Evaluation of reverse transcription loop-mediated isothermal amplification assay for the detection of severe fever with thrombocytopenia syndrome in clinical laboratories: A single-center study
Wen Tian, Yuanyuan Zhang, and Shuying Geng contributed equally to this study and are co-first authors also.
Abstract
Severe fever with thrombocytopenia syndrome (SFTS) is an acute infectious disease prevalent in East Asia with a high mortality rate (5%–30%). Reverse transcription loop-mediated isothermal amplification (RT-LAMP), a rapid nucleic acid-based diagnostic technique, is a useful alternative for the clinical diagnosis of SFTS, particularly in resource-limited hospitals or rural clinics in SFTS virus-endemic regions. However, the actual clinical sensitivity and specificity of RT-LAMP remain unclear. This study evaluated the field application of RT-LAMP. This prospective field study included 130 patients with laboratory-confirmed SFTS from Yantai, Shandong Province, China. Two sets of RT-LAMP primers were validated, and one set of RT-LAMP assays was optimized for field detection. Nucleic acids of serially collected serum/plasma samples were identified using quantitative reverse transcription polymerase chain reaction (RT-qPCR) and RT-LAMP. In laboratory tests, we optimized the detection time of primer set 2 for the RT-LAMP to 60 min. Notably, the onsite testing of 279 plasma samples from patients with SFTS revealed that the sensitivity and specificity of the test were 81.9% and 96.3%, respectively. We also analyzed samples with different durations of the disease, and our study showed that the sensitivity of RT-LAMP detection at the beginning of admission was 89.92%. Univariate analysis showed that the detection rate of RT-LAMP was similar to that of RT-qPCR in the first 5 days of the disease course and was lower than that of RT-qPCR on Days 6 and 14–15 of the disease course. The positive detection rate in patients aged ≥ 65 years was significantly higher than that in younger age groups. RT-LAMP is a simple, suitable, and rapid clinical detection method of SFTS onsite screening. It is more suitable for screening patients in the early stages of the disease and analyzing samples obtained from patients aged ≥ 65 years before the 6th day of the disease course.
1 INTRODUCTION
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging acute infectious tick-borne disease caused by SFTS virus (SFTSV), also known as Dabie bandavirus, which belongs to the genus Phlebovirus of the family Bunyaviridae.1 The virus is a spherical virion that comprises three RNA segments: large (L), medium (M), and small (S). The main clinical manifestations of SFTS include fever, thrombocytopenia, leukopenia, vomiting, and diarrhea, and it can even cause multiorgan failure with a high fatality rate (approximately 5%–30%).1-4 Based on the disease presentation, the clinical course of SFTS infection can be divided into four phases: incubation, acute, multiple organ failure, and recovery phases.5 Previous studies have shown that during the acute phase, patients with confirmed SFTS infection have a high viral load in their serum, which is associated with their prognosis.6 Moreover, a high viral load is one of the risk factors for mortality.7 Most patients with severe SFTS experience multiple organ dysfunction, shock, diffuse intravascular coagulation, and eventually, death.2, 8 To the best of knowledge, effective anti-SFTSV interventions are not currently available. After the detection of its first case in China in 2009, the number of SFTS endemic areas has gradually increased to more than seven countries in East Asia, including China, Vietnam, South Korea, Pakistan, Myanmar, Thailand, and Japan.4, 8-14 Moreover, the number of patients with SFTS has been gradually increasing every year since 2009, with >19 000 cases were diagnosed worldwide in 2021.4, 9-12, 15 High mortality rates, drug shortages, and an increasing number of cases have raised a global health concern about the possibility of an SFTS pandemic. Thus, a rapid and accurate diagnostic method is warranted for the prevention and treatment of SFTS.
Several methods have been developed for the diagnosis of SFTS. Owing to the high sensitivity and specificity of quantitative reverse transcription polymerase chain reaction (RT-qPCR), it is considered the gold standard for virus detection.16 Notably, performing RT-qPCR requires sophisticated instruments, skilled personnel, and a considerable time.16, 17 However, 97% of patients with SFTS are farmers living in areas with limited medical resources,17 which hinders the applicability of RT-qPCR in detecting SFTS; therefore, RT-qPCR is unsuitable for onsite SFTS testing. Conversely, loop-mediated isothermal amplification assay (LAMP)—another nucleic acid-based detection technique—does not require sophisticated equipment and expensive reagents, facilitating its applicability in rural regions with limited resources and primary hospitals with low medical service capacity. A set of six primers was designed to target the viral sequence using LAMP, and the process could be completed in 20–60 min at a constant temperature. The results of LAMP can be determined by observing the change in the color of mixture with the naked eye, eliminating the need for highly trained technicians.17 Previous studies have evaluated the detection of SFTS using reverse transcription LAMP (RT-LAMP) and reported the sensitivity of 84.9%–99%16-19 and specificity as high as approximately 99% (95% confidence interval: 0.93–1.00).20
These studies suggest that RT-LAMP as a viable detection method with a wide range of clinical applications. However, in clinical practice, the relationship between the application procedures of RT-LAMP in the disease process has not been evaluated, and results of RT-LAMP have not been compared among samples collected from patients during different periods. In this study, we evaluated the practicality of RT-LAMP for the clinical diagnosis of SFTSV and explored the application procedures of RT-LAMP using samples collected during different stages of SFTS. We subsequently analyzed the effect of the length of the disease course, age, and gender on the detection rate of RT-LAMP. According to our findings, RT-LAMP detection rates are influenced by the course of the disease and age of patients. RT-LAMP is the most effective method to analyze clinical samples obtained from older patients with a shorter course of disease. We believe that our study findings will lay a solid foundation for future clinical applications of RT-LAMP.
2 MATERIALS AND METHODS
2.1 Collection of clinical samples
All patients from Yantai Qishan Hospital with laboratory-confirmed SFTS from 2021 to 2022 were included in this study. Yantai Qishan Hospital is a general hospital dedicated to treat patients with infectious diseases and is located in an SFTSV-endemic region of Shandong Province. The participants were diagnosed according to the following criteria: exhibiting clinical symptoms of SFTS, such as fever and gastrointestinal symptoms; leukopenia; low platelet (PLT) count; and positivity for SFTSV RNA which was detected using RT-qPCR. Serum samples and clinical data were longitudinally collected from 130 hospitalized patients from hospital admission to discharge or death. All clinical information, including the patients' general information, laboratory test data, and epidemiological data, and clinical outcomes, were carefully collected and uploaded to an electronic cloud storage system. The onset time of SFTS was defined as the time when symptoms of SFTS first appeared. Adult patients with hemorrhagic fever with renal syndrome (HFRS) and human immunodeficiency virus (HIV) were assessed to determine the specificity of RT-LAMP.
2.2 Virus and RNA extraction
Professor Ruiyuan Cao kindly provided our laboratory with inactivated SFTSV strains that were isolated and subcultured from the plasma of patients with laboratory-confirmed SFTS. SFTSV RNA extractions from serum were performed using the MagaBio Plus Virus DNA/RNA Purification Kit (Hangzhou Bioer Technology Co., Ltd.). The volume of the extracted RNA fragments was 70 μL. Nucleic acids were stored at −80°C to prevent repeated freezing and thawing, which would affect the results.
2.3 RT-qPCR
The Fluorescence PCR Kit for SFTSV RNA (Sun Yat-Sen University Daan Gene Co., Ltd.) was used to perform one-step RT-qPCR. The RNA system contained 17 μL SFTS A solution, 3 μL SFTS B solution, and 5 μL RNA, and the total system volume was 25 μL. The reaction conditions for RT-qPCR are listed as follows: preheating at 50°C for 15 min, heating at 95°C for 15 min, followed by 45 cycles at 95°C for 15 s, 55°C for 45 s, and finally extended at 45°C for 10 s. LightCycler 96 (Roche) was used in the experiment. A cycle threshold (Ct) value less than 35 was considered positive.
2.4 RT-LAMP system optimization
The Loopamp RNA amplification kit (Eiken Chemical Co., Ltd.) was used for RT-LAMP. Overall, 25 μL of the reaction system was required, which consisted of 12.5 μL reaction mix, 0.4 μL forward and backward inner primers each, 0.2 μL forward and backward loop primers each, 0.1 μL F3 and B3 primers each, 1 μL enzyme mix, 4.1 μL water, 1 μL fluorescence visual detection reagent (Eiken Chemical Co., Ltd.), and 5 μL RNA. Two sets of RT-LAMP primers that had previously been proven to be highly sensitive and specific were used.16, 19 To improve the adaptability of the RT-LAMP primers to the study population, we optimized the experimental conditions of the primer series. For set 1,16 the experiment time was increased from 40 to 60 min and reaction temperature was maintained at 63°C. For set 2,19 the experiment time was set to 60 min and reaction temperature was maintained at 65°C. The development of bright-green color detected via visual inspection indicated a positive reaction. Ultraviolet light irradiation can also be performed to determine the result. Moreover, a qualitative judgment of the product can also be obtained by fluorimetry.
2.5 Detection limit, sensitivity, and specificity of RT-LAMP
To detect the inactivated virus in our laboratory, one-step RT-qPCR was performed. The virus was serially diluted to obtain a 10-fold dilution with the concentration of the prepared viral nucleic acid ranging from 104 to 100 50% tissue culture infectious dose (TCID50)/mL, to distinguish sensitivities of the two sets of primers. To verify the specificity of the two sets of primers and determine the occurrence of cross-reactions, RNA was extracted from the plasma of patients with HFRS and HIV. Each sample was replicated three times. The two sets of primer systems were also verified using plasma RNA samples obtained from patients with SFTS; these samples were collected in 2019 and 2020 and stored in our laboratory.
2.6 Statistical analysis
Statistical Package for the Social Sciences 25.0 (International Business Machines Corporation) was used to perform statistical analysis. The T test was used to analyze continuous variables with a normal distribution and homogeneous variance, which were expressed as mean ± standard deviation. Variables with a non-normal distribution were analyzed using the Mann–Whitney test and expressed as median (interquartile range: P25, P75). Binary scalars were analyzed using the χ2 test and expressed as constituent ratios. Graphs of the results were constructed using GraphPad 8.0 (GraphPad Software).
3 RESULTS
3.1 Data of patients with SFTS
We included patients with SFTSV infection who presented to Qishan Hospital from July 2021 to August 2022 and prospectively recorded their clinical data. A total of 130 hospitalized patients with SFTS were enrolled, including 45 and 85 patients who were hospitalized in 2021 and 2022, respectively. Of these, 15 patients died postoperatively, and the mortality rate was 11.54%, which is consistent with those reported in previous studies. All 15 patients died due to multiple organ failure caused by cardiac injury, renal failure, and lung infection. The mean age of the deceased patients was 71.93 ± 5.71 years, which was higher than that patients in the survivor group (p < 0.05, T test). Our study population included 73 male patients; however, no statistically significant difference was found in the sex of patients between the deceased and survivor groups (p > 0.05, χ2 test). Generally, patients presented to the hospital on the seventh day of disease onset. The median hospitalization time in the deceased group was 6 days (range, 4–8 days), which was shorter than that in the survivor group (p < 0.05, Mann–Whitney U test). Detailed clinical characteristics of the patients are shown in Table 1.
| Variables | Total (n = 130) | Dead (n = 15) | Survival (n = 115) | p Value |
|---|---|---|---|---|
| Age (years) | 69 (58.75, 75.00) | 71.93 ± 5.71 | 66.43 ± 10.95 | 0.005a |
| <50, N | 9 (6.92%) | 0 (0.00%) | 9 (78.26%) | |
| 50−60, N | 24 (18.46%) | 0 (0.00%) | 24 (20.87%) | |
| 60−70, N | 33 (25.38%) | 5 (33.33%) | 28 (24.35%) | |
| 70−80, N | 53 (40.77%) | 8 (53.33%) | 45 (39.13%) | |
| >80, N | 11 (8.46%) | 2 (13.33%) | 9 (7.83%) | |
| Gender | ||||
| Male, N | 73 (56.15%) | 5 (33.33%) | 52 (45.22%) | 0.38b |
| Female, N | 57 (43.85%) | 10 (66.67%) | 63 (54.78%) | |
| Days to onset at admission | 7.00 (5.00, 8.00) | 6.73 ± 2.74 | 7.10 ± 2.78 | 0.64a |
| Days in hospital | 11.00 (7.00, 14.00) | 6.00 (4.00, 8.00) | 11.00 (8.00, 14.00) | 0.001c |
| Collected year | ||||
| 2021, N | 45 (34.62%) | 6 (40.00%) | 39 (33.91%) | 0.64b |
| 2022, N | 85 (65.38%) | 9 (60.00%) | 76 (66.09%) | |
| Laboratory test | ||||
| WBC (3.5–9.5 × 109/L) | 3.59 (2.59, 5.07) | 3.34 (2.21, 5.52) | 3.60 (2.61, 4.97) | 0.93c |
| PLT (125–350 × 109/L) | 59.00 (40.00, 79.00) | 46.00 (32.00, 61.00) | 60.00 (42.25, 83.75) | 0.08c |
| ALT (9–50 U/L) | 58.70 (38.00, 119.20) | 71.00 (51.80, 144.70) | 55.60 (35.56, 110.55) | 0.30c |
| AST (15–40 U/L) | 115.30 (67.60, 202.40) | 280.60 (100.60, 553.60) | 106.65 (60.93, 174.48) | 0.002c |
| LDH (80–285 U/L) | 529.00 (360.50, 931.50) | 894.00 (529.00, 1732.00) | 490.50 (331.75, 894.00) | 0.001c |
| CK (0–190 U/L) | 375.00 (142.50, 1015.00) | 816.00 (521.00, 1645.00) | 352.50 (127.50, 932.00) | 0.01c |
- Note: ± indicates that data meet the normal distribution, using the mean ± standard deviation.
- Abbreviations: ALT, alanine aminotransaminase; AST, aspartate aminotransferase; CK, creatine phosphokinase; LDH, lactate dehydrogenase; PLT, platelet; WBC, white blood cell count.
- a T test.
- b χ2 test.
- c Mann–Whitney U test.
We also collected and compared the laboratory test data of the patients. White blood cell count (WBC), PLT, and alanine aminotransaminase levels did not significantly differ between the two groups (p > 0.05, Mann–Whitney U test). However, patients in the deceased group had higher aspartate aminotransferase, lactate dehydrogenase, and creatine phosphokinase levels (p < 0.05, Mann–Whitney U test).
3.2 Evaluation of the RT-LAMP primer sets
We screened two sets of RT-LAMP primers. Primer set 1 was designed based on the S fragment, whereas primer set 2 was designed based on the L fragment. The design of primer set 1 was published by the team of Professor Huang in China in 2014, and that of primer set 2 was published by the team of Professor Baek in 2018. Both sets of primers had high sensitivity and specificity.16, 19
The detection limit for each primer set was determined using 10-fold-diluted viral RNA isolated from an SFTSV culture, with the viral load ranging from 104 to 100 TCID50/mL. The results were determined based on the color change observed through visual inspection (Figure 1A,B), fluorescence via UV light irradiation (Figure 1C,D), and fluorescence intensity measured using a fluorimeter (Figure 1E,F) were consistent. We repeated the same procedure 10 times. The detection limits of primers set 1 and 2 were 103 and 102 TCID50/mL, respectively. Finally, we tested the specificity of the two primer sets using RNA extracted from the serum of 20 patients with HFRS and 20 patients with HIV. The RNA samples did not demonstrate RT-LAMP specific amplification, indicating that both primer sets were highly specific.
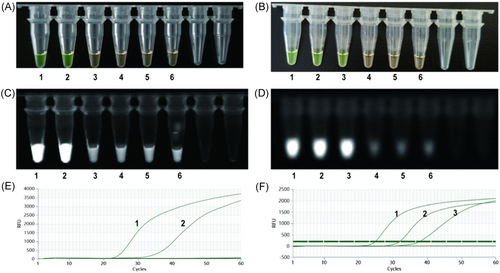
3.3 Laboratory validation of RT-LAMP
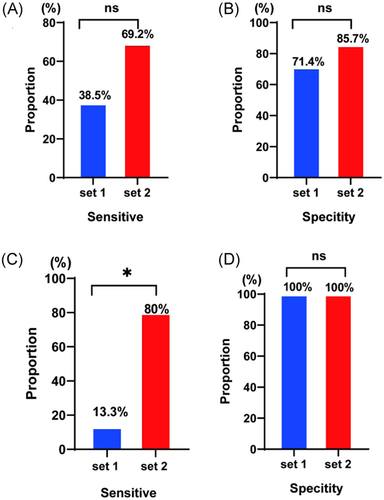
To validate the two primer sets in the clinical samples with different SFTSV loads, we used the clinical serum samples of patients with SFTS previously collected from Qishan Hospital in 2019 and 2020. Compared with the RT-qPCR results, the gold standard method, primer set 1 had a sensitivity of 38.5% (Figure 2A) and specificity of 71.4% (Figure 2B) for the samples collected in 2019, whereas it had a sensitivity of 13.3% (Figure 2C) and specificity of 100% (Figure 2D) for the samples collected in 2020. Primer set 2 had a sensitivity of 69.2% (Figure 2A) and specificity of 85.7% (Figure 2B) for the samples collected in 2019, whereas it had a sensitivity of 80% (Figure 2C) and specificity of 100% (Figure 2D) for the samples collected in 2020. Based on the lowest detection limit as well as the laboratory-validated sensitivity and specificity, we concluded that primer set 2 was more suitable for use at Qishan Hospital.
3.4 Onsite evaluation of RT-LAMP
Following the verification of detection limit, sensitivity, and specificity of RT-LAMP, primer set 2 was used for onsite testing at Qishan Hospital. We collected serum samples from patients with SFTS at Qishan Hospital between June 2021 and October 2022 for onsite verification of RT-LAMP. We performed RT-qPCR on all serum samples as the gold standard for diagnosis. Overall, 279 samples, including 89 and 190 samples collected in 2021 and 2022, respectively, were collected from 130 patients with SFTS. RT-qPCR revealed that 199 samples were positive (53 in 2021 and 146 in 2022) with a median viral load of 1270.5 TCID50/mL (range 53.6–12096.8). Compared with the gold standard RT-qPCR, RT-LAMP had a sensitivity of 81.9%, specificity of 96.3%. Details about the sensitivity and specificity of the assays are presented in Table 2. At the same time, we performed statistical analysis of samples from patients with different stages of disease. The results showed that the sensitivity and specificity of RT-LAMP detection were 89.92% and 90.91% for samples at the beginning of admission, as shown in Table S1. For later longitudinal samples from the same patient, the review found sensitivity and specificity of 70% and 97.1%, respectively, as shown in Table S2.
| RT-LAMP | RT-qPCR | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 163 | 3 | 166 |
| Negative | 36 | 77 | 113 |
| Total | 199 | 80 | 279 |
- Abbreviations: RT-LAMP, reverse transcription loop-mediated isothermal amplification; RT-qPCR, quantitative reverse transcription polymerase chain reaction.
3.5 Factors influencing RT-LAMP results
To compare the onsite sensitivity of RT-LAMP for clinical samples with that reported in previous studies, we analyzed several possible influencing factors, such as disease course, sex, and age. A consistent relationship was observed between the RT-LAMP results and disease course. We compared the day-by-day positive rates of RT-qPCR and RT-LAMP from disease onset. The positive rate of RT-LAMP did not vary significantly from that of RT-qPCR within the first 5 days after onset (p > 0.05, χ2 test). However, a significant difference was found between the two detection methods on Days 6 and 14–15 (p < 0.05, χ2 test). Furthermore, RT-qPCR was more effective than RT-LAMP in detecting SFTS (Figure 3A,B). Upon grouping the patients on the basis of age, we found that the RT-LAMP positive rate was high in patients aged ≥ 65 years (Figure 3C), and the differences between RT-LAMP and RT-qPCR were significant (p < 0.05, χ2 test). Additionally, we compared the positive rates of RT-LAMP between the sexes (Figure 3D) and found no significant difference (p > 0.05, χ2 test). Finally, we confirmed the age of patients and disease course affected the positive rate of RT-LAMP (Figure 4).
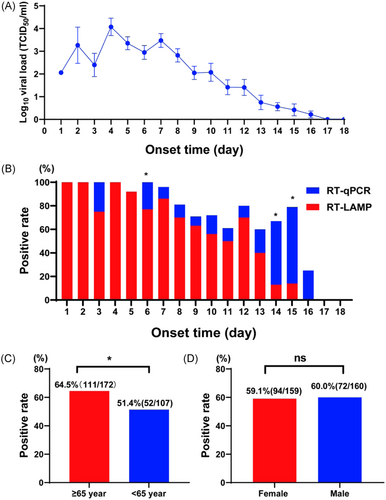
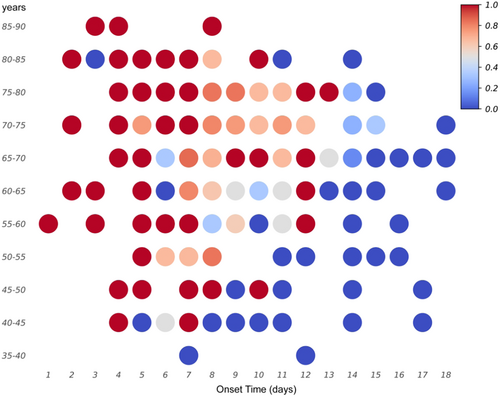
4 DISCUSSION
It is generally known that early and accurate diagnosis of infectious cases is crucial to prevent transmission and provide treatment. RT-LAMP is a rapid nucleic acid-based detection technique that facilitates the rapid onsite diagnosis of infectious diseases. Most individuals with SFTS live in remote areas or work outdoors.17, 21 In addition to tick bites, human-to-human transmission, nosocomial infections, and secondary deaths due to SFTS have been increasingly reported, making the application of RT-LAMP to detect SFTS critical.22-25
Furthermore, many factors can affect its gene-based clinical detection; thus, developing a systematic assessment method is important, especially if it is to be used in conjunction with established clinical methodologies. Several factors may impact methodological evaluations, such as the date of clinical sample collection, disease course, disease severity, transport of the sample, and manipulation of the sample by the technician.26, 27
In our study, the relevant laboratory conditions were optimized by screening primers, and two sets of primers were evaluated to select the primer group that was most suitable for this study. Based on the minimum detection limit, sensitivity and specificity of RT-LAMP primer sets for laboratory-preserved samples, primer set 2 was selected for conducting experimental studies. We found that the detection limit of RT-LAMP primers in primer set 2 was 102 TCID50/mL, which was lower than that of the RT-qPCR. We also found that RT-LAMP had extremely high specificity and demonstrated no specific cross-amplification reactions for HFRS and HIV samples.
However, the sensitivity and specificity of the laboratory test were lower than those reported in previous studies, which may be attributed to the following reasons. First, as the used samples were cryopreserved, their quality may have been compromised during collection, transportation, and preservation, which may have degraded the RNA fragment of interest. Second, the viral load in the studied samples was low, with approximately 60% samples having a viral load below the minimum detection limitation of RT-LAMP; moreover, 29.0% samples were negative for SFTS. Finally, we did not know the genotype of patients whose samples were collected from Yantai Qishan Hospital, which may not have matched the target sequence of the primer we designed.
We accurately recorded the outcomes of our laboratory experiments and implemented pertinent improvements to address any conjectural aspects. Subsequently, we conducted an onsite evaluation at an SFTS-specific hospital. At the same time, we included a large number of patients for two consecutive years, and a large number of longitudinal samples of patients were collected to explore the factors affecting the field detection rate of RT-LAMP. Using RT-qPCR as the gold standard, we performed onsite testing of 279 plasma samples and found that the sensitivity and specificity of RT-LAMP were 81.9% and 96.3%, respectively. After conducting further analysis on the samples that initially tested negative for RT-LAMP upon admission, it was discovered that 12 samples had a viral load with a Ct value distribution of 32–34, which fell below the detection threshold. And the average length of hospital stay for patients was 9 days. The insensitivity of the RT-LAMP assay may be due to the fact that patients' viral loads are below the limit of detection of RT-LAMP because of the mild condition or the long disease course at the time of admission. The low susceptibility of RT-LAMP during the course of the disease may be due to the treatment or the patient's own immune system, which reduces the viral load in the patient's blood.
Next, we conducted related cause analysis. Few studies have assessed the viral load of SFTS, and it can be used to assess the outcome of clinical patients and is closely related to the production of cytokines.7 Yang et al.28 found that the viral load of SFTS is correlated with the disease course. By continuously collecting longitudinal samples from patients with SFTS in Yantai from 2021 to 2022, we observed that the viral load varied with the time elapsed after disease onset and thus explored the pattern of change in the viral load across the different stages of the disease. We found that the samples collected on Day 4 after disease onset had the highest viral load. Notably, the disease burden gradually decreased within 8–13 days and after 14 days of disease onset, which is consistent with the temporal pattern of disease staging described by Liu et al.29 The results of this study further demonstrate that the viral load of SFTS varies depending on the disease stage.
While exploring the effect of disease course on RT-LAMP detection rates, we found that there were fewer positive tests on the third day after the onset of the disease. Moreover, the detection rate of RT-LAMP was lower than that of RT-qPCR after Day 6 of the course of the disease. After the initiation of the recovery phase of SFTS, some studies29, 30 have shown that after it occurs 13 days of the disease course, the detection rate of RT-LAMP significantly decreased, which may be related to the decrease in viral load during this period.
In our study, we found that the positive detection rate of RT-LAMP was correlated with age. Some studies found that age and high viral load were associated with poor disease outcomes.30, 31 Similarly, viral load was correlated with age in our study. Another study reported that the clinical manifestations of SFTS depend on the host's immune response.32 In older or immunocompromised patients, the virus may escape the host's immune surveillance or it cannot be cleared by the host's immune system.33, 34
Therefore, we propose the following factors that might contribute to the low sensitivity of RT-LAMP in this study. First, based on hospital field research, we enrolled patients who developed SFTS during the summer and autumn epidemic in Yantai Qishan Hospital in 2021 and 2022. Patients who met the criteria were included. Therefore, among admitted patients, factors such as young age of patients with prolonged disease duration or the occurrence of rapid nucleic acid negative rotation time may decrease the viral load of the sample below the detection limit of RT-LAMP. Second, the positive criterion for the RT-qPCR Test Kit used in this study was having a Ct < 35, which was lower than that of the RT-LAMP detection limit used in this study, resulting in the reduction of viral loads in some samples that may not have been detectable. In studies of coronavirus disease 2019, RT-LAMP was also shown to be associated with the viral load of the sample, and it was more sensitive to samples with Ct < 30 than those Ct > 30.35 Finally, RT-LAMP method has some limitations. This assay involves the use of multiple pairs of complex primers, which may increase the reaction rate but also limit the selection of target sites and specificity of results.36 The detection of changes in the color observed with the naked eye may affect the judgment of a weakly positive sample.37
Our study showed that the detection limit of RT-LAMP was affected by both age and disease course. We found that patients aged ≥ 65 years with a disease onset time of less than 6 days are more suitable for RT-LAMP assessment. Therefore, for clinical application, RT-LAMP may provide accurate results for patients with fever and outdoor work history as well as for patients with decreased WBC and PLT counts. RT-LAMP is widely used for pathogen detection, but its effectiveness has not been clinically proven, which is the purpose of our study. We found that RT-LAMP is more suitable for point-of-care clinical screening, but has a lower sensitivity for detection of disease of the whole course of disease. In this study, we evaluated the clinical use of RT-LAMP, and found that samples with low viral load could not be tested positive. This may result in RT-LAMP being undetectable on clinical screening in patients with mild disease, prolonged duration, or younger age, and is not suitable for long-term clinical disease detection.
In this single-center study, a total of 130 patients were included. However, to mitigate potential bias resulting from variations in the duration and severity of the patients' diseases, it is crucial to expand the study to include a larger number of patients from multiple centers. Viral genome mutation can result in the failure of nucleic acid amplification. The SFTSV genotype of the patients enrolled in this study should have been defined to explain the lower sensitivity of the RT-LAMP primer set used. Simultaneously, the RT-LAMP assay is constantly being updated and can be combined with other molecular diagnostic techniques to improve the accuracy of the assay, which also applies to SFTS.38, 39 Furthermore, to improve the accuracy of RT-LAMP, doctors in primary hospitals should distinguish the results according to the age and course of the disease while confirming the diagnosis of patients with suspected symptoms. The diagnosis of other patients with suspected symptoms and those with similar symptoms but a negative RT-LAMP test can be confirmed by performing recommending alternative tests.
5 CONCLUSION
Our study evaluated the clinical applicability of RT-LAMP to diagnose SFTS. This technique was found to be a fast and effective method for field detection in our study. RT-LAMP is suitable for the initial screening in the field, not for the continuous monitoring of the whole disease process. And then, the clinical detection rate of RT-LAMP was influenced by age and duration of disease, with the best results obtained in patients aged ≥ 65 years with a disease duration of less than 6 days.
AUTHOR CONTRIBUTIONS
Wei Zhang, Zhihai Chen, and Chuan Song designed the study. Wen Tian, Yuanyuan Zhang, and Shuying Geng performed the experiments and wrote the manuscript. Jianxin Wang, Wenjuan Ji, and Yanli Xu collected samples. Xu Gao, Xin Li, Ling Lin, and Yuanni Liu analyzed data.
ACKNOWLEDGMENTS
The authors would like to thank all the medical staff of the Department of Infectious Diseases and Laboratory of Qishan Hospital for their help in this study. This work was supported by National Natural Science Foundation of China (82072295), Funding Project for Open Research Projects of Beijing Key Laboratory of Emerging Infectious Disease Research (DTKF202201), the Major Project of the National Key Technology R&D Program (2017ZX103 05501-005), Changjiang Scholar Program of Chinese Ministry of Education, Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202006), and Beijing Hospitals Authority's Ascent Plan (DFL20221601).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was conducted according to the guidelines of the Declaration of Helsinki. The study was approved by the Ethics Committee of the Beijing Ditan Hospital of Capital Medical University (NO.DTEC-KY2022-022-01), and each participant signed an informed consent form.
Open Research
DATA AVAILABILITY STATEMENT
The data of results presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.




