Verifying AXL and putative proteins as SARS-CoV-2 receptors by DnaE intein-based rapid cell–cell fusion assay
Quan Fang and Xiaobai He contributed equally to this study.
Abstract
As the understanding of the mechanisms of SARS-CoV-2 infection continues to grow, researchers have come to realize that ACE2 and TMPRSS2 receptors are not the only way for the virus to invade the host, and that there are many molecules that may serve as potential receptors or cofactors. The functionality of these numerous receptors, proposed by different research groups, demands a fast, simple, and accurate validation method. To address this issue, we here established a DnaE intein-based cell-cell fusion system, a key result of our study, which enables rapid simulation of SARS-CoV-2 host cell infection. This system allowed us to validate that proteins such as AXL function as SARS-CoV-2 spike protein receptors and synergize with ACE2 for cell invasion, and that proteins like NRP1 act as cofactors, facilitating ACE2-mediated syncytium formation. Our results also suggest that mutations in the NTD of the SARS-CoV-2 Delta variant spike protein show a preferential selection for Spike-AXL interaction over Spike-LDLRAD3. In summary, our system serves as a crucial tool for the rapid and comprehensive verification of potential receptors, screening of SARS-CoV-2–neutralizing antibodies, or targeted drugs, bearing substantial implications for translational clinical applications.
1 INTRODUCTION
The novel coronavirus (SARS-CoV-2) contains a linear, single-stranded positive RNA genome and a capsid. It is the seventh identified coronavirus to infect humans. The virus can infect both humans and animals, and it has caused a global pandemic since its outbreak in late 2019.1 It is currently understood that SARS-CoV-2 invades the human body through the upper respiratory tract.2 The spike protein (S) is the most crucial surface protein for the SARS-CoV-2 ability to infect, and consists of two functional subunits, S1 and S2. The S1 subunit includes an N-terminal structural domain (NTD) and a C-terminal structural domain (CTD), with the receptor-binding domain (RBD) located in the CTD. The S2 subunit contains the elements necessary for membrane fusion, such as the fusion peptide (FP), fusion peptide proximal region (FPPR), and two heptapeptide repeats (HR1 and HR2).3 During the infection of host cells by SARS-CoV-2, the RBD of the S protein recognizes and binds to the host cell membrane receptor, the S1/S2 linkage region is cleaved by transmembrane proteases, and then the S2 subunit is released. The FP in the S2 subunit inserts into the host cell membrane, causing a conformational change in the S protein and the formation of a six-helix structure by the HR segments, thereby facilitating the fusion of the viral and host cell membranes.4, 5 As the RBD domain of the S protein has an important role in the infection of host cells by SARS-CoV-2, it is a crucial target for neutralizing antibodies.
The cell–cell fusion model has been used to simulate the fusion of SARS-CoV-2 and host cells.6 Tests for cell–cell fusion include staining, which is not suitable for large-scale screening of receptors and drugs, and the detection of cell–cell fusion through the transcriptional activation of reporter gene expression.7, 8 The latter method has been used in large-scale screenings, but it is time-consuming for the activation of the signal protein transcription. It typically takes between 24 and 48 h from the time of syncytium formation to the time that the reporter gene is detected. Therefore, it is necessary to optimize the screening time.
Inteins are protein segments that can self-excise from the primary protein sequence, leaving the flanking protein sequences to rejoin to form a functional protein.9 The DnaE intein is a type of intein that undergoes a two-step splicing reaction, which involves an initial cleavage step followed by an intramolecular transesterification step.10, 11 It has potential uses in the development of protein splicing–based reconstruction of reporter proteins (e.g., luciferase and GFP).
In our study, we used the self-splicing ability of the DnaE intein to develop a reporter system that monitors the interaction between the SARS-CoV-2 S protein and human receptors and coreceptors, which induces cell–cell fusion. Our constructed model enables the rapid verification of cell–cell fusion mediated by SARS-CoV-2 and its mutants with host receptors.
2 METHODS
2.1 Plasmid construction
The cDNAs of candidate SARS-CoV-2 receptors, including ACE2 (NM_001371415.1), TMPRSSR2 (NM _001135099.1), AXL (NM_001278599.2), LDLRAD3 (NM_001304263.2), CLEC4G (NM_001244856.2), CD147 (NM_001322243.2), NRP1 (NM_001024628.3), and TMEM30A (NM_001143958.2), were purchased from Addgene and cloned into pcDNA3.1 vector.
The original SARS-CoV-2 S gene (NC_045512.2), the Delta variant (B.1.617.2) S gene, and the Omicron variant (B.1.1.529) S gene sequences were codon-optimized and synthesized by Generay Biotech. The S gene sequences were cloned into pcDNA3.1 vector. The recombinant plasmids were extracted using an endotoxin-free plasmid extraction kit (#DP118, TIANGEN Biotech, Beijing, China), and the sequences were confirmed via Sanger sequencing (Shangya Biotech). The primers that were used are listed in the supplementary data (Supporting Information: Tables S1 and S2).
The DnaE intein–based reporter plasmids pcDNA3.1-DcRc, pcDNA3.1-RnDn, pcDNA3.1-DcEc, and pcDNA3.1-EnDn have been reported previously.9
2.2 Cell culture and recombinant plasmid transfection
HEK293 cells (ThermoFisher Scientific) were cultured in DMEM supplemented with 10% Fetal Bovine Serum (FBS, #12007 C, Sigma) and 1× penicillin/streptomycin, and then maintained in a 37°C incubator with 5% CO2. Plasmids were transfected into cells using Lipofectamine 3000 (ThermoFisher Scientific) in accordance with the manufacturer's instructions. In brief, the cells were seeded to 50% confluence in a 24-well plate before transfection. One microliter of Lipofectamine 3000 reagent was diluted in 25 μL Opti-MEM medium. A total of 500 ng of plasmids was diluted in 25 μL Opti-MEM medium and then mixed with 1 μL P3000. The diluted DNA was added to the diluted Lipofectamine 3000, mixed, and incubated for 10 min. After that, the DNA–lipid complex was added to the cells.
2.3 Cell–cell fusion assay
Cell–cell fusion assays were performed using 48-well plates. HEK293 cells were transfected and incubated for 48 h. The transfected cells were designed to express the SARS-CoV-2 spike protein coupled with half of the DnaE intein reporter protein (referred to as Cell A), or to express the putative SARS-CoV-2 receptor along with the other half of the DnaE intein reporter protein (referred to as Cell B). Following incubation, cells were digested with trypsin and resuspended in culture medium. Cell A and Cell B were mixed in a 1:1 ratio and seeded into the wells of a 48-well plate, achieving a cell density of approximately 80%. After 4–6 h of coculture at 37°C, syncytium formation was monitored using GFP fluorescence or chemiluminescence (Renilla luciferase). Further details on the methods used for observation and quantification of cell fusion can be found in the relevant sections of this manuscript.
2.4 Pseudovirus infection assay
SARS-CoV-2 pseudovirus expressing both GFP and firefly luciferase was purchased from Yeasen (#11906ES). For the infection assay, HEK293 cells were seeded to 30% confluence in a 96-well plate before infection. The frozen pseudovirus was thawed on ice and added to the wells at a titer of 1 × 106 TU/mL. For HEK293-ACE2 cells, the pseudovirus was added to infect the cells, and the culture medium was replaced with fresh medium after 6 h. After 48 h, the infection efficiency was determined by observing GFP expression with fluorescence microscopy and by using the manufacturer's instructions of the firefly luciferase reporter gene assay kit to detect luciferase activity.
2.5 Chemiluminescence detection
The Renilla Luciferase Reporter Gene Assay Kit (#RG016, Beyotime) was used to detect syncytium formation–induced chemiluminescence signal. After removal of the cell culture supernatant, the cells were collected by adding 100 µL of Renilla lysis buffer and then centrifuging at 12,000 rpm at room temperature for 5 min. Fifty microliters of the supernatant was transferred to an opaque white 96-well plate. The assay buffer and luciferase substrate were mixed at a ratio of 100:1 to form the assay working solution, and 50 µL of the solution was added to each sample well. The sample and solution were mixed for less than 10 s before chemiluminescence signal detection using a SpectraMax microplate reader (Molecular Devices).
The Firefly Luciferase Reporter Gene Assay Kit (#RG005, Beyotime) was used to measure the pseudovirus infection efficiency. After removal of the cell culture supernatant, the cells were collected by adding 100 µL of luciferase reporter gene lysis buffer and centrifuging at 12,000 rpm at room temperature for 5 min. Twenty microliters of the supernatant was transferred to an opaque white 96-well plate. One hundred microliters of the equilibrated luciferase assay reagent at room temperature was added to each sample and mixed thoroughly. The mixture was then incubated at room temperature for approximately 5 min, and the chemiluminescent signal was detected using a SpectraMax microplate reader (Molecular Devices) with a detection time of 2 s per well.
2.6 Molecular docking analysis of receptors to S protein
Information on the target proteins AXL (PDB ID: 4RA0), LDLRAD3 (PDB ID: 7FFN), and ACE2-spike (PDB ID:7A98) was obtained from the RCSB database (https://www.rcsb.org/). All of the protein structures were processed using the Molecular Operating Environment (MOE 2019.1) platform with Amber10 as the standard choice. The processing involved the removal of water and ions, protonation, the addition of missing atoms and complementary missing groups, and protein energy minimization. After that, HDOCK was used to predict binding complexes between two proteins, with a hybrid docking strategy. Every protein was set to rigid, and the docking contact site was set to full surface. The resulting conformation after docking was set at 100, and the docking score was calculated according to the knowledge-based iterative scoring function ITScorePP, with a more negative score indicating a more likely binding model. The most negative energy conformations were selected using the scoring function, and then they were optimized with the Minimization module in the MOE 2019.1 software platform to address the potential for unreasonable spatially structured contacts in rigid docking. The force field for energy minimization was set to Amber10:ETH, and the solvation model was chosen as water molecules. The optimization methods were divided into a two-step Steepest Descent and a Conjugate Gradient, both with a maximum number of 5000 iterations. Finally, the results were visualized and analyzed with Pymol 2.1 software.
2.7 Statistical analysis
GraphPad Prism 6.0 was used for data collation and statistical analysis. The experimental data were presented as mean ± standard deviation, and the independent-samples t-test was used to analyze the compared groups. A p-value of < 0.05 was considered to be statistically significant.
3 RESULTS
3.1 Developing a rapid screening model for cell fusion simulating SARS-CoV-2 infection
In our prior work, we developed a rapid, reliable, and reproducible cell fusion assay based on a DnaE intein reporter system to mimic the HIV virus infection of host CD4+ cells.9 In the current study, we aimed to develop a rapid screening model for cell fusion simulating SARS-CoV-2 infection, which is shown in Figure 1. To achieve this, we transfected one part of HEK293 cells with SARS-CoV-2 S genes and half of the DnaE intein reporter system (pcDNA3.1-DcEc plasmids or pcDNA3.1-DcRc), and the other part of HEK293 cells with putative host receptor genes and the other half of the DnaE intein reporter system (pcDNA3.1-EnDn plasmids or pcDNA3.1-RnDn). After 48 h, we cocultured the cells for an indicated time and then detected syncytium formation.
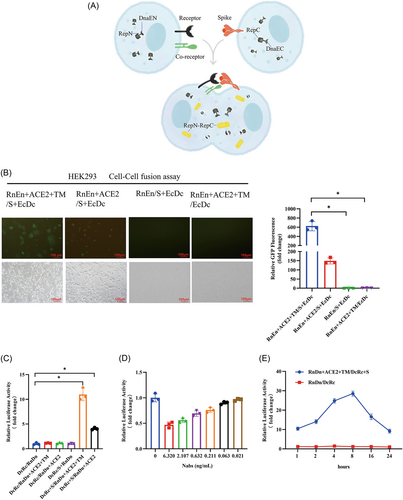
Establishment of the rapid screening model for cell fusion simulating SARS-CoV-2 infection. (A) The figure depicts the SARS-CoV-2 spike-based cell–cell fusion model, where the successful binding between the S and receptor proteins is indicated by the detection of the DnaE intein self-splicing–induced reconstitute reporter protein (RepN-RepC). (B) Syncytium formation was detected by observing the reconstitution of GFP. HEK293 cells were transfected with plasmids expressing the genes as indicated. After 48 h, the cells were cocultured for 6 h to induce cell–cell fusion. Fluorescence was observed under a fluorescent microscope at 100× magnification, and the scale bars indicate 100 µm. *indicates p < 0.05. (C) Syncytium formation was detected by observing the reconstitution of Renilla luciferase activity. HEK293 cells were transfected with plasmids expressing the indicated genes. After 48 h, the cells were cocultured for 6 h to induce cell–cell fusion. The cells were collected, and the Renilla luciferase activity was measured using a Renilla Luciferase Reporter Gene Assay Kit, as described in Section 2. *indicates p < 0.05. (D) Syncytium formation was blocked by the addition of neutralization antibodies (Nabs) (#40592-R001, SinoBiological Biotech). HEK293 cells expressing SARS-CoV-2 S protein were preincubated with different concentrations of Nabs for 30 min before coculturing with HEK293 cells expressing ACE2 and TMPRSS2. Syncytium formation was detected by observing the reconstitution of Renilla luciferase activity. (E) The kinetics of syncytium formation was detected. The reconstitution of Renilla luciferase activity was observed at the indicated time points. Compared with the control (RnDn/DcRc).
To validate our cell–cell fusion model, we used the first reported SARS-CoV-2 receptor ACE212 and the necessary protease TMPRSS213 as the fusion assay receptor. Our results demonstrated that the absence of any of these proteins in the cell–cell fusion model affected its performance, and the successful detection of reporter proteins, such as GFP or luciferase (Figure 1B,C), was only possible when all four of the proteins (S, ACE2, and two DnaE reporter proteins) were present. Notably, HEK293 cells express TMPRSS2 at low levels,14 and overexpressing TMPRSS2 led to a significant increase in the signal, thus indicating its pivotal role in syncytium formation. Further, as shown in Figure 1D, the addition of neutralization antibodies was able to block syncytium formation in a concentration-dependent manner.
Syncytium formation has been observed in lung tissue from patients with COVID-19, and it is believed to contribute to the lung damage and respiratory failure associated with severe disease.15, 16 To investigate the kinetics of syncytium formation in our cell–cell fusion assay, we performed a time course experiment. We found that the syncytium formation signal was detectable at as early as 1 h and reached a maximum at 8 h, after which time the signal decreased significantly at 16 and 24 h (Figure 1E). This suggests that the syncytium formation–induced cell damage may impair the assay signal over time. We took advantage of the DnaE intein reporter system and set the detection time at 6–8 h to optimize the assay.
3.2 Verification of AXL and other proteins as SARS-CoV-2 receptors
We conducted a search of published articles and identified over 40 proteins reported as potential SARS-CoV-2 receptors or coreceptors (see Supplementary Table S3). Many of these putative receptors are also used by other viruses to enter host cells, thus suggesting that SARS-CoV-2 may use similar mechanisms for cell entry. To validate these reported receptors, we utilized our screening model to test the ability of the identified proteins to induce syncytium formation. Among the proteins tested were AXL, a representative candidate due to its presence in multiple research papers as a SARS-CoV-2 receptor,17, 18 and CD147, a controversial receptor for SARS-CoV-2.19, 20 We also evaluated CLEC4G and LDLRAD3 as representative candidates, as they were initially identified through large-scale screening but have not yet been independently verified.21
As shown in Figure 2A, only the cells expressing AXL and LDLRAD3 cocultured with cells expressing SARS-CoV-2 showed a significant increase in luciferase activity, thereby indicating that AXL and LDLRAD3, but not CD147 and CLEC4G, were able to interact with SARS-CoV-2 S protein and induce syncytium formation. Coexpression with TMPRSS2, similar to that with ACE2, AXL, and LDLRAD3, resulted in more syncytium formation (Figure 2B). Furthermore, our co-immunoprecipitation experiments provide direct evidence of interaction between SARS-CoV-2 S protein and both AXL and LDLRAD3 (Supporting Information: Figure S1).
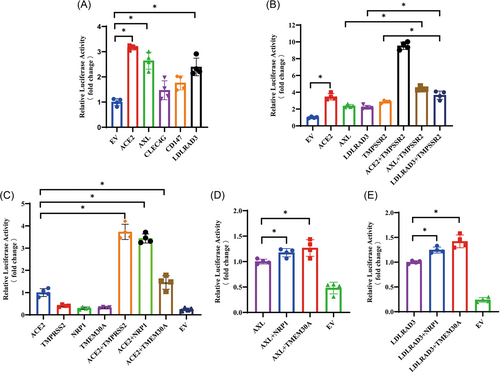
We also evaluated two putative cofactors, NRP122, 23 and TMEM30A,21, 24 which have been identified in several research papers. HEK293 cells expressing NRP1 or TMEM30A alone did not induce syncytium formation when cocultured with cells expressing the S protein. However, when coexpressed with ACE2, NRP1 exhibited a threefold increase in luciferase activity, while TMEM30A showed only a weak but significant increase in luciferase activity (Figure 2C). To further validate their role as cofactors, we cotransfected HEK293 cells with NRP1 or TMEM30A with our validated SARS-CoV-2 receptors AXL or LDLRAD3. As shown in Figure 2D,E, both NRP1 and TMEM30A resulted in a slight yet significant increase in luciferase activity induced by AXL or LDLRAD3, thus suggesting their potential role as cofactors in SARS-CoV-2 infection.
3.3 Validation of the synergistic effect of AXL and ACE2 during syncytium formation in our model
We confirmed AXL and LDLRAD3 as receptors, although these receptors have a much weaker ability than ACE2 to induce syncytium formation. Both AXL and LDLRAD3 have been reported to bind to the NTD of the spike protein S1 subunit,18, 21 while ACE2 binds to the RBD of the spike protein S1 subunit. The question then arose as to whether ACE2 and these receptors have a synergistic effect. Using molecular docking, we analyzed whether the S protein has the binding sites and space for ACE2 and AXL/LDLRAD3 binding simultaneously. As shown in Figure 3A and Table 1, we used an ACE2–S protein complex and AXL to perform the docking assay, and the binding score of AXL to the ACE–S complex was −278.42 kcal/mol, with the binding sites of the S protein primarily located in the NTD region (Table 1). However, we did not find an interaction interface on the NTD surface of S1 for LDLRAD3 without potential steric clashes (Figure 3B). Instead, we found poses in the RBD area for LDLRAD3 (Figure 3B) with the interaction residents (TYR-449, TYR-489, and GLN493) on the S1 RBD domain, thus suggesting that LDLRAD3 may bind to a site on the S protein that competes with ACE2 (Supporting Information: Figure S2, Table S4). To further validate our findings, we employed ColabFold, a fast implementation of AlphaFold-Multimer,25 to predict the structure of S protein-receptor complexes. Despite a relatively low prediction score, this computational prediction pointed towards distinct binding modes for AXL and LDLRAD3, aligning with our observations from the docking studies (Supporting Information: Figure S3).
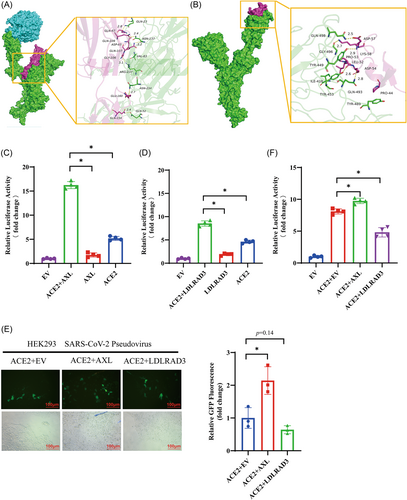
Synergistic effect of AXL and ACE2 during syncytium formation. (A, B) The interface between the spike and receptors. The binding mode of the complex AXL and LDLRAD3 with the ACE–Spike complex. The backbone of protein is rendered and colored in green (Spike), cyan (ACE2), and red (AXL (A) or LDLRAD3 (B). (C, D) HEK293 cells were transfected with the indicated receptors and cocultured with HEK293 cells expressing SARS-CoV-2 spike protein. Syncytium formation was detected by measuring Renilla luciferase activity, with the luciferase activity of the EV set at 1. (E, F) SARS-CoV-2 pseudovirus infected HEK293 cells expressing the indicated receptors. The infection efficiency was determined by observing GFP expression and fold change using fluorescence microscopy (E) and by using the instructions of the firefly luciferase reporter gene assay kit to detect luciferase activity, with the luciferase activity of the EV set at 1 (F). Magnification 200× under fluorescent microscope. The scale bars indicate 100 μm. *indicates p < 0.05.
| Protein1 | Protein2 | Binding energy (kcal/mol) | Contact sites (protein1) | Contact sites (protein2) | Combination type |
|---|---|---|---|---|---|
| ACE2–Spike | AXL | −278.42 | ASN-137, GLU-52, ASN-234, ARG-237, VAL-83, GLN-23 | GLN-107, GLN-109, GLN-154, GLU-160, VAL-26, GLY-106, ASP-65, GLN-67 | Hydrogen bond, hydrophobic interaction |
| Spike | LDLRAD3 | −206.52 | GLY-496, TYR-453, GLN-493, GLN-498, TYR-449, ILE-418, TYR-489 | LYS-58, PRO-53, ASP-54, ASP-57, LEU-52, PRO-44 | Hydrogen bond, hydrophobic interaction |
To further investigate the synergistic effect of AXL and LDLRAD3 with ACE2, we cotransfected cells with AXL or LDLRAD3 and ACE2 and performed the cell–fusion assay. As shown in Figure 3C, AXL alone induced a twofold increase in luciferase activity; ACE2 induced a fivefold increase; and AXL plus ACE2 induced over a 15-fold increase in luciferase activity, providing strong support for the idea that AXL and ACE2 act synergistically as SARS-CoV-2 receptors. For LDLRAD3, as shown in Figure 3D, we observed that LDLRAD3 alone induced a twofold increase in luciferase activity; ACE2 induced a fivefold increase; and LDLRAD3 plus ACE2 induced over an eightfold increase in luciferase activity, thus suggesting a weak synergistic effect between LDLRAD3 and ACE2.
We also attempted to use a SARS-CoV-2 pseudovirus infection assay to detect the synergistic effect of AXL and LDLRAD3 with ACE2. Unexpectedly, we did not observe infection in the cells expressing AXL or LDLRAD3, nor in HEK293 cells expressing CLEC4G or CD147 (Supporting Information: Figure S4). We then used the SARS-CoV-2 pseudovirus to infect the cells expressing AXL + ACE2 or the cells expressing LDLRAD3 + ACE2. As shown in Figure 3E,F, AXL enhanced pseudovirus infection in ACE2-expressing cells, while LDLRAD3 decreased pseudovirus infectivity in ACE2-expressing cells.
3.4 Verifying SARS-CoV-2 variants pathogenicity in our model
The pseudovirus infection assay is commonly used to evaluate the transmission capacity of SARS-CoV-2, while the cell–cell fusion assay provides insights into the virus pathogenicity.26-28 With the emergence of novel coronavirus variants, there has been a transition from high pathogenicity (Delta variants) to high transmissibility (Omicron variants). To investigate this, we constructed the recombinant plasmids containing the Delta and Omicron spike genes and applied them to our model. As shown in Figure 4A,B, the Delta group exhibited significantly enhanced GFP fluorescence and luciferase activity compared with the wild-type (WT) S group, while the opposite was observed in the Omicron group, consistent with the variants' pathogenicity.29
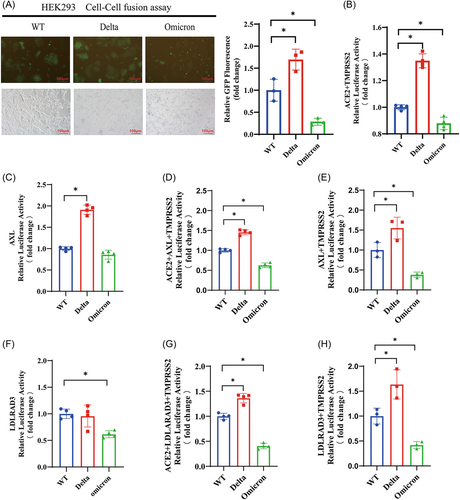
To further investigate the pathogenicity of the variants, we assessed the ability of our confirmed receptors AXL and LDLRAD3 to mediate cell fusion with cells expressing the Delta and Omicron S proteins. The results showed that the likelihood of AXL mediating cell fusion with cells expressing the Delta S protein was significantly higher, while its ability to mediate fusion with cells expressing the Omicron S protein did not show significant changes compared with the WT group (Figure 4C). When coexpressed with ACE2 and TMPRSS2, the AXL/ACE2 complex mediated a similar extent of cell fusion with the variants as ACE2 alone (Figure 4D,E vs. Figure 4B).
In contrast, the likelihood of LDLRAD3 mediating cell fusion with cells expressing the Delta S protein did not show significant changes, while its fusion ability with cells expressing the Omicron S protein was much weaker compared with the original strain (Figure 4E). When coexpressed with ACE2 and TMPRSS2, the LDLRAD3/ACE2 complex also mediated a similar extent of cell fusion with the variants as ACE2 alone (Figure 4G,H vs. Figure 4B).
These observations that the Delta variant showed enhanced cell fusion compared with the WT SARS-CoV-2, while the Omicron variant showed decreased cell fusion ability suggest possible differences in the infectivity and spread of these variants, which could potentially influence their pathogenicity. However, direct experimental evidence regarding the pathogenicity of these variants is still needed to validate these conclusions.
4 DISCUSSION
Although the COVID-19 epidemic has largely ended in China30 and is expected to end in the United States in May 2023,31 the World Health Organization still considers COVID-19 to be a major international health threat due to the emergence of new variants of SARS-CoV-2, such as BQ.1, BQ.1.1, XBB, and other Omicron subvariants that have recently emerged and are able to substantially escape neutralizing antibodies.32 Notably, SARS-CoV-2 has undergone convergent evolution, with multiple new variants incorporating R346T and other mutations in the RBD region.33, 34 These mutations impair RBD–ACE2 binding and may allow the virus to enter host cells using alternative receptors.
In this study, we compiled a list of over 40 potential receptors for SARS-CoV-2 that have been reported to date; however, the majority of these receptors have not been validated by third-party studies. To address this, we developed a rapid, reliable, and reproducible cell fusion assay based on a DnaE intein reporter system to validate these putative receptors. Using this assay, we confirmed that ACE2 is the dominant receptor for SARS-CoV-2, and NRP1, a coreceptor of SARS-CoV-2, could significantly increase infections. More importantly, we found that AXL, an alternative receptor that binds to the NTD of the S protein, could synergistically work with ACE2 to facilitate virus entry into cells. These results are consistent with prior reports.17, 18, 21
We utilized our model to investigate whether the SARS-CoV-2 variants exhibited a differential ability to mediate cell-cell fusion via various potential receptors. We found that the highly pathogenic Delta variant significantly enhanced cell-cell fusion when AXL or ACE2 were expressed, but not when LDLRAD3 was expressed. Conversely, the less pathogenic Omicron variant demonstrated diminished cell–cell fusion ability in the presence of all tested receptors. While our cell–cell fusion assay data suggests a potential influence of these variants on receptor utilization and infectivity, we acknowledge that this finding is yet to be substantiated by direct experimental evidence. This interesting observation warrants further investigation.
We also attempted to dissect the impact of specific point mutations on the fusion-promoting abilities of these variants, but the results did not align with our initial expectations. Our findings suggested that mutations in the RBD region affect the fusion-promoting abilities even when the receptors that recognize the NTD are involved, indicating that the interaction between these domains is complex and interdependent (Supporting Information: Figure S5). Our observations hint at a potential synergistic effect of multiple variant sites within both RBD and NTD domains on the fusion abilities, rather than being driven by individual point mutations.
In this study, we have presented an innovative assay model for investigating SARS-CoV-2 mediated cell–cell fusion. Our approach circumvents the requirement for viral particle preparation by using multiple reporter proteins and offers a significantly reduced experimental timeline compared to traditional methods, which often require 24–48 h to detect syncytium formation. In contrast, our method can achieve this in a much reduced timeframe of 1–6 h, offering a more efficient alternative for large-scale receptor and drug screening. The model is not only suitable for a conventional laboratory setup, but also adaptable for high-throughput platforms, facilitating large-scale receptor and drug screenings. This enables large-scale receptor and drug screenings, potentially facilitating the identification of antiviral drugs and neutralizing antibodies against SARS-CoV-2. While our model has certain limitations, such as potential low fluorescence background and inability to discern late-stage fusion factors, the benefits in terms of simplicity, time efficiency, and adaptability for large-scale screening constitute a valuable contribution to the current repertoire of techniques for studying SARS-CoV-2.
In summary, our rapid, reliable, and reproducible cell fusion assay is an effective approach for verifying the binding receptors of SARS-CoV-2. It is a crucial instrument for tracking the emergence of highly pathogenic variants and for high-throughput screening.
AUTHOR CONTRIBUTIONS
Quan Fang, as the first author, took the responsibility for article writing, implementing the research process, collecting and collating data, and submitting the manuscript. Xiaobai He, as the co-first author, contributed equally in implementing the research process, collecting and collating data. Xiaoguang Zheng, Yu Fu, Ting Fu, Jingyi Luo, and Jiajing Lan collected information and implemented the research the process. Yaoqiang Du, Jun Yang, Yongneng Luo, and Xiaopan Chen implemented the research process. Naiming Zhou and Zhen Wang provided guidance for thesis. Jianxin Lyu, as the cocorresponding author, provided guidance for thesis and financial support. Linjie Chen, as the corresponding author, took the responsibility for proposing research topics, designing research plans, guidance for thesis, financial support and technical support and reviewed the final draft. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This research was funded by the projects from Natural Science Foundation of Zhejiang (LQ21H200006, LXZ22H300001), the Science Technology Department of Zhejiang Province (2022C03186, 2022C03024), Zhejiang Provincial Medical and Health Science and Technology Program (2020KY107, 2021KY132), and the Key Discipline of Zhejiang Province in Public Health and Preventive Medicine (First Class, Category A, Hangzhou Medical College), the Major Science and Technology Medicine and Healthcare in Zhejiang (WKJ-ZJ-2017). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




