Evolution of neutralizing antibodies through vaccination and breakthrough infections in the era of COVID-19 endemicity
Jinyoung Yang, Hye-Jin Kim, and Jun-Won Kim shared the first authorship.
Jinyoung Yang, Hye-Jin Kim, Jun-Won Kim, Byoungguk Kim, and Jae-Hoon Ko contributed equally to this study.
Abstract
Despite a high vaccination rate, the COVID-19 pandemic continues with immune-evading Omicron variants. The success of additional antigenic stimulation through breakthrough infection (BI) and updated vaccination in overcoming antigenic imprinting needs to be determined. Participants in a long-term follow-up cohort of healthcare worker (HCW) vaccinee were categorized according to their infection/vaccination status. Anti-SARS-CoV-2 spike/nucleocapsid protein antibodies were measured, and plaque reduction neutralization tests (PRNTs) against wild-type (WT), BA.5, BN.1, and XBB.1.5 were conducted. The neutralization activity of intravenous immunoglobulin (IVIG) products was evaluated to assess the immune status of the general population. Ninety-five HCWs were evaluated and categorized into seven groups. The WT PRNT ND50 value was highest regardless of infection/vaccination status, and groups with recent antigenic stimulation showed high PRNT titers overall. Groups with double Omicron stimulation, either by BI plus BA.4/5 bivalent vaccination or repeated BI, exhibited significantly higher BA.5 and BN.1 PRNT to WT PRNT ratios than those with single Omicron stimulation. Overall group immunity was estimated to be boosted in January 2023, reflecting the effect of the BA.4/5 bivalent booster and additional BIs, but slightly declined in June 2023. A substantial increase in the antibody concentrations of IVIG products was noticed in 2022, and recently produced IVIG products exhibited a substantial level of cross-reactive neutralizing activity against emerging variants. Neutralizing activity against emerging variants could be enhanced by repeated antigenic stimulation via BI and/or updated vaccination. Overall group immunity was elevated accordingly, and IVIG products showed substantial activity against circulating strains.
1 INTRODUCTION
Despite the rapid development of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the coronavirus disease 2019 (COVID-19) pandemic has continued with immune-evading variants.1-3 In response to the rapid spread of Omicron subvariants in 2022, bivalent mRNA vaccines containing spike protein sequences of both wild-type (WT) SARS-CoV-2 and Omicron subvariants (either BA.1/2 or BA.4/5) were developed.4-7 However, the additive effect of bivalent vaccines (in terms of overcoming the antigenic imprinting of WT SARS-CoV-2 primed by the initial vaccine series) has been controversial.5 Furthermore, more than 50% of the total population has experienced breakthrough infection (BI) despite the high coverage of the third dose, and the actual vaccination rate for the bivalent vaccine has remained below 30%, reflecting questions about the additive effects of the vaccine after a BI.8, 9
Currently, the epidemic waves of Omicron subvariants are continuing, and the introduction of monovalent vaccines against XBB.1.5 is scheduled.10 To establish a vaccination strategy for the upcoming COVID-19 endemic era, we used long-term follow-up data from a healthcare worker (HCW) vaccinee cohort to evaluate whether BIs and additional vaccination for updated strains could overcome antigenic imprinting.1-3, 11, 12 Furthermore, to validate whether the group immunity observed in the HCW cohort is similar to that in the general population, we measured anti-SARS-CoV-2 antibody concentrations and neutralization activity against the circulating strains in serial intravenous immunoglobulin (IVIG) products produced from pooled plasma collected from anonymous healthy donors.
2 METHODS
2.1 Study population and specimen collection
Participants in a long-term follow-up cohort were recruited from a previous HCW vaccinee cohort.1-3 The cohort was divided into three groups by prime vaccine series: two doses of ChAdOx1 (ChAd; Vaxzevria®; AstraZeneca), two doses of BNT162b2 (BNT; Comirnaty®; Pfizer), and ChAd-BNT heterologous vaccination. All participants also received a third dose of mRNA vaccine in the winter of 2021. HCWs who experienced SARS-CoV-2 infections before the third dose were excluded from this study. BIs were primarily diagnosed by a positive rapid antigen test (RAT) or reverse transcription-polymerase chain reaction (RT-PCR) of respiratory specimens (molecular diagnosis). HCWs who did not have apparent clinical symptoms and did not undergo RAT or RT-PCR testing were diagnosed using the following serologic diagnostic criteria: (1) positive conversion of antinucleocapsid antibody (Nab, for those without a previous infection history) or an increasing COI (cut-off index) of Nab (for those with a previous infection) and (2) an antispike protein antibody (Sab) concentration more than 1.5-fold higher than expected.2, 3, 13 Those criteria are based on previous observations in RT-PCR-confirmed cases, and the expected Sab concentration was calculated from the waning equation derived in previous analyses.2, 3 The infecting variant type of each BI was considered the dominant strain circulating during that outbreak period without further testing. Sampling was performed in January and June 2023, and previous data were also used for the analyses.1-3 This study was approved by the Institutional Review Board of Samsung Medical Center (SMC 2021-01-165), and all participants provided written informed consent.
2.2 Laboratory procedures
The semiquantitative measurement of Sab was performed using an Elecsys® Anti-SARS-CoV-2 S kit (Roche Diagnostics) with an electrochemiluminescence immunoassay (ECLIA) method in cobas e analyzers. The positive cut-off value for the Sab concentration was 0.8 U/mL, and the linear range was 0.4–250 U/mL. Automated dilution was performed for up to a 1:50 dilution using the analyzer, and additional manual dilutions of up to 1:200 were conducted for saturated specimens. The numeric results from the kit in U/mL and those from the World Health Organization in BAU/mL are considered equivalent14-16 and exhibited good linear correlation with the neutralizing antibody titer.17, 18 The measurement of Nab was performed with an Elecsys® Anti-SARS-CoV-2 kit (Roche Diagnostics) utilizing the ECLIA method. A Nab COI ≥ 1.0 was considered positive, and high sensitivity and specificity in detecting SARS-CoV-2 infection were validated.18, 19 To assess neutralizing activity, plaque reduction neutralization tests (PRNTs) were conducted for the WT, BA.5, BN.1, and XBB.1.5 strains of SARS-CoV-2 at the Korea Disease Control and Prevention Agency (KDCA) using detailed methods described in previous publications.18, 19 The 50% neutralizing dose (ND50) titer was calculated using the Karber formula.20 A PRNT ND50 larger than 20 was considered positive, according to the standard operating procedure of the KDCA, and a previous publication estimated that a WT PRNT ND50 of 118.25 indicated 50% protective value.1 To test the neutralization activity of IVIG products against the circulating strains, PRNTs against BA.2, XBB.1.16, and XBB.1.9.1 were additionally conducted.
2.3 Statistical analysis
To compare the baseline characteristics and laboratory test results, the Mann–Whitney U test was used for continuous variables, and the χ2 test was used for categorical variables. To compare matched specimens, the Wilcoxon matched-pairs signed rank test was conducted. To evaluate the waning kinetics of antibody titers, linear regression, exponential one phase decay, and segmental linear regression models were used. Antibody titers, including Sab and PRNT ND50, were evaluated on the log10 scale, and correlation equations derived from the linear regression model were used to estimate group immunity. All p values were two-tailed, and values < 0.05 were considered statistically significant. GraphPad Prism version 9.2 (GraphPad Software) was used for all statistical analyses.
3 RESULTS
3.1 The study population and sampling points according to the COVID-19 outbreak situation
The COVID-19 outbreak situation, study population, and sampling points are illustrated in Figure 1, with a detailed description provided in the Supplementary Materials. In brief, the outbreak period was divided into the BA.1/2 period, the BA.5 period, and the BN.1/XBB period. Ninety-five HCWs from the earlier HCW vaccinee cohort were included in the present analyses. Sab and Nab were measured in all collected specimens, and 56 (58.9%) serum samples from the January collection underwent PRNTs. Participants were categorized into seven groups based on their infection/vaccination status at the January 2023 sampling point: Group 0 (n = 11, no BI and no BA.4/5 booster), Group 1 (n = 7, no BI and BA.4/5 booster), Group 2 (n = 31, BA.1/2 BI and no BA.4/5 booster), Group 3 (n = 25, BA.5 BI and no BA.4/5 booster), Group 4 (n = 6, BA.1/2 BI and BA.4/5 booster), Group 5 (n = 9, BA.5 BI and BA.4/5 booster), and Group 6 (n = 6, both BA.1/2 and BA.5 BI and no BA.4/5 booster). These groups were also divided into two categories based on the number of stimulations with the Omicron antigen: Groups 1–3 were categorized as single Omicron stimulation, and Groups 4–6 were categorized as double Omicron stimulation. The baseline characteristics of each group are presented in Supporting Information: Tables 1 and 2, and they show no significant differences between the single and double Omicron stimulation groups.
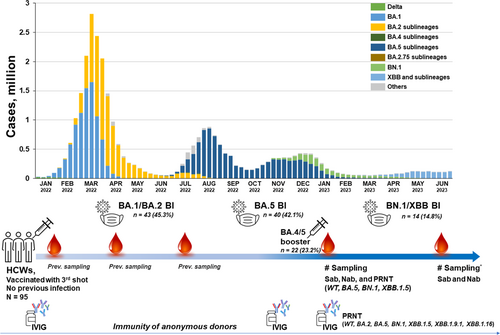
3.2 Neutralizing activities against Omicron subvariants according to infection/vaccination status
Neutralizing activity against WT, BA.5, BN.1, and XBB.1.5 in each of the infection/vaccination groups is presented in Supporting Information: Figures 1–3. Among the 57 serum samples subjected to PRNT, the WT PRNT ND50 value was highest (median 2302, IQR 580–5631), followed BA.5 (median 647, IQR 178–1871), BN.1 (median 381, IQR 93–981), and XBB.1.5 (median 305, IQR 144–526). The WT PRNT ND50 value was highest regardless of the infection/vaccination status, suggesting antigenic imprinting from the prime vaccine series. HCWs who experienced a recent BI (Group 3) or received the additional BA.4/5 booster vaccine after an Omicron BI (Groups 4 and 5) showed high PRNT titers overall. HCWs who had both BA.1/2 and BA.5 BIs (Group 6) experienced the second infection mildly, and 80% of them were diagnosed serologically. The overall PRNT titer in Group 6 was between those in Groups 2 and 3, but the ratio of BN.1 and XBB.1.5 PRNT titers to WT PRNT titer was higher in Group 6 than in the other groups. HCWs who did not experience any BI after the third vaccine dose and did not receive the BA.4/5 bivalent booster vaccine (Group 0) exhibited markedly waned PRNT titers, the median values of which were below the 50% protective level of 118.25,1 but a low level of cross-reactivity to the Omicron subvariants was maintained (Supporting Information: Figure 2).
To investigate the effect of repeated Omicron stimulation on antigenic imprinting, we compared single Omicron stimulation (Groups 1–3) with double Omicron stimulation (Groups 4–6) (Figure 2 and Supporting Information: Figure 4). The Sab and WT PRNT titers did not differ statistically between the two groups, but the BA.5, BN.1, and XBB.1.5 PRNT titers were significantly higher in the double Omicron stimulation groups (all p < 0.01). Due to variations in the interval between the last antigenic stimulation and sampling across groups, we further analyzed the ratio of the Omicron subvariant PRNT titers to the WT PRNT titer to adjust for the overall boosting and waning of titers. The double Omicron stimulation groups exhibited significantly higher BA.5 and BN.1 PRNT to WT PRNT ratios, suggesting that humoral immunity might be enhanced toward variant antigens with repeated stimulation. To substantiate that finding, we conducted two additional analyses. First, a subgroup analysis compared the BI-only groups (Groups 2 and 3) with the BI plus BA.4/5 booster groups (Groups 4 and 5) (Supporting Information: Figure 5). Similarly, the BI plus BA.4/5 booster groups exhibited significantly higher BA.5, BN.1, and XBB.1.5 PRNT titers, along with a higher BA.5 PRNT to WT ratio, than the BI-only groups. Second, paired pre- and poststimulation comparisons were conducted in both the single and double Omicron stimulation groups in individuals with available PRNT test results from the previous analysis (Supporting Information: Figure 6).3 After the first Omicron stimulation, Sab and the WT PRNT and BA.5 PRNT titers increased significantly (all p < 0.01). The fold increase in PRNT titers was higher for BA.5 than for WT, and the BA.5 PRNT to WT PRNT ratio increased significantly after the first Omicron stimulation, suggesting an enhanced response to BA.5. After the second Omicron stimulation, a similar enhancement was observed, but statistical significance was not achieved due to the limited number of paired samples.

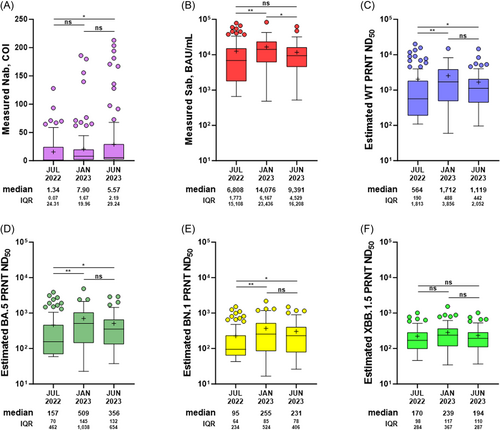
3.3 Estimation of the changing level of group immunity
To evaluate the changing level of group immunity, we compared the sampling points of July 2022, January 2023, and June 2023 (for those who participated in all three sampling points, n = 87; Figure 3). The points represent group immunity produced after the BA.1/2, BA.5, and BN.1/XBB outbreak periods, respectively. Nab titers increased serially, representing the cumulative number of BIs. Sab titers were highest in January 2023, reflecting the effect of the BA.4/5 bivalent booster (19.4%), along with BIs during the BA.5 period (29.9%). In June 2023, a decline in Sab titers was observed, resulting from the combined effect of natural waning and low additional boosting by BIs during the BN.1/XBB period (16.1%). To estimate the overall neutralizing activity at each time point, we used a correlation equation deduced from the measured values (Supporting Information: Table 3). The overall neutralizing activity against WT, BA.5, and BN.1 decreased slightly in June 2023, but it remained significantly higher than that in July 2022. The slow decline in overall neutralizing activity could be associated with increasing accumulation of BI cases. In the analysis of waning kinetics, BI-induced hybrid immunity exhibited a gradual decrease throughout the 16-month follow-up period (Supporting Information: Figure 7).
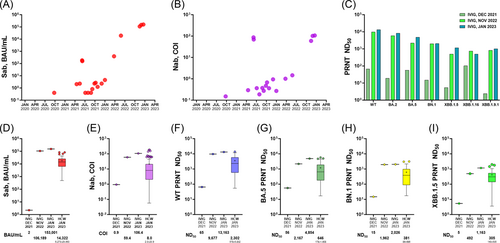
To investigate the level of group immunity in the general population, we analyzed the Sab and Nab of 19 IVIG products that remained after infusion (Figure 4). Most of the IVIG products manufactured in 2020 and 2021 were either Sab-negative or exhibited low titers, but a substantial increase was noticed in those manufactured in 2022. In the lots manufactured after November 2022, Sab titers were higher than 105 BAU/mL. Nab COI increased accordingly. PRNTs against WT, BA.2, BA.5, BN.1, XBB.1,5, XBB1.16, and XBB 1.9.1 were conducted for lots produced in December 2021, November 2022, and January 2023. Recently produced IVIG products exhibited a substantial level of cross-reactive neutralizing activity against the strains (BN.1 and XBB1.5) emerging at the time of production, as well as a high level of activity against strains that had circulated previously (BA.2 and BA.5). When the titer was compared with the median measured in the HCW vaccinee cohort, the IVIG PRNT titers were approximately fivefold higher than the median for the HCW vaccinee cohort. Based on pharmacokinetics, it is expected that the peak plasma concentration of anti-SARS-CoV-2 antibodies delivered by an IVIG infusion at 1 g/kg would be as high as that directly measured in a 10% IVIG product.21, 22 This concentration would be considerably higher than that of antibodies obtained by infusing 500 mL of convalescent plasma (CP) from recently infected or vaccinated individuals.23, 24 Detailed calculations are provided in the Supplementary Materials.
4 DISCUSSION
In the present longitudinal HCW cohort, we subdivided infection/vaccination groups to reflect the domestic COVID-19 outbreak situation. Because HCWs were highly compliant with the infection control policy, they were willing to undergo RAT or RT-PCR if COVID-19-related symptoms were present and shared their diagnoses. In addition, because Sab/Nab titers were tested at regular intervals, subclinical infections could be serologically diagnosed. With additional antigenic stimulation, either by an Omicron BI or bivalent vaccination, the WT PRNT increased to the highest titer, indicating persistent antigenic imprinting.2, 25, 26 However, as repeated exposure to Omicron antigens occurred, the ratios of Omicron subvariant PRNT titers to WT PRNT titer increased. This suggests that neutralizing antibodies evolve and can overcome antigenic imprinting through repeated antigenic stimulation. This phenomenon was most strongly observed for BA.5, as BA.5 BI and BA.4/5 bivalent vaccination took a major antigenic stimulation. The rise of PRNT titers against BN.1 and XBB.1.5 was not as robust as those against BA.5, which suggests that an updated vaccine booster tailored to the circulating strain is needed. This phenomenon of enhanced neutralization ability due to repeated Omicron antigen stimulation was verified in the analysis of paired samples and the subgroup analysis comparing the Omicron BI only and hybrid immunity from Omicron BI and bivalent vaccination groups.
In the estimation of changing group immunity, the overall antibody titers increased in January 2023, reflecting additional BIs and bivalent vaccination, and then slowly declined to June 2023. The robust boosting effect and slow waning of hybrid immunity were presented in a previous study 3 and verified with a longer follow-up period in this analysis. Unlike the vaccine-induced antibody titers of the Group 0 HCWs, which declined rapidly until the inflection point 8 months after vaccination, the hybrid immunity induced by BIs waned slowly without an inflection point over the 16-month follow-up period. Like the slightly decreased antibody titers of June 2023, the group immunity strengthened by hybrid immunity is expected to be maintained for months. However, when new immune-evading variants appear while hybrid immunity is waning, a new epidemic wave could occur. Considering the additive immunogenic effect of bivalent vaccination found in our analyses, additional boosting with an updated vaccine could reduce the scale of such an epidemic.
Enhanced group immunity in the general population was shown by the IVIG products derived from multiple anonymous donors. Neutralizing activity was robust against previously circulating strains (BA.2 and BA.5), and substantial cross-reactivity to variants emerging at the time of IVIG production was noted. This confirms that the strengthening of group immunity through BIs and booster vaccinations is not limited to this HCW cohort but occurs at the population level as well. In particular, IVIG collected before the BN.1/XBB period exhibited cross-reactive neutralizing ability, even against the subsequent circulating strains. This suggests that IVIG products could have a role as a therapeutic modality of passive immunization that can keep up with the changing variants in circulation. According to pharmacokinetics, an IVIG infusion of 1 g/kg would provide considerably more numerous neutralizing antibodies than a 500 mL CP infusion from recently infected or vaccinated individuals. In other words, patients with primary immune deficiency who require IVIG replacement can be expected to develop protective immunity against COVID-19, and IVIG products can be used to treat persistent COVID-19 infection in immunocompromised hosts.27 Although many monoclonal antibody agents have been developed, they were withdrawn from the market due to rapid emergence of variants.28, 29 Although the population requiring passive immunization might not be large, the therapeutic potential of IVIG products based on evolving herd immunity could be crucial for the management of COVID-19 in immunocompromised hosts.
This study has several limitations. First, due to a variety of infection/vaccination statuses during the study period, relatively few specimens from each infection/vaccination group underwent PRNT. Nevertheless, by comparing the single and double Omicron stimulation groups, we found a significant enhancement of neutralizing activity toward the stimulating variant antigen. Second, in the estimation of group immunity at each sampling point, the correlation equations we used had moderate associations overall, and those who were infected during the BN.1/XBB period might have had higher BN.1/XBB PRNT titers than estimated in this study. However, the estimation was based on the measured Sab value and could provide insight into change in group immunity. Also, we substantiated that the group immunity enhanced by BIs and booster vaccination was reflected in IVIG products from multiple anonymous donors. Third, the time interval from the last antigenic stimulation to blood sampling varied by infection/vaccination group. To address that limitation, we mainly compared Omicron subvariant PRNT ratios to the WT PRNT. Lastly, we could not answer whether enhanced neutralizing antibodies against Omicron subvariants would play a protective role against symptomatic infection. The finding that HWCs in group 6 experienced subclinical second infection may suggest protective effect of the hybrid immunity formulated from the prior infection. However, as the number of HCWs in group 6 were small and interval between two BI events were short (6–8 months), it would be difficult to determine the protective effect obtained from the prior infection. A longer follow-up data need to be investigated to evaluate protective effect from symptomatic infections.
5 CONCLUSION
In conclusion, neutralizing activity against emerging variants can be enhanced by repeated antigenic stimulation by BIs and/or updated vaccination. Overall group immunity was elevated accordingly, and IVIG products contained substantial activity against circulating strains.
AUTHOR CONTRIBUTIONS
Jinyoung Yang, Hye-Jin Kim, Jun-Won Kim, Ju-yeon Choi, Gi-eun Rhie, So-Young Lee, Kyong Ran Peck, Byoungguk Kim, and Jae-Hoon Ko were involved in the design of this study. Jinyoung Yang, Kyong Ran Peck, and Jae-Hoon Ko enrolled participants and collected specimens. Hye-Jin Kim, Jun-Won Kim, Jin Yang Baek, Young Jae Lee, Su-Hwan Kim, Hyeonji Jeong, Eun Joo Chung, and Byoung Kwon Park performed the experiments. Jinyoung Yang, Hye-Jin Kim, Jun-Won Kim, Byoung Kwon Park, and Jae-Hoon Ko assembled the data. Jinyoung Yang, So-Young Lee, Byoungguk Kim, and Jae-Hoon Ko were involved in writing. All authors crucially approved and revised the manuscript.
ACKNOWLEDGMENTS
The authors would like to express our gratitude to all participants who participated voluntarily in this study. This study was supported by an intramural fund (##2022-NI-039-01) from the Korea National Institute of Health, research program funds (#2023-ER2603-00 and #2022-ER1902-00) by the Korea Disease Control and Prevention Agency, and a Samsung Medical Center grant (#SMO1230121). We thank Jinseob Kim (Zarathu Co., Ltd.) for advice on statistics.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the Institutional Review Board of Samsung Medical Center (SMC 2021-01-165), and all participants provided written informed consent.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




