cells were blocked with 3% BSA in PBS for 30 min at room temperature and washed with PBS. Then, the cells were incubated with rabbit anti-NP antibodies (1:100 dilution) in 3% BSA overnight at 4°C. The cells were washed three times and subsequently incubated with Alexa Fluor 594-conjugated goat anti-rabbit IgG (1:300 dilution) in 3% BSA for 1 h, at room temperature. The cells were washed three times with PBS, and the cell slides were mounted onto glass slides using antifading mounting medium (with 4′,6-diamidino-2-phenylindole), dried and kept overnight at 4°C. Imaging was performed on a fluorescence microscope at excitation/emission wavelengths of 488/505–550 nm. Images of the fluorescence patterns were merged to visualize colocalization by ImageJ 1.8.0 software.
2.9 Neuraminidase inhibition assay
The NA inhibition assay was performed using Neuraminidase Inhibitors Screen Kit (Beyotime). According to manufacturer's instruction, 70 μL of reaction buffer solution, 10 μL of NA, and 10 μL of various concentrations of the studied compounds were added to each well of 96-well plates. To allow NA-compound mixture interaction, the 96-well plates were mixed for 1 min and incubated at 37°C for 2 min to enable the samples fully react with the NA. Then, 10 μL of fluorescent substrate was added, mixed, and incubated at 37°C for 1 h. The fluorescence was measured by SYNERGY neo2 multimode reader.
2.10 Surface plasmon resonance (SPR) assay
The recombinant NA protein was transferred to PBS buffer through three ultrafiltration substitutions for subsequent amino coupling. His-tagged NA was immobilized on an activated CM5 chip at 50 μg/mL in pH 4.0 acetate buffer, and a blank channel was used as the negative control. Punicalagin and ellagic acid were serially diluted to various concentrations and then flowed through the chip (injection time: 60 s, injection flow rate: 30 μL/min; dissociation time: 180 s, injection flow rate: 30 μL/min) respectively. The KD values were calculated using a steady affinity state model (ellagic acid) or dynamic fitting method (punicalagin) by the Biacore T100 analysis software.
2.11 Statistical analysis
Data are expressed as the mean ± SD. Statistical differences were analyzed by student's t test analysis using the SPSS software. A value of p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Reliability of VAUM
The surface proteins of H1N1 include hemagglutinin (HA), neuraminidase (NA), and M2, while the intracellular proteins mainly include matrix-1 (M1), NS1, nuclear export protein (NEP), nucleoprotein (NP), and polymerase complexe subunits PB1 and PB2. The representative inhibitors of H1N1 mainly include NA inhibitors (oseltamivir, zanamivir, peramivir),8 M2 inhibitor (amantadine),9 viral RdRP inhibitors (favipiravir and baloxavir marboxil),10, 11 and HA inhibitor (ING-1466).32 ING-1466, a group 1 influenza A viruses (H1N1 and H5N1) HA inhibitor,33, 34 can form multiple binding interactions in the specific hydrophobic pocket on HA to inhibit the viral entry of H1N1.32 Oseltamivir acid can bind to NA to inhibit the release of H1N1.35 Favipiravir can selectively inhibit the RdRP, thus blocking the replication of H1N1.10 First, two H1N1 surface protein inhibitors, oseltamivir acid and ING-1466, and a nonsurface protein inhibitor, favipiravir, were used to investigate whether VAUM could accurately identify compounds that targeted two different viral surface proteins, and to ensure that it could not be used to identify compounds that bind to internal proteins of H1N1.
The recognition ability of VAUM was evaluated by using oseltamivir, ING-1466, and favipiravir with a blank solution of H1N1 as the negative control. As shown in Figure 1A–C,I, oseltamivir acid and ING-1466 peaks significantly increased compared with the control group. Conversely, the peak area of favipiravir had no significant difference compared with the control group. This data showed that VAUM recognized compounds that bind to the H1N1 surface proteins HA and NA, whereas favipiravir, which acts on the internal protein (RdRP) of H1N1, was not recognized by VAUM.
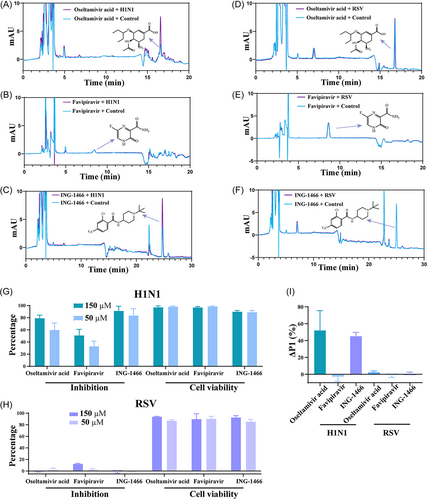
Oseltamivir acid and ING-1466, which are specific anti-H1N1 drugs, have no report of their inhibitory effect on RSV. Favipiravir can block the replication of various viruses, but has no inhibitory effect on RSV surface proteins.10 The anti-H1N1 and anti-RSV activity of oseltamivir acid, ING-1466, and favipiravir were detected. As shown in Figure 1G,H, oseltamivir acid, ING-1466, and favipiravir all had good inhibitory effect on H1N1, but poor inhibitory effect on RSV. These results indicated that oseltamivir acid, ING-1466, and favipiravir did not act on RSV surface proteins.
The recognition ability of VAUM was evaluated by using oseltamivir acid, ING-1466, and favipiravir with a blank solution of RSV as the negative control. As shown in Figure 1D–I, the peak area of oseltamivir acid, ING-1466, and favipiravir had no significant difference compared with the control group. The results further indicate that VAUM can only recognize compounds that act on the surface proteins of the virus, but not those that act on the internal proteins of the virus or those that have no inhibitory effect on the virus.
3.2 Optimization of conditions for screening antiviral compounds in TC by VAUM
Additionally, we used VAUM to screen antiviral compounds in HMs. We further optimized our VAUM screening conditions using TC, which is known to have anti-H1N1 activity.36 To obtain the best screening performance for active compounds in TC, sample concentration and incubation time were investigated.
Sample concentration will affect the screening of active compounds. At low concentrations, trace compounds are difficult to identify because they may not be detected by HPLC or LC/MS, or can easily be competitively replaced by high-content compounds. Extremely high sample concentrations may increase the likelihood of interference from inactive compounds in HMs and further increase the probability of false–positive results. In this study, three concentrations of TC (2.5, 5, 10 mg/mL) were investigated. As shown in Figure 2A–C,F, a total of five active compounds were found in TC, the number of active compounds found in TC had little relationship with the concentration of TC. The ΔP1 value of compounds 1 and 2 had little correlation with the concentration of TC, possibly because compounds 1 and 2 had higher ΔP1 value at lower concentrations of TC. The ΔP1 value of compound 3 increased with increasing TC concentration (although it decreases at 10 mg/mL). The ΔP1 value of compound 4 increased with increasing TC concentration. The ΔP1 value of compound 5 was less than 20% (although the ΔP1 value is slightly greater than 20% at the concentration of TC at 2.5 mg/mL), due to this, compound 5 was no longer considered in subsequent target screening and pharmacological tests. Based on the results mentioned above, the optimal concentration of TC was selected as 10 mg/mL.
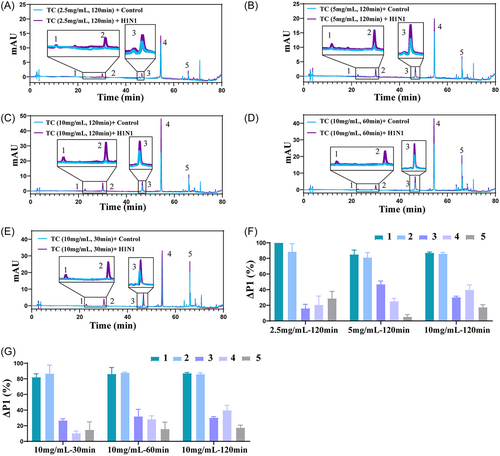
Incubation time can also affect screening results. Short incubations may result in compounds not being able to tightly bind to the virus, while prolonged incubation is unnecessary. In this study, three incubation times (30, 60, 120 min) were investigated. The ΔP1 values of compounds 1, 2, and 3 had little correlation with incubation time (Figure 2C–E,G), indicating that compounds 1, 2, and 3 were able to bind tightly to the surface protein of H1N1 in a short time. The ΔP1 value of compound 5 was less than 20%. The ΔP1 value of compound 4 increased with the increase of incubation time, and the ΔP1 value exceeds 20% when the incubation time was 60 min. These results indicated that 60 min was sufficient for the screening of active compounds in TC.
3.3 NA was identified as the main target of active compounds in TC
Four active compounds against H1N1 were screened in TC by VAUM. These four compounds may act on the three surface proteins of H1N1. HA, NA, M2 inhibitors were added to co-incubate with H1N1 for 60 min, then TC was added for co-incubation with H1N1 for 60 min. The target protein of active compounds in TC was determined by VAUM with competitive or noncompetitive inhibition. If there was a competitive or noncompetitive inhibition relationship between the active compounds and the inhibitors, then the inhibitor group would have reduced the binding of the active compounds with H1N1 compared to the control group, and thus will show a decrease in the peak area of the active compounds. If there was neither competitive nor noncompetitive inhibition relationship between the active compounds and the inhibitors, further pharmacological experiments will be needed to find the target of its action.
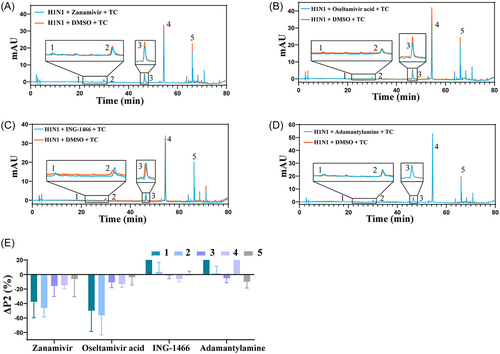
As shown in Figure 3, after the addition of zanamivir, the ΔP2 value of compounds 1, 2, 3, and 4 were all less than −10%. After the addition of oseltamivir acid, the ΔP2 value of compounds 1, 2, 3, and 4 were all less than −10%. After ING-1466 was added, the ΔP2 value of compound 1, 2, and 5 was greater than 0%, and the ΔP2 value of compound 3 and 4 was greater than −10%. These results indicated that the addition of ING-1466 promoted rather than inhibited the binding of compounds 1, 2, and 5 to the surface proteins of H1N1. After amantadine was added, the ΔP2 value of compounds 1, 2, and 4 was seen to be greater than 0%, and the ΔP2 value of compound 3 was greater than −10%. These results indicated that amantadine promoted rather than inhibited the binding of compounds 1, 2, and 4 to the surface proteins of H1N1. These results indicate that the target of compounds 1, 2, 3, and 4 is likely NA.
3.4 Identification and efficacy verification of active compounds acting on NA
First, based on the comparison of standard substances, compounds 1, 2, and 4 were identified as punicalagin α, punicalagin β, and ellagic acid by HPLC (Figure 4A). These results were confirmed by LC/MS, showing that compounds 1, 2, and 4 were indeed punicalagin α, punicalagin β, and ellagic acid respectively. Through LC/MS it was also identified that compound 3 may be chebulanin (Table 1). As the standard substance was not available, we did not consider chebulanin in the follow-up experiment.

| No. | tR(min) | Molecular formula | [M-H]−m/z exptl. (theor.) | Error(ppm) | Major MS/MS ions | Identification |
|---|---|---|---|---|---|---|
| 1a | 1.76 | C48H28O30 | 1083.0582(1083.0592) | −0.9233 | 781.0527, 600.9894, 575.0100, 300.9986, 270.9883 | Punicalagin α |
| 2a | 2.29 | C48H28O30 | 1083.0585(1083.0592) | −0.6463 | 781.0527, 721.0313, 600.9896, 575.0101, 300.9986, 270.9884 | Punicalagin β |
| 4a | 4.48 | C14H6O8 | 300.9988(300.9989) | −0.3322 | 283.9960, 257.0087, 245.0089, 229.0138, 201.0185, 185.0234 | Ellagic acid |
| 1b | 1.70 | C48H28O30 | 1083.0579(1083.0592) | −1.2003 | 781.0544, 600.9898, 575.0099, 300.9987, 270.9883 | Punicalagin α |
| 2b | 2.36 | C48H28O30 | 1083.0591(1083.0592) | −0.0923 | 781.0535, 721.0326, 600.9897, 575.0102, 300.9988, 270.9884 | Punicalagin α |
| 4b | 4.21 | C14H6O8 | 300.9988(300.9989) | −0.3322 | 283.9962, 257.0088, 245.0087, 229.0136, 201.0184, 185.0233 | Ellagic acid |
| 3b | 2.77 | C27H24O19 | 651.0829 (651.0828) | 0.1536 | 481.0620, 275.0198, 247.0243, 169.0129 | Chebulanin |
- Abbreviation: TC, Terminalia chebula.
- a Reference substance.
- b TC.
It was determined by CPE that TC, punicalagin, and ellagic acid had dose-dependent protective effects on cells infected with H1N1 (Figure 4B–D). A virus yield reduction assay was used to determine that TC, punicalagin, and ellagic acid could inhibit the life cycle of H1N1 (Figure 4E).
The life cycle of the virus (fusion, replication, translation, and release) was used to determine whether punicalagin and ellagic acid acted on NA. NA is responsible for cleaving the terminal sialic acid from the host receptors in the viral replication cycle.35 In the multistep life cycle of the virus, the amount of NP protein is reduced regardless of the stage of the drug's action during viral entry, replication, or release phase. However, in a viral replication cycle, if the drug affects the viral entry or replication phase of the virus, it will reduce the content of NP. If the drug acts during the release phase of the virus, the content of NP will not be affected. As shown in Figure 4F, the NP content was detected by WB at 8 and 24 h postinfection, respectively. The results showed that 8 h after adding punicalagin and ellagic acid, there was no significant difference in NP content compared to the H1N1 group. However, 24 h after adding punicalagin and ellagic acid there is a significant reduction in NP content. To make the results more visual, we performed immunofluorescence (IF) staining (Figure 4G,H), and the results were consistent with WB. This indicates that the main target of punicalagin and ellagic acid is NA. Previous research by our team also supports this view.37
3.5 Verification of NA as the target of anti-H1N1 compounds in TC
To investigate whether NA activity was directly inhibited by punicalagin, ellagic acid, or TC, an NA inhibition assay was carried out following the manufacturer's instructions. The results showed that punicalagin, ellagic acid, and TC inhibited NA activity in a dose-dependent manner (Figure 5A).
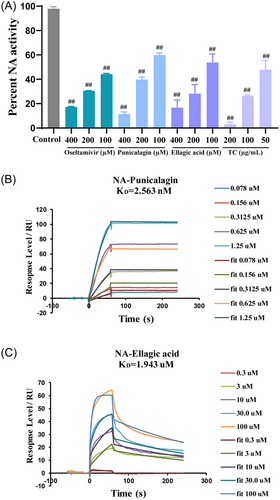
To verify whether punicalagin and ellagic acid directly bind to NA, SPR, a robust biophysical method, was used to evaluate the binding affinities of punicalagin and ellagic acid to NA. The results indicated that both punicalagin and ellagic acid could directly bind to NA in a dose-dependent manner (Figure 5B,C). The binding of punicalagin to NA was in accordance with the slow dissociation mode with KD value of 2.563 nM. The binding of ellagic acid to NA was in fast binding and fast dissociation mode with KD value of 1.943 μM. This result is consistent with that of the anti-H1N1 compounds of TC screened by VAUM. Compared with ellagic acid, punicalagin has a higher ΔP1 value. After the addition of zanamivir and oseltamivir acid, respectively, punicalagin had a lower ΔP2 value than ellagic acid. In summary, these results showed that the target of punicalagin and ellagic acid anti-H1N1 was likely NA, which also shows that VAUM is feasible and reliable.
4 DISCUSSION
We have developed a highly efficient VAUM for systematic screening of virus-targeting compounds in complex matrices such as HMs. VAUM is an improved screening method based on traditional affinity ultrafiltration methods. The biggest advantage of the traditional affinity ultrafiltration method is that it can quickly identify active compounds from a large number of mixed compounds such as HMs. However, the traditional affinity ultrafiltration method for screening antiviral compounds in HMs is limited. At present, most methods have been optimized to screen NA inhibitors by affinity ultrafiltration. Their anti-H1N1 activity is then verified by pharmacological tests. However, this approach is limited because there are many other functional proteins in the virus that are not targeted, and the method is prone to producing false positive results. Compared to the traditional affinity ultrafiltration method, the advantages of this method are as follows: (1) VAUM replaces the protein monomer with the virus, which is more consistent with the physiological state of the virus. The complete exposure of protein monomers to small molecule mixtures in traditional affinity ultrafiltration methods increases the possibility that the compound binding site is not the active site. However, VAUM can reduce this probability. (2) Compared with the traditional affinity ultrafiltration method, VAUM can screen compounds with affinity by targeting multiple proteins (viral surface proteins). (3) Compared with the production of recombinant proteins, the production of viruses is more economical, less time-consuming, and less labor-intensive. However, VAUM also has its own limitations: (1) Although VAUM can target more functional proteins than traditional affinity ultrafiltration methods, it is also limited to the surface proteins of the virus, and the proteins in the membrane of the virus cannot be targeted. (2) Due to the use of live virus, this method needs to be carried out in a laboratory with the appropriate biosafety measures corresponding to the biosafety level of the virus. Despite this, it is undeniable that VAUM greatly improves the screening efficiency of antiviral compounds in complex matrices, such as HMs. VAUM improves the accuracy, expands the application range, and reduce the cost and labor of traditional affinity ultrafiltration method.
Positive and negative control experiments showed that VAUM has excellent recognition ability and reliability. We successfully identified four compounds from TC that can bind to H1N1 surface proteins. We identified the viral NA as the target of the four compounds by the addition of HA, NA, and M2 inhibitors in a competitive or noncompetitive manner. Three compounds were identified by HPLC and LC/MS as punicalagin α, punicalagin β, and ellagic acid. Through a pharmacological experiment we showed that punicalagin and ellagic acid could inhibit the release of H1N1 and provide protection against the cytopathic effect caused by H1N1. The mechanism of action was verified, and our results showed that punicalagin and ellagic acid could bind to NA to inhibit NA activity. Therefore, VAUM can be used to screen compounds targeting viruses in complex systems such as HM, helping to elucidate the mechanism of drug action and develop virus-targeting drugs from natural products.
5 CONCLUSION
In summary, the traditional affinity ultrafiltration method has been modified and improved to screen for active antiviral compounds in HMs. Here, we report for the first time that VAUM is directly applicable for screening of antiviral compounds in HMs. Furthermore, we show that VAUM is accurate, reliable, labor-saving, and economical. We believe that VAUM has the potential to be a reliable method for the screening of antiviral components in HMs.
ACKNOWLEDGMENTS
We are grateful for the support of the Collaborative Innovation Center for Antiviral Traditional Chinese Medicine in the Shandong Province. This work was supported by the National Natural Science Foundation of China (No. 82274204), Major Basic Program of Natural Science Foundation of Shandong Province (ZR2021ZD17), the Project for Development of TCM Science and Technology in Shandong Province (M-2022145), and Special Emergency research and development of Social Benefiting Technology Program, Qingdao (Grant No. 23-7-8-smjk-3-nsh).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data that support the findings of this study are presented in the manuscript. Data are available from the corresponding authors upon reasonable request.




