Low antibody levels associated with significantly increased rate of SARS-CoV-2 infection in a highly vaccinated population from the US National Basketball Association
Yonatan H. Grad and Christina DeFilippo Mack shared co-senior authorship.
Abstract
SARS-CoV-2 antibody levels may serve as a correlate for immunity and could inform optimal booster timing. The relationship between antibody levels and protection from infection was evaluated in vaccinated individuals from the US National Basketball Association who had antibody levels measured at a single time point from September 12, 2021, to December 31, 2021. Cox proportional hazards models were used to estimate the risk of infection within 90 days of serologic testing by antibody level (<250, 250–800, and >800 AU/mL1), adjusting for age, time since last vaccine dose, and history of SARS-CoV-2 infection. Individuals were censored on date of booster receipt. The analytic cohort comprised 2323 individuals and was 78.2% male, 68.1% aged ≤40 years, and 56.4% vaccinated (primary series) with the Pfizer-BioNTech mRNA vaccine. Among the 2248 (96.8%) individuals not yet boosted at antibody testing, 77% completed their primary vaccine series 4–6 months before testing and the median (interquartile range) antibody level was 293.5 (interquartile range: 121.0–740.5) AU/mL. Those with levels <250 AU/mL (adj hazard ratio [HR]: 2.4; 95% confidence interval [CI]: 1.5–3.7) and 250–800 AU/mL (adj HR: 1.5; 95% CI: 0.98–2.4) had greater infection risk compared to those with levels >800 AU/mL. Antibody levels could inform individual COVID-19 risk and booster scheduling.
1 INTRODUCTION
The effectiveness of COVID-19 vaccination for preventing SARS-CoV-2 infection wanes,1, 2 and although additional vaccine doses reduce the risk of incident SARS-CoV-2 infection,3 the optimal schedule for these booster doses remains unclear.4-6 Additionally, many factors—such as age, comorbidity and history of SARS-CoV-2 infection—impact immune response to SARS-CoV-2, suggesting that, although a one-size-fits-all time-based booster schedule may be the only practical approach currently, such a schedule ignores important individual-level heterogeneity7, 8 Identifying an objective correlate of immunity, could help health care providers understand infection risk and the optimal booster timing to protect against SARS-CoV-2 infection.
SARS-CoV-2 antibody levels are a potential correlate of immunity that can be measured through serologic tests. Several of these tests have received Emergency Use Authorization from the Food and Drug Administration (FDA) since March 2020,9 but none yet have full FDA approval to quantify immunity to SARS-CoV-2 infection, and it is possible that new tests are currently needed to account for the continuing evolution of SARS-CoV-2 variants. The FDA has indicated that more research is needed, and antibody testing is not currently recommended to assess a person's level of immunity after COVID-19 vaccination.10 While high SARS-CoV-2 antibody levels up to 4 weeks after primary series vaccinations are associated with decreased infection risk,11, 12 there remains a gap in the understanding of antibody level trajectories over longer periods of time since vaccination.
This study aimed to measure the relationship between antibody levels and incident SARS-CoV-2 infection among a cohort of individuals who received all doses of a SARS-CoV-2 primary vaccine series (hereafter referred to as vaccinated) and to evaluate the utility of serologic testing to estimate SARS-CoV-2 risk before receiving a booster. This was accomplished by (1) describing antibody levels by relevant clinical factors including time since vaccination, vaccine type, booster status, and prior SARS-CoV-2 infection, and (2) evaluating the association between antibody levels and rate of subsequent SARS-CoV-2 infection.
2 METHODS
All US National Basketball Association (NBA) players and team staff, regardless of vaccination and SARS-CoV-2 infection history, were encouraged to have their antibody levels measured once before the start of the 2021-2022 Season. All individuals underwent assessment of their SARS-CoV-2 antibody levels using the DiaSorin LIAISON SARS-CoV-2 TrimericS IgG assay (the “TrimericS Assay”) which was developed to measure antibodies against the WA-1 spike protein and received FDA Emergency Use Authorization on May 19, 2021.13 At the time, the TrimericS assay was chosen for its high correlation to neutralizing antibodies compared to other commercially available platforms.14-17 Individuals were included in this analysis if they met the following criteria: aged 18 and older who voluntarily had their SARS-CoV-2 antibody levels measured using the TrimericS Assay from September 12, 2021, through December 31, 2021 and completed their primary SARS-CoV-2 vaccine series (either one dose of the J&J/Janssen vaccine or two doses of an mRNA vaccine given 3–4 weeks apart) by time of serologic testing. Those who received a vaccine dose (primary or booster) or had a confirmed COVID-19 infection in the 14 days before their antibody test were excluded. If an individual had more than one antibody test during the study period, only the first test was included in these analyses. Relevant demographic and clinical characteristics (age, time since receipt of primary vaccine, and prior SARS-CoV-2 infection) were collected as part of the NBA occupational health program, as described.3, 18, 19
The Advarra institutional review board determined the study met the criteria for exemption status. Individuals signed health information authorizations allowing collection, storage, and use of health information by the NBA for monitoring purposes, including disclosure to medical experts.
2.1 Antibody level assessment
The TrimericS assay reports qualitative results (detected and not detected) and quantitative values in Arbitrary Units (AU) per milliliter (mL) when antibody levels are detected. Antibody levels ≥13 AU/mL are considered detectable; however, only values between 13 and 800 AU/mL are reported by the assay. Levels above this range are not quantitated and are reported only as >800 AU/mL20 which could represent values substantially greater than 800 AU/mL. In this study, individuals were categorized into three groups (<250, 250–800, and >800 AU/mL) based on their quantitative antibody value. The cutoff of 250 was selected to denote a group with potentially higher risk of SARS-CoV-2 infection based on correlation with virus neutralization assays for wildtype and Delta SARS-CoV-2 strains.21-24 These assays showed that an authentic neutralizing titer of 100 was associated with a 50% protective neutralization level for wild type.23 This corresponded to a cutoff of ≥189.09 AU/mL (95% confidence interval [CI]: 147.61–235.75; Supporting Information: Figure 1) on the TrimericS assay, as described previously.24 Based on this information, 250 AU/mL was considered a conservative upper bound for this group. The cutoff of 800 AU/mL was selected because it is the upper limit for which the assay reports quantitative values.
2.2 SARS-CoV-2 outcome definition
The primary outcome of interest was defined as a SARS-CoV-2 infection within 90 days following antibody measurement. Any individual with at least one positive test (reverse transcription polymerase chain reaction [PCR] or rapid diagnostic) and clinical confirmation was defined as having a confirmed incident SARS-CoV-2 infection, as previously described.18 During the study period, surveillance testing for vaccinated individuals was required in the following situations: (1) if an individual was experiencing symptoms; (2) if required by team or league physician or a government authority; (3) during the Thanksgiving Holiday (November 28, 2021 through November 20, 2021); and (3) on all game days that occurred after December 1, 2021 (for individuals with J&J/Janssen primary series) or December 12, 2021 (for individuals with mRNA vaccine primary series).
When possible, at least one positive PCR sample from each confirmed case was sent for genomic sequencing. In some cases, SARS-CoV-2 sequencing was unsuccessful due to inadequate sample volume or low viral load. In these scenarios, cases before December 3, 2021, were assumed to be Delta and those on or after February 1, 2022, to be Omicron. Cases occurring between December 3, 2021, through January 31, 2022, when both variants were circulating in this cohort, were considered not sequenced.
2.3 Statistical analysis
Descriptive statistics were calculated to characterize the demographic and clinical characteristics of the analytic cohort overall and stratified by booster receipt at time of serologic testing. Antibody levels were then summarized by demographic and clinical factors—including vaccine type, booster status, and days since the last dose of primary series—separately for individuals who were boosted and those who were unboosted at time of testing.
To evaluate the association between antibody levels and rate of subsequent incident SARS-CoV-2 infection, a time-to-event analysis was conducted among the subset of individuals who were unboosted at the time of serologic testing. In the case of multiple infections, only the first infection was included in these analyses. Unadjusted time (days) from serologic testing to the first SARS-CoV-2 infection by antibody level (<250, 250–800, and >800 AU/mL) was estimated using life tables and Kaplan–Meier curves. In both analyses, individuals who received a booster within 90 days after serologic testing were censored on the date of booster receipt. Cox proportional hazards regression was then used to estimate the relative rate (with 95% CIs) of the first SARS-CoV-2 infection within 90 days of serologic testing. The rate of SARS-CoV-2 infection was estimated among the <250 and 250–800 AU/mL groups compared to the >800 AU/mL group (reference group), overall and stratified by primary vaccine type. Individuals who received a booster within 90 days after serologic testing were censored on the date of their booster. Models were adjusted for age (years) and time since the last vaccine dose (months), both of which were modeled as continuous variables, as well as SARS-CoV-2 infection before antibody measurement (yes and no/unknown). The assumption of proportional hazards was confirmed by evaluating plots of the survival function and log(−log(survival)) versus log(time) and models with time-dependent covariates for each independent variable of interest (e.g. age, time since last vaccine dose).
Several analyses were performed to test the sensitivity of the observed hazard ratios to the operational definitions for exposure and outcome used in the primary analysis. First, Cox proportional hazards models were used to model the rate of SARS-CoV-2 infection within 90 days using different cut-points for the antibody categories. Then an analysis was performed to evaluate how the estimated association between antibody levels and rate of first SARS-CoV-2 infection may have been influenced by the selection of 90 days for the follow-up period. In this analysis, Cox proportional hazard regression was used to model the rate of infection within 60 days of serologic testing among the <250 and 250–800 AU/mL groups compared to the >800 AU/mL group. Finally, because the predominant SARS-CoV-2 variant transitioned from Delta (B.1.617.2 and AY lineages) to Omicron (initially B.1.1.529, BA.1, and BA.1.1) during the follow-up period, a sensitivity analysis was conducted using Cox proportional hazards regression models to estimate variant-specific hazard ratios for Delta and Omicron infections. In these models, Omicron infections (or Delta) were modeled as a competing risk. Infections that could not be sequenced were also modeled as a competing risk. In all sensitivity analyses, individuals who received a booster during the follow-up period were censored on the date of receipt. All analyses were conducted using SAS Enterprise Guide version 8.2 and R version 4.2.0.
3 RESULTS
3.1 Cohort characteristics
From September 12, 2021, through December 31, 2021, a total of 2388 vaccinated adults underwent antibody testing via the TrimericS Assay. Of these, 54 (2.3%) received a primary vaccine or booster dose, and 11 (0.5%) had a confirmed SARS-CoV-2 infection in the 14 days before their serologic test and were excluded. This resulted in an analytic cohort of 2323 individuals (Figure 1). The cohort was 78.2% male, 68.1% were aged ≤40 years, and 56.4% were vaccinated (primary series) with the Pfizer-BioNTech mRNA vaccine (Table 1). Antibody levels for 92% of the cohort were measured from September 12, 2021, through October 12, 2021. Among the analytic cohort, 2248 (96.8%) individuals were vaccinated but not boosted at the time of serologic testing, while 75 (3.2%) individuals had received a SARS-CoV-2 booster (monovalent) by the time of serologic testing. The majority of vaccinated unboosted individuals (77.2%) had completed their primary vaccine series 4–6 months before antibody measurement, while the majority (77.3%) of boosted individuals had completed their primary vaccine series more than 7 months before serologic testing
| Characteristics | Overall n = 2323 | Vaccinateda unboosted at serologic test, n = 2248 | Boosted at serologic test, n = 75 |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 218 (9.4) | 211 (9.4) | 7 (9.3) |
| Male | 1817 (78.2) | 1754 (78.0) | 63 (84.0) |
| Not reported | 288 (12.4) | 283 (12.6) | 5 (6.7) |
| Age in years, n (%) | |||
| 18–30 | 926 (39.9) | 915 (40.7) | 11 (14.7) |
| 31–40 | 654 (28.2) | 637 (28.3) | 17 (22.7) |
| 41–50 | 409 (17.6) | 389 (17.3) | 20 (26.7) |
| 51–60 | 245 (10.5) | 230 (10.2) | 15 (20.0) |
| 61–94 | 89 (3.8) | 77 (3.4) | 12 (16.0) |
| Time since last dose of primary vaccine series, n (%) | |||
| 0–3 months | 285 (12.3) | 284 (12.6) | 1 (1.3) |
| 4–6 months | 1751 (75.4) | 1735 (77.2) | 16 (21.3) |
| 7–14 months | 287 (12.4) | 229 (10.2) | 58 (77.3) |
| SARS-CoV-2 infection before serologic test, n (%)b | |||
| Infection at any time point before serologic test | 390 (16.8) | 388 (17.3) | 2 (2.7) |
| Infection before last dose of primary series | 290 (74.4) | 289 (74.5) | 1 (50.0) |
| Infection after last dose of primary series | 100 (25.6) | 99 (25.5) | 1 (50.0) |
| Primary vaccine series type, n (%) | |||
| Vector—J&J/Janssen | 493 (21.2) | 483 (21.5) | 10 (13.3) |
| mRNA—Moderna | 520 (22.4) | 504 (22.4) | 16 (21.3) |
| mRNA—Pfizer-BioNTech | 1310 (56.4) | 1261 (56.1) | 49 (65.3) |
| Qualitative antibody test result, n (%) | |||
| Detected | 2262 (97.4) | 2187 (97.3) | 75 (100.0) |
| Not detected | 61 (2.6) | 61 (2.7) | 0 (0.0) |
| Antibody level category, n (%) | |||
| >800 AU/mL | 594 (25.6) | 524 (23.3) | 70 (93.3) |
| 250–800 AU/mL | 714 (30.7) | 709 (31.5) | 5 (6.7) |
| <250 AU/mLc | 1015 (43.7) | 1015 (45.2) | 0 (0.0) |
| Antibody level, AU/mL | |||
| Median | 309.0 | 293.5 | >800.0 |
| IQR: 25th, 75th percentile | 125.0, >800.0 | 121.0, 740.5 | Not availabled |
- a An individual is considered Vaccinated if they have received all doses of a SARS-CoV-2 primary vaccine series (either one dose of the J&J/Janssen vaccine or two doses of an mRNA vaccine given 3–4 weeks apart) and at least 14 days have passed since they received the last dose. For example, if the final primary series dose was June 1, 2021, then the individual would be considered vaccinated on June 16, 2021.
- b Individuals with at least one positive test and clinical confirmation from March 2020 through 14 days before their serologic test were considered to have a prior SARS-CoV-2 infection. Individuals with a SARS-CoV-2 infection within the 14 days before their serologic test were excluded from the analytic cohort.
- c Includes “not detected” results, which were reported as an antibody level of “<13 AU/mL.”
- d When antibody levels were described as a continuous variable, values >800 were treated as 800 AU/mL and those with values <13 as 13 AU/mL. The IQR is “Not Available” when the 25th and 75th percentile were >800.0 AU/mL since these values exist beyond the reportable limit of detection authorized by the FDA for the TrimericS assay.
3.2 Antibody levels by clinical characteristic
Among the 2248 individuals who were vaccinated but unboosted at serologic testing, 2187 (97.3%) had antibody levels detected (Table 1). Among these individuals, the median level was 293.5 AU/mL, with a wide interquartile range (IQR): 121.0–740.5. Antibody levels were highest among those within 4 months of their primary series (median: >800 AU/mL; IQR: 335.5 to >800) and lower among individuals who had completed their primary series >7 months before their serologic test (median: 141.0 AU/mL; IQR: 71.4–338.0; Figure 2A). Antibody levels varied by vaccine type, with J&J/Janssen vaccine recipients having lower levels (median: 95.8 AU/mL; IQR: 24.5–438.0) than Pfizer-BioNTech (median: 285.0 AU/mL; IQR: 138.0–748.0) and Moderna (median: 463.0 AU/mL; IQR: 256.5 to >800.0) vaccine recipients (Table 2). Even among the individuals vaccinated <4 months before antibody measurement, J&J recipients had a lower median level at 114 AU/mL (IQR: 26.2–800.0) compared to mRNA recipients who had a median level of >800 AU/mL (Figure 2B–D, Table 2).
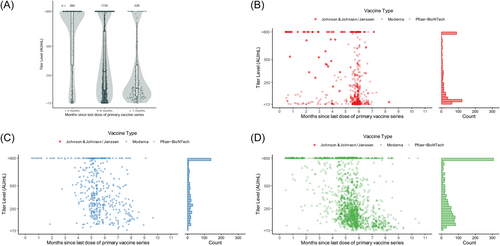
Antibody levels by primary series vaccine type and time since vaccination. Includes only individuals who were unboosted at serologic test. Antibody levels >800 were treated as 800 AU/mL and <13 as 13 AU/mL. Figure (A) shows antibody levels by months since primary vaccine series completion. The shaded area shows the kernel density estimate. Figure (B–D) shows levels by month since primary vaccine series completion, stratified by primary series type.
| Characteristic | Vaccinated unboosted at serologic test | Boosted at serologic Test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| J&J/Janssen | Moderna | Pfizer-BioNTech | |||||||||
| n (%) | Median | IQR | n (%) | Median | IQR | n (%) | Median | IQR | n (%) | Medianb | |
| Overall | 483 | 95.8 | 24.5–438.0 | 504 | 463.0 | 256.5 to >800.0 | 1261 | 285.0 | 138.0–748.0 | 75 | >800.0 |
| Age in years | |||||||||||
| 18–40 | 376 (77.8) | 117.0 | 31.5–462.0 | 304 (60.3) | 558.0 | 336.5 to >800.0 | 872 (69.2) | 356.5 | 180.5 to >800.0 | 28 (37.3) | >800.0 |
| 41–60 | 100 (20.7) | 32.8 | 13.4–342.5 | 177 (35.1) | 309.0 | 183.0–576.0 | 342 (27.1) | 174.0 | 87.2–425.0 | 35 (46.7) | >800.0 |
| 61–94 | 7 (1.4) | 28.5 | <13.0 to 196.0 | 23 (4.6) | 243.0 | 103.0–638.0 | 47 (3.7) | 90.1 | 33.5–214.0 | 12 (16.0) | >800.0 |
| Time since last primary series dose | |||||||||||
| 0–3 months | 73 (15.1) | 114.0 | 26.2 to >800.0 | 38 (7.5) | >800.0 | 791.0 to >800.0 | 173 (13.7) | >800.0 | 527.0 to >800.0 | 1 (1.3) | >800.0 |
| 4–6 months | 406 (84.1) | 91.7 | 23.2–365.0 | 396 (78.6) | 463.0 | 282.0–800.0 | 933 (74.0) | 267.0 | 145.0–578.0 | 16 (21.3) | >800.0 |
| 7–14 months | 4 (0.8) | 138.0 | 83.5–471.0 | 70 (13.9) | 241.5 | 139.0–492.0 | 155 (12.3) | 105.0 | 59.7–260.0 | 58 (77.3) | >800.0 |
| Time between serologic test and previous SARS-CoV-2 infectionc | |||||||||||
| No known prior infection | 379 (78.5) | 49.0 | 19.0–285.0 | 438 (86.9) | 413.5 | 242.0–768.0 | 1043 (82.7) | 234.0 | 121.0–538.0 | 73 (97.3) | >800.0 |
| 0–5 months | 33 (6.8) | >800.0 | 349.0 to >800.0 | 20 (4.0) | >800.0 | 508.0 to >800.0 | 62 (4.9) | >800.0 | Not availablea | 1 (1.3) | >800.0 |
| 6–18 months | 71 (14.7) | 321.0 | 189.0–627.0 | 46 (9.1) | >800.0 | 504.0 to >800.0 | 156 (12.4) | 714.0 | 363.5 to >800.0 | 1 (1.3) | >800.0 |
- Abbreviation: IQR, interquartile range.
- a When antibody levels were described as a continuous variable, values >800 were treated as 800 AU/mL and those with values <13 as 13 AU/mL The IQR is “Not Available” when the 25th and 75th percentiles were >800.0 AU/mL since these values exist beyond the reportable limit of detection authorized by the FDA for the TrimericS assay.
- b Individuals with at least one positive test and clinical confirmation from March 2020 through 14 days before their serologic test were considered to have a prior SARS-CoV-2 infection. Individuals with a SARS-CoV-2 infection within the 14 days before their serologic test were excluded from the analytic cohort.
- c The interquartile range was not reported for boosted individuals as all values for the 25th and 75th percentiles were beyond the reportable limit of detection ( 800 AU/mL) authorized by the FDA for the TrimericS assay. The range of antibody levels among individuals boosted at the time of serologic testing was 628.0 to >800.0 AU/mL; 93% of individuals boosted before serologic testing had antibody levels >800.0 AU.
The median antibody level among the 75 people who were boosted by the time of their serologic test was >800.0 AU/mL and there was no variation in antibody level across clinical characteristics in this group (Table 2).
3.3 Rate of SARS-CoV-2 infection by antibody level
Only the 2248 individuals who were vaccinated but unboosted at the time of serologic testing were included in the time-to-event analyses. Among these individuals, 136 (6.0%) had a confirmed SARS-CoV-2 infection within 90 days following serologic testing and before receiving a booster, while 178 (7.9%) did not have an infection or receive a booster during the follow-up period. The remaining 1,934 (86.0%) individuals received a booster during the 90-day follow-up period and were censored on the date of booster receipt. The median follow-up time among these individuals was 49 days (IQR: 38.0–61.0 days); 338 (17.5%) were censored within the first 30 days.
The proportion of individuals who experienced a SARS-CoV-2 infection over the 90-day follow-up period was lower among the 1015 individuals with antibody levels <250 AU/mL than the 524 individuals with levels >800 AU/mL (5.1% vs. 8.6%). Infections among individuals with low antibody levels, however, tended to occur earlier in follow-up: 67.3% of infections among individuals with antibody levels <250 AU/mL occurred before Day 70, coinciding with the start of the Omicron wave in the United States, compared to 26.7% of infections among individuals with levels >800 AU/mL (Figure 3, Table 3). Because infections among those with levels <250 AU/mL occurred earlier in follow-up, the crude rate of infection among these individuals was 2.1 (95% CI: 1.4–3.1; Table 4) times higher than among those with levels >800 AU/mL. This relative rate of infection increased after adjusting for age, time since the last primary vaccine dose, and SARS-CoV-2 infection (adj hazard ratio [HR]: 2.4; 95% CI: 1.5–3.7). Similar patterns were observed when stratified by primary vaccine type (Table 4), although the adjusted relative rate of infection was not statistically significant among recipients of the Moderna and J&J/Janssen vaccines.
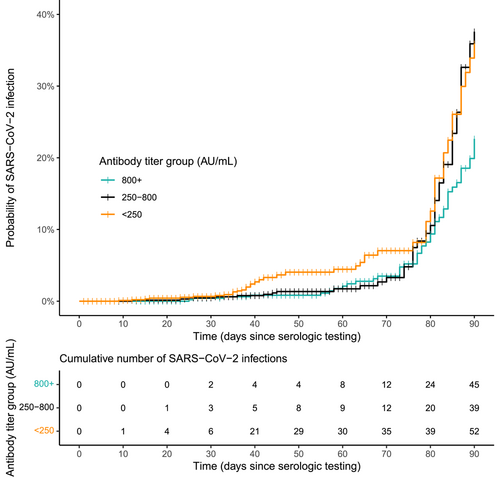
| Antibody level category | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time interval (days) | <250 AU/mL | 250–800 AU/mL | >800 AU/mL | ||||||||||||
| Number of individuals infected, n = 52 | Number of individuals censored, n = 963 | Effective sample sizea | Interval incidence rate | Cumulative incidence rate | Number of individuals infected, n = 39 | Number of individuals censored, n = 970 | Effective sample size* | Interval incidence rate | Cumulative incidence rate | Number of individuals infected, n = 45 | Number of individuals censored, n = 479 | Effective sample size* | Interval incidence rate | Cumulative incidence rate | |
| 0 | 0 | 0 | 1015 | 0.000 | 0.000 | 0 | 0 | 709 | 0.000 | 0.000 | 0 | 0 | 524 | 0.000 | 0.000 |
| 1, 10 | 1 | 64 | 983 | 0.001 | 0.000 | 0 | 15 | 701.5 | 0.000 | 0.000 | 0 | 7 | 520.5 | 0.000 | 0.000 |
| 11, 20 | 3 | 79 | 910.5 | 0.003 | 0.001 | 1 | 39 | 674.5 | 0.001 | 0.000 | 0 | 21 | 506.5 | 0.000 | 0.000 |
| 21, 30 | 2 | 78 | 829 | 0.002 | 0.004 | 2 | 22 | 643 | 0.003 | 0.001 | 2 | 13 | 489.5 | 0.004 | 0.000 |
| 31, 40 | 15 | 128 | 724 | 0.021 | 0.007 | 2 | 70 | 595 | 0.003 | 0.005 | 2 | 20 | 471 | 0.004 | 0.004 |
| 41, 50 | 8 | 258 | 516 | 0.016 | 0.027 | 3 | 185 | 465.5 | 0.006 | 0.008 | 0 | 85 | 416.5 | 0.000 | 0.008 |
| 51, 60 | 1 | 161 | 298.5 | 0.003 | 0.042 | 1 | 128 | 306 | 0.003 | 0.014 | 4 | 77 | 335.5 | 0.012 | 0.008 |
| 61, 70 | 5 | 90 | 172 | 0.029 | 0.046 | 3 | 88 | 197 | 0.015 | 0.018 | 4 | 47 | 269.5 | 0.015 | 0.020 |
| 71, 80 | 4 | 61 | 91.5 | 0.044 | 0.073 | 8 | 65 | 117.5 | 0.068 | 0.033 | 12 | 73 | 205.5 | 0.058 | 0.035 |
| 81, 90 | 13 | 44 | 35 | 0.371 | 0.114 | 19 | 58 | 48 | 0.396 | 0.098 | 21 | 136 | 89 | 0.236 | 0.091 |
- a Censored individuals are assumed to drop out of the risk set halfway through the interval; thus, the effective sample size equals the number of individuals in the risk set at the start of the interval minus half of the number of individuals censored during the interval. All censored individuals were censored due to receipt of booster.
| Antibody level category | |||
|---|---|---|---|
| <250 AU/mL | 250–800 AU/mL | >800 AU/mL | |
| Overall | |||
| n | 1015 | 709 | 524 |
| Number (%) infected | 52 (5.1) | 39 (5.5) | 45 (8.6) |
| Crude HR (95% CI) | 2.1 (1.4–3.1) | 1.4 (0.93–2.2) | REF |
| Adjusted HR (95% CI) | 2.4 (1.5–3.7) | 1.5 (0.98–2.4) | REF |
| J&J/Janssen | |||
| n (%) | 317 (65.6) | 80 (16.6) | 86 (17.8) |
| Number (%) infected | 22 (6.9) | 3 (3.8) | 5 (5.8) |
| Crude HR (95% CI) | 2.1 (0.78–5.5) | 0.98 (0.23–4.1) | REF |
| Adjusted HR (95% CI) | 2.2 (0.82–5.8) | 0.91 (0.22–3.8) | REF |
| Moderna | |||
| n (%) | 124 (24.6) | 242 (48.0) | 138 (27.4) |
| Number (%) infected | 6 (4.8) | 6 (2.5) | 7 (5.1) |
| Crude HR (95% CI) | 2.9 (0.97–8.7) | 1.0 (0.35–3.1) | REF |
| Adjusted HR (95% CI) | 3.2 (1.0–10.1) | 1.2 (0.38–3.5) | REF |
| Pfizer-BioNTech | |||
| n (%) | 574 (45.5) | 387 (30.7) | 300 (23.8) |
| Number (%) infected | 24 (4.2) | 30 (3.8) | 33 (11.0) |
| Crude HR (95% CI) | 1.8 (1.0–3.1) | 1.7 (1.0–2.8) | REF |
| Adjusted HR (95% CI) | 2.0 (1.1–3.6) | 1.8 (1.1–2.9) | REF |
- Note: Models were adjusted for age (years), months since last vaccination dose, and SARS-CoV-2 infection before serologic test; REF = the >800 AU/mL group was considered the reference category in these models.
- Abbreviations: CI, confidence interval; HR, hazard ratio.
The 709 individuals with antibody levels of 250–800 AU/mL had an elevated, although not significantly different relative rate of infection compared to the >800 AU/mL group (adj HR: 1.5; 95% CI: 0.98–2.4; Table 4). When stratified by vaccine type, the infection rates among recipients of the Moderna and J&J/Janssen vaccines were also not statistically significantly different; however, among recipients of the Pfizer-BioNTech vaccine, the rate of infection was significantly higher among those with levels of 250–800 AU/mL compared to the >800 AU/mL group (adj HR: 1.8; 95% CI: 1.1–2.9; Table 4).
Sensitivity analyses using other thresholds such as <125, 125–249, <200, and >750 AU/mL were evaluated but did not meaningfully change results (Supporting Information: Table 1). Results were also robust to varying follow-up periods as demonstrated by an analysis that showed a similar relative rate of infection over 60 days comparing individuals with antibody levels <250 and >800 AU/mL (adj HR: 2.8; 95% CI: 1.2–7.0). In this shorter follow-up period, the rate of infection was more similar between those with levels 250–800 and >800 AU/mL (adj HR: 0.94; 95% CI: 0.35–2.5; Supporting Information: Table 2).
Individuals with antibody levels <250 AU/mL were more likely to experience SARS-CoV-2 infections earlier in the follow-up period, while Delta was predominant, comprising 67.3% of infections among this group (Supporting Information: Table 3). In contrast, 79.5% and 88.9% of infections among those in the 250–800 and >800 AU/mL groups, respectively, were from the Omicron variant. Notably, there were no Delta infections among individuals with levels >800 AU/mL; thus, the rate of Delta infections could not be estimated for this group. When compared to individuals with antibody levels of 250–800 AU/mL, however, those with levels <250, had a significantly higher relative rate of Delta infection (adj HR: 7.0; 95% CI: 2.6–18.9). Results from the variant-specific model for Omicron indicated that the rate of infection was not significantly different between individuals with levels <250 AU/mL and those with >800 AU/mL (adj HR: 1.2; 95% CI: 0.59–2.3; Supporting Information: Table 2). In contrast, those with levels of 250–800 AU/mL had a significantly higher relative rate of infection from omicron compared to those with >800 AU/mL (adj HR: 1.8; 95% CI: 1.1–2.9).
4 DISCUSSION
In this cohort of vaccinated individuals from the NBA, antibody levels among the 2248 individuals who were vaccinated but unboosted at time of serologic testing were lower among individuals who were further from their primary vaccine series. The 75 patients who had received a booster at time of serologic testing all had antibody levels of >800 AU/mL. When evaluating the rate of SARS-CoV-2 infection among individuals who were unboosted at time of serologic testing, low antibody levels were associated with a significantly increased rate of infection in the subsequent 90 days. These associations persisted across vaccine types, although the associations were not statistically significant among recipients of the Moderna and J&J/Janssen vaccines.
Regardless of antibody level, the rate of infection increased rapidly after Day 70, which, for most of the cohort, coincided with the beginning of the Omicron wave for the United States in December 2021. While the relative rate of Omicron infection among individuals with levels >800 AU/mL was lower than those with lower antibody levels, they were more likely to experience an infection during the Omicron wave. Individuals with levels >800 AU/mL may have been more likely to experience an Omicron than a Delta infection due to the timing of the follow-up period which coincided with the Omicron wave for a large proportion of participants. Many individuals may have had higher antibody levels and were less susceptible to infection earlier during the follow-up period when Delta was predominant but subsequently had lower antibody levels by the time the Omicron wave began, when they were more susceptible to SARS-CoV-2 infection regardless of the variant. Alternatively, as the Omicron variant is characterized by substantial immune escape compared to earlier variants,21, 25-27 the antibodies to the founder WA-1 strain as measured by the TrimericS assay may have had less relevance for Omicron than Delta. Previous reports have shown reduced correlation between measured anti-spike antibodies and neutralizing antibodies for Omicron infections when compared to the wildtype strain.15, 27
Vaccine-induced antibody levels wane over time, but earlier studies28-30 assessed a relatively short window—generally up to 4 months postvaccination. Since then, additional research31-37 confirmed that antibody titers continue to decrease up to 12 months after SARS-CoV-2 vaccination and observed reduced neutralization activity to emerging variants including Delta and Omicron. Although antibody levels in this study were measured at only one time-point, results are consistent with this prior research,31-34 as unboosted individuals for whom more time had passed between receipt of their primary vaccine and serologic testing (≥7 months) tended to have lower antibody levels than those vaccinated within 4 months of testing.
Understanding effective correlates of protection against COVID-19 is important, and to do so, additional research and large, representative studies will be required. It is also important to further examine the durability of immunity, for example, how long someone may remain in a high or low antibody group, and whether the protective effect from infection remains robust across SARS-CoV-2 variants. The degree to which specific serological assays are able to measure antibody levels may change as variants evolve; for example, antibody levels measured by tests developed against the wild-type strain (WA-1) spike protein have been shown to have weaker correlation with Omicron-specific neutralizing antibody titers.27, 38 The utility of these correlates may also encourage diagnostics manufacturers to develop, and the FDA to authorize, antibody tests that are specific to neutralizing antibodies rather than overall IgG levels.
This study has several limitations. Data on additional confounders, including pre-existing health conditions, genetics, and level of exposure to SARS-CoV-2, were not collected and therefore could not be controlled for in the Cox proportional hazards regression models. The results presented in this analysis may therefore be subject to residual confounding; for example, individuals with behaviors that result in higher risk of SARS-CoV-2 exposure may be more likely to have higher antibody levels and may also be more likely to be infected; this could lead to attenuation in observed associations. Our findings are based on a single antibody timepoint; multiple longitudinal data points could better characterize the trajectory of antibody decline following vaccination and quantify the level of immunity leading up to the time of infection. A large proportion of individuals (86.0%) were censored during follow-up upon receipt of booster with 33% censored within the first 60 days. Sensitivity analyses showed that results from a 60-day follow-up period analysis did not differ greatly from the primary analysis with a 90-day follow-up period and demonstrated a slightly stronger association between antibody level and infection risk. Additionally, the proportion of censored individuals was similar across all three antibody groups suggesting that this censoring did not introduce substantial selection bias. As this is a largely healthy, young, mostly male cohort, these results may not generalize to other populations, underscoring the need for further studies in more diverse cohorts. Finally, the results from these analyses may be specific to the timeframe observed during the end of the period of Delta variant dominance in late 2021 and early in the Omicron wave of 2021–2022 and thus may not generalize to other time periods during the pandemic or to other SARS-CoV-2 variants. Specifically, while high antibody levels are expected to predict lower risk of infection in most settings, the precise relationship between antibody level and the risk of infection will depend on the antibody assay used, population, and epidemiological setting.
5 CONCLUSION
Answers to the question, “when should one get a SARS-CoV-2 booster dose?” remain uncertain. Recommendations currently attempt to account for individual-level risks through proxy factors that include age, time since vaccination, vaccine type, and immunocompromised status.39 Moreover, whether the logic for a Fall booster, like for seasonal influenza, will hold depends on (1) whether COVID-19 establishes a wintertime peak in transmission and (2) whether the duration of the immune response to the booster matches the duration of COVID-19 transmission. In the meantime, findings from this study suggest that serologic testing can inform individual-level assessments of susceptibility to infection and therefore may serve as a useful guidepost for decision-making on when to get a booster.
AUTHOR CONTRIBUTIONS
Caroline G. Tai and Christina DeFilippo Mack had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Christina DeFilippo Mack and Yonatan H. Grad are co-senior authors of this work. All authors were responsible for the concept and design of manuscript, drafting of manuscript, and critical revision of the manuscript for important intellectual content. Christina DeFilippo Mack, Caroline G. Tai, Rachel M. Lucia, Miriam J. Haviland, and Yonatan H. Grad were responsible for acquisition, analysis, or interpretation of data. Caroline G. Tai, Rachel M. Lucia, and Miriam J. Haviland performed the statistical analysis. Christina DeFilippo Mack, Caroline G. Tai, Miriam J. Haviland, and Rachel M. Lucia were responsible for administrative, technical, or material support, and Christina DeFilippo Mack, Caroline G. Tai, and Yonatan H. Grad provided supervision of the manuscript.
ACKNOWLEDGMENTS
The authors thank Joseph Fauver (University of Nebraska Medical Center) for contributing his expertise in genomic surveillance; Tempus Labs for their sequencing support; the NBA Players Association, the NBA Team Physicians Association, and medical and athletic training staff for the collection of these data; and the analytic and operational teams at the NBA (David Weiss, Miheer Mhatre, Patrick Clifton, Peter Meisel, Rachel Davis, Kelly Hogan, Caroline Coughlan, and Anton Arellano) and IQVIA (Kristina Zeidler, Gabriel Farkas, Kendall Knuth, Madeline Johnson, Erin Johnson, Riju Shrestha, Rahul Gondalia, Kelly Brotherhood, Sarah Connolly, Radhika (Melody) Samant, Julie Griffith, Jim Dunn, Marcin Mazurek, Tiffany Koch, and Michael Booth) for their tireless work on the NBA COVID-19 monitoring program. This work was funded by the NBA in the interest of players, staff, and community health.
CONFLICT OF INTEREST STATEMENT
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. CDM, CGT, MJH, and RML report full-time employment by IQVIA which is in a paid consultancy with the NBA. DJA reports receiving institutional support from the CDC and the Agency for Healthcare Research and Quality; receiving royalties from UpToDate; and ownership of Infection Control Education for Major Sports. JD, YHG, SMK, MM, NDG, and LLM report consulting fees from the National Basketball Association. YDG also reports grants from the CDC, NIH, and Family Smith Foundation as well as consulting fees and participation on the Day Zero and Decoy Therapeutics Advisory Board. SMK is a paid consultant for Moderna Therapeutics. NDG is a paid consultant for Tempus Labs and reports funding from the NBA, grants from the CDC and NIH, and payments or honoraria for lectures, presentations, speaker's bureaus, manuscripts, or educational events from Moderna. No authors had other conflicts of interest to report. No author received direct, individual payment for this work.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.




