High Torque teno virus load and outcome of patients undergoing allogeneic hematopoietic cell transplantation
Abstract
Quantification of Torque teno virus (TTV) load emerged as a marker of immunosuppression. Associations of TTV load with complications and survival after allogeneic hematopoietic cell transplantation (allo-HCT) were controversial in published studies. In this prospective study, we aimed to identify factors influencing TTV load after allo-HCT and to determine whether the TTV load is associated with complications or outcomes. Seventy allo-HCT recipients were included. TTV DNA load was quantified in 469 plasma samples of 70 patients from Day (D) 15 before D120 after transplantation. The influence of transplant characteristics on TTV load and the associations of TTV load with viral infections, acute graft versus host disease, mortality, and relapse were analyzed. More than 80% of patients were TTV DNA positive from D30 after transplantation onwards. Median TTV load increased between D30 and D60 post-transplantation. Patients with lymphoid malignancies had higher TTV load than those with myeloid malignancies. Myeloablative conditioning was associated with higher TTV loads. Patients with no measurable residual disease at transplant had higher TTV loads. High TTV load at D90 post-transplantation was associated with lower overall survival and at D120 post-transplantation was associated with higher relapse rate. In conclusion, TTV load at time points later than D90 after allo-HCT may be useful to assess prognosis.
1 INTRODUCTION
Allogeneic hematopoietic cell transplantation (allo-HCT) is a widely used treatment for hematologic disorders. Owing to the conditioning regimen, pre-transplant disease treatment, the period of aplasia, and immunosuppressive therapy, infections are frequent complications after allo-HCT, accounting for approximately 24% of mortality post-allo-HCT.1, 2 Another major complication is acute graft versus host disease (aGvHD) that occurs in 20%–80% of allo-HCT recipients.3 A biomarker that allows to monitor the degree of immunosuppression and to predict complications or outcomes after allo-HCT would be useful for patient management.
Torque teno virus (TTV) is a single-stranded DNA virus of the Anelloviridae family, genus Alphatorquevirus. The majority of humans are persistently infected with this virus.4 The TTV DNA load increases in case of immunosuppression.5 In solid organ transplant recipients, TTV DNA load is useful for monitoring the degree of immunosuppression.6 A TTV DNA load higher than 3.15 log10 copies/mL at 1 month after renal transplantation was associated with an increased risk of infections.7 Following lung or liver transplantation, a higher TTV DNA load was observed in patients who later developed infections, for example, cytomegalovirus (CMV) infections.8-10 A lower TTV DNA load was observed in patients with acute rejection after kidney, lung, and liver transplantation.8, 10, 11 These studies proposed that the TTV DNA load allows to predict the risk of complications after solid organ transplantation.7, 10
In the context of allo-HCT, the usefulness of TTV DNA load monitoring remains to be determined. Some studies found that TTV DNA load correlated with infectious complications after allo-HCT and/or aGvHD whereas others did not confirm these findings.12-16 The objectives of the current study were to determine factors influencing TTV load after allo-HCT and to investigate the association of TTV load with CMV, human adenovirus (HAdV), human herpesvirus 6 (HHV6), Epstein–Barr virus (EBV) or BK virus infections and aGvHD as well as survival and relapse.
2 METHODS
2.1 Patients and specimens
For this prospective study, 70 adult patients undergoing allo-HCT at Lille University Hospital between April 2016 and December 2018 were included. The patients had blood specimens collected at day (D)15 before and at D0, D15, D30, D60, D90, and D120 after transplantation. Plasma specimens were stored at −80°C. According to their clinical course, patients were divided into patients with viral infections, aGvHD, both of these, or none of these (control group). Clinical data, including underlying disease, donor type, HLA-matching and type of aGvHD prophylaxis, conditioning, the delay to engraftment, and risk status, were collected from hospital charts. Follow-up for the outcomes of death and relapse was performed until D400 after transplantation.
2.2 Ethical considerations
The study was a non-interventional research study with no additional clinical procedures and was carried out in accordance with the Declaration of Helsinki. In accordance with French legislation, written information was provided, and consent was obtained from each patient. The study complied with the requirements of the French Commission Nationale Informatique et Libertés (no. DC-2008-642).
2.3 Clinical protocols
Myeloablative conditioning (MAC) consisted mostly of Cyclophosphamide (Cy, 3 g/m2) in combination with total body irradiation (TBI, 8–12 Gy) or Fludarabine with TBI (8–12 Gy) or Fludarabine (150–160 mg/m2) with high-dose Busulfan (9.6–12.8 mg/kg). Reduced intensity regimen (RIC, included non-myeloablative regimen and reduced toxicity regimen) consisted mostly of Fludarabine (150–160 mg/m2), associated with low dose busulfan (6.4 mg/kg). T-cell depletion was in vivo with anti-thymocyte globulin (ATG such as thymoglobulin 5 mg/kg) in peripheral stem cell recipients or cyclophosphamide (50 mg/kg) on D3 and D4 in the set of haplo-identical transplant.
aGvHD prophylaxis consisted of ciclosporin (up to 3–6 months after transplant in the absence of aGvHD) and methotrexate (15 mg/m2 on D1, then 10 mg/m2 on D3 and D6) except for haploidentical transplant in which ciclosporin and mycophenolate mofetil (MMF) were used. The treatment of aGvHD consisted of corticosteroids as first line and mostly ruxolitinib as a second line treatment.
Antiviral prophylaxis consisted of valaciclovir 500 mg twice daily.
Patients were monitored for CMV (Herpesviridae family, subfamily betaherpesvirinae, genus cytomegalovirus), human adenovirus (HAdV) (Adenoviridae family, genus Mastadenovirus), Human Herpesvirus 6 (HHV-6) (Herpesviridae family, subfamily betaherpesvirinae, genus roseolovirus, species HHV-6) and Epstein-Barr Virus (EBV) (Herpesviridae family, subfamily gammaherpesvirinae, genus lymphocryptovirus) DNA in peripheral blood once weekly from D15 before transplant until D100 after transplantation. BK virus (Family Polyomaviridae, genus Betapolyomavirus) detection was performed in urine in case of functional urinary symptoms, or systematically in case of micro- or macro-hematuria (in the haploidentical graft setting until D60 with a weekly systematic urine strip detection). Preemptive therapy of CMV was performed if virus load exceeded 3.5 log10 IU/mL. First-line treatment consisted of Ganciclovir 5 mg/kg/day for 14 days. Second-line treatment consisted of Foscarnet 120 mg/kg/day for 14 days. Preemptive therapy for PTLD prevention was given if the EBV load exceeded 4 log10 UI/mL. It consisted of two to four injections of Rituximab (375 mg/m2), once weekly, guided by EBV viremia.
aGVHD was diagnosed and graded following the MAGIC criteria.17 Patients presenting grades II–IV aGvHD, necessitating systemic treatment with corticoids or other drugs were included in the group aGvHD.
2.4 Definitions
Viral infections were defined as CMV, HAdV, HHV6, or EBV DNAemia that prompted the initiation of antiviral or Rituximab treatment. In case of BK virus, infection was defined as BK virus DNAuria or BK virus DNAemia associated with hemorrhagic cystitis.
High TTV load was defined as a TTV load above the 75th percentile.
Engraftment was defined as absolute neutrophil count of 0.5 × 109/L, and platelet count of 20 × 109/L, with transfusion independency and graft donor chimerism of at least 90% at D28.
The risk group was classified as high-risk versus non-high-risk group regarding the presence or not of the underlying disease at the time of transplantation. The presence of the underlying disease was defined by the absence of complete remission (CR), or by the presence of measurable residual disease (MRD) in case of CR.
2.5 CMV, EBV, adenovirus, HHV6, and BK virus DNA detection
For detection and quantification of viral DNA, nucleic acid extraction was performed with Versant kPCR Molecular systems SP and the Versant sample preparation 1.2 Reagents (Siemens Healthcare Diagnostic). kPCR PLX® CMV, EBV, HAdV, HHV6, and BK Virus DNA Assays (Siemens Healthcare Diagnostics) were used on Versant kPCR Molecular Systems AD (Siemens Healthcare Diagnostics).
2.6 Quantification of TTV load
DNA were extracted from 200 µL of EDTA plasma with the QIAamp DNA Blood Mini Kit (Qiagen) with an elution volume of 50 µL.
For quantification of TTV, the R-gene assay (REF. 69-030B, bioMérieux) was used. Results were expressed as log10 copies per mL of plasma.
2.7 Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics 22 (IBM) and GraphPad Prism (GraphPad Software) software. Analysis of quantitative variables was performed by using Mann–Whitney U test. Associations with mortality and relapse up to D400 were assessed with the Chi-square test or Fisher's exact test as appropriate. Kaplan–Meier analysis was used to analyze the impact of TTV load above the 75th percentile at different time points on the probability of mortality or relapse up to D400. Kaplan–Meier analyses were restricted to patients alive at the respective time of TTV load determination. A p-value < 0.05 was considered statistically significant.
3 RESULTS
Seventy patients who underwent allo-HCT between April 2016 and December 2018 were included in the study (Table 1). TTV DNA load was quantified in 469 plasma samples of 70 patients from D15 before until D120 after transplant.
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 41 (58.6) |
| Female | 29 (41.4) |
| Age, years (median, min–max) | 54 (19–73) |
| Underlying hematological disease | |
| Lymphoid malignancies | |
| Hodgkin's lymphoma | 1 (1.4) |
| Non-Hodgkin's lymphoma | 6 (8.6) |
| Multiple myeloma | 2 (2.9) |
| ALL | 13 (18.6) |
| Myeloid malignancies | |
| AML | 27 (38.6) |
| CML | 1 (1.4) |
| Myelodysplastic syndrome | 13 (18.6) |
| Myelofibrosis | 3 (4.3) |
| Others (aplastic anemia, dentritic cell leukemia, systemic mastocytosis) | 4 (5.7) |
| Donor type | |
| Matched-related donor | 14 (20.0) |
| Matched unrelated donor | 40 (57.1) |
| Mismatched unrelated donor | 5 (7.1) |
| Haploidentical | 11 (15.7) |
| Conditioning regimen | |
| Myeloablative | 36 (51.4) |
| Reduced intensity regimen | 34 (48.6) |
| Stem cell source | |
| Peripheral blood | 37 (52.9) |
| Bone marrow | 33 (47.1) |
| Virus infection | 33 (47.1) |
| CMV | 16 (22.9) |
| EBV | 4 (5.7) |
| HHV6 | 3 (4.3) |
| BKV | 3 (4.3) |
| Multiple viruses | 7 (10.0) |
| Acute GvHD | 26 (37.1) |
| Virus infection and acute GvHD | 13 (18.6) |
| Outcome | |
| Relapse | 12 (17.1) |
| Death | 15 (21.4) |
- Abbreviations: ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; BKV, BK virus; C ML, chronic myeloid leukemia; CMV, cytomegalovirus; EBV, Epstein–Barr virus; HHV6, human herpesvirus 6; GvHD, graft versus host disease.
Patients were classified into four groups according to their complications until D120 after transplantation: virus infection, aGvHD, virus infection plus aGvHD or none of these complications (control group). Patients were monitored for viral infections/reactivations, including CMV, EBV, HHV6, HAdV, and BK virus. Thirty-three patients (47.1%) had at least one viral infection. CMV infection was most frequent and occurred in 16 patients (22.9%), followed by EBV in 4 patients (5.7%), HHV6, and BKV in 3 patients each (4.3%). Seven patients (10%) had more than one viral infection (Table 1). Twenty-six patients (37.1%) developed aGvHD requiring systemic treatment (grades II–IV). Thirteen patients (18.6%) suffered from both viral infection and aGvHD (Table 1).
3.1 TTV detection and quantification in allo-HCT patients
All patients who went on to develop aGvHD had detectable TTV before transplantation, whereas 66.7% of controls and 47.7% of patients who went on to develop both viral infection and aGvHD had detectable TTV DNA before transplantation. The percentage of TTV-positive plasma specimens was statistically different between the four groups before allo-HCT (Figure 1A, p = 0.001, chi-square test), but not at the day of transplantation or later time points. More than 80% of patients were TTV DNA positive from D30 after transplantation onwards, without significant differences between the groups.
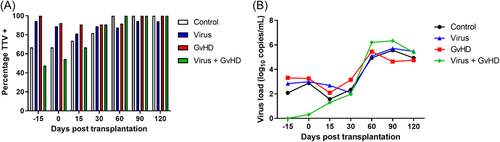
Median TTV load did not differ between the four groups but increased significantly between D30 and D60 post-transplantation (p < 0.0001; Mann–Whitney U test, Figure 1B).
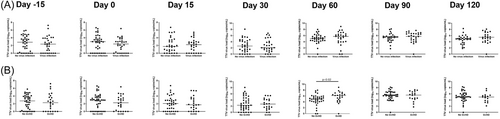
We hypothesized that if the TTV load reflected the degree of immunosuppression, a high TTV load would be associated with infectious complications, whereas a low TTV load would be associated with aGvHD. However, no statistically significant differences in TTV load were observed in patients with virus infections compared to patients without virus infections at different time points (Figure 2A). Next, TTV load was compared between patients with and without aGvHD. TTV load was significantly different according to aGvHD status at D60 after transplantation (No aGvHD: Median [interquartile range, IQR] 4.97 [4.18–5.74] vs. aGvHD: 6.18 [4.44–6.79] log10 copies/mL, p = 0.02) (Figure 2B).
3.2 Factors impacting on TTV load
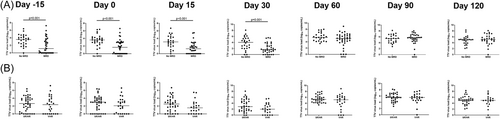
Next, we analyzed other factors that could impact TTV load. The type of underlying malignancy influenced TTV load before transplantation and at D90 and D120 after transplantation (Figure 3A), where patients with lymphoid malignancies had higher TTV loads (median [IQR] 3.29 [2.02–4.85], 6.27 [5.43–6.86], and 5.61 [4.79–6.48] log10 copies/mL, before transplantation and at D90 and D120, respectively) than patients with myeloid malignancies (median 1.92 [0–3.81], 5.37 [4.47–5.79], and 4.93 [3.62–5.30] log10 copies/mL, before transplantation and at D90 and D120, respectively) (p = 0.03; p = 0.01; p = 0.01).

MAC was also associated with higher TTV loads at D30 and D60 after transplantation (median [IQR] 3.01 [1.67–5.11] and 5.41 [4.47–6.45] log10 copies/mL, at D30 and D60, respectively) compared to RIC (median [IQR] 1.94 [0.82–3.52] and 4.81 [3.87–5.90] log10 copies/mL, at D30 and D60, respectively) (p = 0.048 and 0.05, respectively) (Figure 3B). Furthermore, patients with no MRD at the time of transplantation had higher TTV load from D15 before until D30 after transplantation (p </= 0.001) but not at later time points (Figure 4A). Risk status did not influence TTV load (Figure 4B).
Origin of transplanted cells, degree of histocompatibility, and type of aGvHD prophylaxis did not significantly influence TTV load (data not shown).
3.3 Association of TTV load with outcome
We first analyzed whether a high TTV load influenced time to engraftment. The median time to engraftment was not significantly different in patients with a TTV load above the 75th percentile compared to patients with TTV load below the 75th percentile before transplantation and at D0 and D15 post-transplantation (data not shown). We next analyzed whether TTV load was associated with patients' outcomes death or relapse. First, the associations of different characteristics with outcomes survival and relapse up to D400 were analyzed (Supporting Information: Tables S1 and S2). TTV load above the 75th percentile at D90, as well as aGvHD, was associated with outcome death (p = 0.03 and p = 0.008, respectively) (Supporting Information: Table S1). Lymphoid versus myeloid malignancies showed a trend toward a higher mortality rate (p = 0.07) (Supporting Information: Table S1). Next, Kaplan–Meier analysis was performed to compare the overall survival in patients with a TTV load above the 75th percentile to patients with TTV load below the 75th percentile. TTV load above the 75th percentile at D15 before, at the day of transplantation as well as at D15 and D30 after transplantation did not have an impact on the outcome death (data not shown). However, a TTV load above the 75th percentile at D60 and D120 after transplantation showed a trend to a higher mortality (p = 0.08 and 0.06, respectively) (Figure 5A,C). A TTV load above the 75th percentile at D90 after transplantation was associated with a higher mortality (p = 0.02) (Figure 5B).
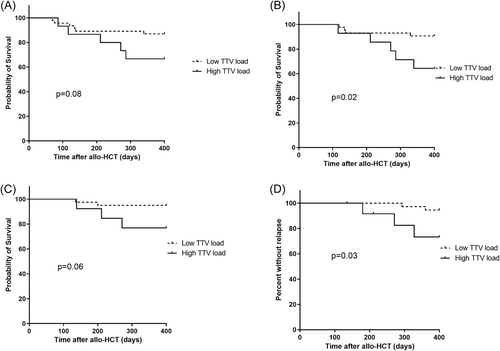
As far as the outcome relapse was concerned, MAC was associated with lower probability of relapse (p = 0.008) and a TTV load above the 75th percentile at D120 after transplantation showed a trend toward a higher probability of relapse (p = 0.09) (Supporting Information: Table S2). In Kaplan–Meier analysis TTV load above the 75th percentile at early time points after transplantation did not have an impact on the outcome relapse (data not shown), but a TTV load above the 75th percentile at D120 after transplantation was associated with a higher probability of relapse (p = 0.03) (Figure 5D).
4 CONCLUSIONS
TTV load has emerged as an indicator of the degree of immunosuppression in the context of solid organ transplantation8, 10, 18; therefore, one could expect that a high TTV load predisposes to infectious complications, whereas a low TTV load predisposes to organ rejection. Indeed, an association of TTV load with infections or rejection after solid organ transplantation has been found in several studies.4, 9, 11, 19, 20 These findings led to the initiation of clinical trials that investigate the usefulness of TTV load to guide immunosuppressive therapy in solid organ transplant patients, for example, VIGILung (NCT04198506) and TTV GUIDE TX (https://cordis.europa.eu/project/id/896932).21, 22
The role of TTV load in allo-HCT is less clear. Previous studies yielded contradictory results (reviewed in reference 18), some reporting association of TTV load with infectious complications or aGvHD early after allo-HCT 13, 15 whereas others found no such association.12, 16 TTV load measured at a median of 6 months after allo-HCT was found to reflect T cell function and differed between patients with viral infections when compared to patients without viral infections.23 TTV kinetics were suggested to mirror immunological reconstitution early after allo-HCT whereas TTV load would mirror the degree of immunosuppression in the long-term period after allo-HCT.18
In our cohort, both the percentage of patients with detectable TTV in plasma and the TTV load increased after allo-HCT (Figure 1) confirming findings from studies that found a decline of TTV load after conditioning and an increase after allo-HCT.14, 24, 25
In accordance with recent publications, we found a higher TTV load in patients with lymphoid malignancies compared to patients with myeloid malignancies at certain time points (Figure 3A).12, 16, 25 We also found a higher TTV load in patients with the absence of MRD compared to patients with MRD (Figure 4A) from D15 before until D30 after transplantation. MAC was reported to be associated with higher TTV loads than reduced intensity conditioning.12 In our cohort, this finding was confirmed and reached significance at D30 and D60 after allo-HCT (Figure 3B). In contrast to the study by Pradier and colleagues, we did not find an impact of donor type on TTV load.25
We found no association between TTV load and viral infections up to D120 post-transplant, in accordance with results from Schmitz et al.,12 and in contrast to a previous study, that found lower TTV load in patients who developed high-level CMV DNAemia, but it should be noted that CMV infections were more frequent in the latter cohort.13 TTV load was also associated with infections at 6 months or more after transplantation in recent studies.23, 25 These discordant findings are probably due to the dynamics of TTV load after allo-HCT that is influenced by multiple factors (e.g., the type of malignancy, the conditioning regimen, immune reconstitution, development of GvHD needing systemic therapy), especially in the early post-transplant period.18
TTV load was not different in patients with aGvHD compared to patients without aGvHD in our cohort at most time points up to D120 post-transplant as observed by Schmitz et al.12 We observed a significantly higher TTV load in patients with aGvHD at D60 after allo-HCT (Figure 2B). Interestingly, a recent study also found a higher TTV load in patients with aGvHD at D60 after transplantation, although the finding did not reach statistical significance.14 Another study found that high TTV load at D120 was associated with a higher probability of developing subsequent GvHD.25 The finding of a higher TTV load in patients with aGvHD at D60 after transplantation is intriguing. Most of the patients developed aGvHD before D60 (19/26, 73%). Therefore, the higher TTV load that we observed at D60 in patients with aGvHD may be secondary to the higher degree of immunosuppression caused by the systemic aGvHD treatment.
Concerning patient outcomes, we found that a high TTV load at D90 after allo-HCT was associated with a higher mortality within 400 days after allo-HCT. These results are in agreement with the results of a recent study that found an association of high TTV load at D100 post-transplant with lower overall survival.25 We also found that a high TTV load at D120 was associated with the probability of relapse, confirming a trend towards a higher TTV load in patients with compared to patients without relapse observed recently.25 These findings suggest that TTV load could be a useful marker predictive of the outcome at later time points post-allo-HCT. Nevertheless, high TTV load is not independent of other factors that have an impact on outcome, for example, type of malignancy and aGvHD and/or its treatment. Future studies are needed to address whether high TTV loads were associated with poorer outcomes because high TTV loads reflected both impaired antitumour and anti-infectious immunity or resulted from other characteristics associated with adverse outcomes. Our study has a number of limitations: The number of patients in our study was relatively low and this limits the power of the study. Statistical analysis was restricted to univariable analysis due to the limited patient number. TTV load measurements were not performed at time points later than D120 post-transplantation. Chronic GvHD was not investigated because it typically develops after D100 post-transplantation.26
Taken together, our results suggest that TTV load at early time points after transplantation is influenced by the type of malignancy, the disease status, and the conditioning regimen. At time points later than D90 after allo-HCT, TTV load was associated with the probability of relapse and mortality and may therefore be useful to assess the prognostics of allo-HCT patients. Future studies should be performed to corroborate and extend upon these findings; specifically, studies should extend TTV load measurements to time points later than D120 after allo-HCT to further investigate their association with long-term outcomes. Furthermore, prospective multicentre studies enabling the inclusion of a larger patient number would be useful to enable multivariate analyses.
AUTHOR CONTRIBUTIONS
Micha Srour designed the study, collected data, interpreted the data, and wrote the manuscript. Corentin Grenier performed experiments, collected and interpreted data, and edited the manuscript. Leonardo Magro collected data, interpreted the data, and edited the manuscript. Didier Hober reviewed the manuscript and provided resources. Ibrahim Yakoub-Agha designed the study, collected data, interpreted the data, and edited the manuscript. Ilka Engelmann designed the study, collected data, performed the statistical analyses, interpreted the data, and wrote the manuscript. All authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank the technicians of the virology laboratory for excellent technical assistance and the clinical research assistants for help with data collection. The authors thank P. Bourgeois, bioMérieux, France, for providing the kits for TTV DNA load measurements. This research was funded by the Budget Program Innovation of Centre Hospitalier Universitaire de Lille and Santélys.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon reasonable request from the corresponding author with the permission of Lille University Hospital.




