Trends in the incidence of cirrhosis in global from 1990 to 2019: A joinpoint and age-period-cohort analysis
Abstract
Cirrhosis remains a major public health concern globally; the burden of cirrhosis should be further clarified worldwide and helped us to understand the current situation of cirrhosis. In the present study, we estimate DALYs and mortality rates attributable to several major cirrhosis risk factors and use joinpoint and age-period-cohort methods to determine the trends of cirrhosis incidence and deaths in the global population in the 1990–2019 period. Globally, from 1990 to 2019, the incidence of cirrhosis, deaths due to cirrhosis, and cirrhosis DALY cases increased from 1274 (103, 95% uncertainty interval [UI]: 1027.2–1548.5) to 2051.6 (103, 95% UI: 1661.4–2478.1), 1013 (103, 95% UI: 948.9–1073.9) to 1472 (103, 95% UI: 1374.6–1578.7), and 34727.7 (103, 95% UI: 32383.0–37132.8) to 46189.4 (103, 95% UI: 43027.1–49551.3), respectively. Hepatitis virus was the most important cirrhosis mortality risk factor. Globally, hepatitis virus infection (HBV+HCV) accounted for more than 45% of the incidence of cirrhosis cases and about 50% of cirrhosis deaths. Importantly, from 1990 to 2019, the proportion of cirrhosis incidence due to HBV decreased from 24.3% to 19.8%, whereas that due to alcohol use increased from 18.7% to 21.3%. Additionally, the proportion of NAFLD-induced cirrhosis incidence increased from 5.5% to 6.6% over the same period. Our findings on the global disease burden of cirrhosis provide a valuable resource for developing targeted prevention strategies.
Abbreviations
-
- AAPC
-
- average APC
-
- APC
-
- annual percentage change
-
- ASR
-
- age standardized rate
-
- CI
-
- confidence interval
-
- DALYs
-
- disability-adjusted life years
-
- EAPC
-
- estimated annual percentage change
-
- GBD
-
- Global Burden of Diseases, Injuries, and Risk Factors Study
-
- GHDx
-
- Global Health Data Exchange
-
- HBV
-
- hepatitis B virus
-
- HCV
-
- hepatitis C virus
-
- NAFLD
-
- nonalcoholic fatty lives disease (NAFLD)
-
- NASH
-
- nonalcoholic steatohepatitis
-
- RR
-
- rate ratio
-
- SDI
-
- socio-demographic index
-
- UI
-
- uncertainty interval
-
- YLDs
-
- years lived with disability
-
- YLLs
-
- years of life lost
1 INTRODUCTION
Cirrhosis is caused by various liver injury mechanisms, which induce necrotizing inflammation and fibrosis. It is characterized by hepatocyte degeneration and necrosis and loss of fibrotic tissue and regenerative nodules in liver parenchyma, ultimately leading to liver function failure.1 The initial asymptomatic phase of cirrhosis can be compensated, but once it progresses to the symptomatic phase, the patient develops complications characteristic of decompensated cirrhosis, such as ascites, esophageal variceal bleeding, and hepatic encephalopathy, which may increase patient death.2 Once decompensation occurs, the mortality risk increases to nearly 10 times that in the general population.3 The etiology of cirrhosis differs geographically. Chronic hepatitis C virus (HCV) infection, alcoholism, and nonalcoholic fatty lives disease (NAFLD) are the most important etiological factors in Western countries, whereas in sub-Saharan Africa and most parts of Asia cirrhosis is mostly due to chronic hepatitis B virus (HBV) infection.4 NAFLD is the most common cause of cirrhosis in Western countries, with a prevalence of 30% in the United States, 2%–44% in European countries (including obese children), and 42.6%–69.5% in Type 2 diabetes patients.5, 6 Evidence now links NAFLD to cardiovascular disease, diabetes, and cancers, representing a high-burden disease for patients and healthcare systems.7
Deaths due to cirrhosis accounted for 2.4% of total deaths globally in 2017, with Egypt reporting the highest age-standardized deaths due to cirrhosis and Singapore the lowest.8 Cirrhosis-related mortality decreased from 20.0 deaths per 100 000 person-years in 1980 to 15.8 deaths per 100 000 person-years in 2010.9 Although viral hepatitis is the main cause of cirrhosis-related mortality in Asia, mortality is declining due to universal HBV vaccination programs and viral hepatitis treatment.10 A French study found that 0.3% of screened males (aged >40 years) had liver cirrhosis.10 As the only viable option for decompensated cirrhosis, 5500 liver transplants are needed each year in Europe to improve survival of patients.6 It is estimated that alcoholic liver disease accounts for 48% of all liver cirrhosis deaths, and about 15%–20% of patients consuming excessive alcohol develop liver cirrhosis in their lifetime.11, 12 The incidence of alcohol-induced cirrhosis disability-adjusted life years (DALYs) per 100 000 people is highest in Central Asia, followed by Eastern Europe with 456.1 DALYs per 100 000 people.13 Four countries (China, India, Nigeria, and Indonesia) account for 50% of all HBV infections globally.14 An estimated 2.1%–6.0% of patients with chronic HBV progress to liver cirrhosis annually.15 In 2015, the incidence rate of cirrhosis due to HCV infection was 1.1% worldwide.16 Although the global HCV prevalence rate has decreased significantly, this decline has been offset by the increasing prevalence of injection drug use, especially in Western countries.17 Nonalcoholic steatohepatitis, an inflammatory subtype of NAFLD, affects an estimated 3%–6% of the US population, and about 20% of the patients progress to cirrhosis, which exacerbates liver-specific and overall mortality rates.18 Previous studies investigating the global burden of cirrhosis have reported a decrease or constant death rate from 1990 to 2017 in all Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) regions except Eastern Europe and Central Asia.8 In the present study, using data from GBD study 2019, we provide estimates of the incidence and mortality of cirrhosis from 1990 to 2019, as well as age-standardized morbidity, mortality, and DALYs rate using data. Moreover, we estimate DALYs and mortality rates attributable to several major cirrhosis risk factors and use joinpoint and age-period-cohort methods to determine the trends of cirrhosis incidence and deaths in the global population in the 1990–2019 period.
2 MATERIALS AND METHODS
2.1 GBD source
Age-standardized and annual incidence of cirrhosis from 1990 to 2019 by region, sex, country, and risks were downloaded from the Global Health Data Exchange query tool.19 Based on the socio-demographic index (SDI), 204 countries and territories were categorized into five regions: high, high-middle, middle, low-middle, and low. In addition, the world was geographically divided into 21 regions. The general ways for the GBD 2019 and patterns for estimations of cirrhosis burden were sought from GBD 2017 Cirrhosis Collaborators.8 DALYs were calculated by summing years lived with disability and years of life lost. Rates were standardized to the GBD world population and reported as age-standardized DALY rates, age-standardized incidence rates, and age-standardized death rates per 100 000 people. As a good composite measure of social development, including education, income, and fertility, SDI is closely correlated with health outcomes.8 Age-standardized rate (ASR) and estimated annual percentage change (EAPC) were used to quantify the trends of cirrhosis incidence, deaths, and DALYs rate.20 ASR trend was used to better reflect the change in disease patterns in the population and establish targeted preventive strategies for cirrhosis. EAPC summary was used to quantify the trends of ASR among different populations in a period.21 Drug use defined as use of cannabis, opioids, or amphetamines, or use of injecting drugs and alcohol use defined as any alcohol consumption. Definition cirrhosis and other chronic liver diseases are conditions in which liver cells are destroyed and replaced by fibrosis. Decompensated cirrhosis occurs when the liver can no longer compensate for the damage, and is marked by profound symptoms, health loss, and typically death in a few years. GBD 2019 analysis was conducted in MR-BRT of five case-series studies that reported on both NASH and cryptogenic cases as causes of cirrhosis to adjust studies only reporting on cryptogenic cases but not NASH.22
2.2 Joinpoint regression analysis
The determination of changes in recent disease trends is useful in the analysis of cancer mortality and incidence data. To describe such continuous changes, joinpoint regression analysis was performed on the age-adjusted rates to estimate piecewise log-linear time calendar trends by country using NCI Joinpoint Regression software (version 4.9.0.0). Using Joinpoint regression model analysis, a long-term trend line was cut into significant trend sections characterized by continuous linearity through the model fitting.23 The temporal trends were fitted with one to five joinpoints to choose the best model for cirrhosis incidence and death rates during 1990–2019 in the GBD study. To characterize the trend of the incidence rate of cancer over time, the best fitting logarithmic linear regression model was used to estimate the annual percentage change (APC), average APC (AAPC), and the corresponding 95% confidence interval (CI) for each trend and to determine the significant connection point.
2.3 Age-period-cohort analyses
Age period cohort analysis is used to analyze disease trends by age, population-level demographic changes (period), and initial life or generational exposures (cohort) effects.24 Investigating age, period, and cohort effects at the same time is significant as the characteristics of patients change with age. In the present study, we used the NCI's age period cohort analysis online tool (http://analysistools.nci.nih.gov/apc/) to explore the relationship of the observed incidence rates with age, period, and cohort effects. Briefly, the number of incidents or deaths by age set was entered in a calendar phase as the number and the relevant person-year at-risk cases as the population. Mortality was assessed based on the age groups and corresponding calendar periods. The mortality of GBD data was estimated using 6 corresponding calendar periods and 13 5-year age groups. The rate ratio (RR) of mortality rates in each calendar period and a reference period was also calculated, adjusting for age and nonlinear cohort effects. Wald Chi-Square tests for estimable functions in the age-period-cohort model.
2.4 Statistical analysis
ASR and EAPC were calculated according to the procedure by Liu et al.25 Both EAPC value and the upper 95% CI boundary were <0, suggesting a downward trend in ASR. In contrast, the EAPC value and the lower boundary of the 95% CI were >0, suggesting a rising trend of ASR. A 95% CI of 0 indicates that ASR has a constant trend. Incidence rates were calculated per 100 000 person-years, and age-adjusted to the 2000 US standard population. All statistical analyses were conducted using R software (R 4.1.2 software). In the Joinpoint software, the p value for a two-sided test that the true APC is zero is calculated based on a t distribution and the AAPC confidence interval is based on the normal distribution. Grid search method (GSM) builds and Monte Carlo permutation test optimizes the model in Joinpoint software. Values with p < 0.05 were considered statistically significant.
3 RESULTS
3.1 Global cirrhosis burden
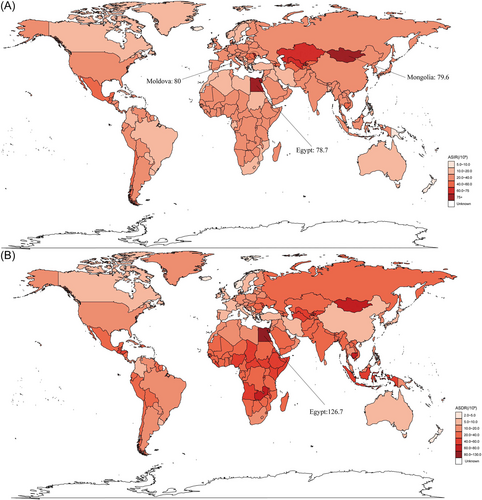
Globally, from 1990 to 2019, the incidence of cirrhosis, deaths due to cirrhosis, and cirrhosis DALYs cases increased from 1274 (103, 95% UI: 1027.2–1548.5) to 2051.6 (103, 95% UI: 1661.4–2478.1), 1013 (103, 95% UI: 948.9–1073.9) to 1472 (103, 95% UI: 1374.6–1578.7), and 34727.7 (103, 95% UI: 32383.0–37132.8) to 46189.4 (103, 95% UI: 43027.1–49551.3), respectively. Meanwhile, the percent prevalence of cirrhosis increased from 20.8% (95% UI: 22.4%–19.2%) to 21.6% (95% UI: 23.6%–20.0%). Globally, there was an increase in the incidence of cirrhosis, deaths due to cirrhosis, and cirrhosis DALY cases from 2017 to 2019, with 53,249, 45,224, and 1,003,128 cases, respectively. In the same period, age-standardized incidence rate (ASIR), age-standardized death rate (ASDR), and DALYs decreased by an average of −0.05% (95% CI −0.07% to −0.02%) per year (from 25.7 per 100 000 in 1990 to 25.3 per 100 000 in 2019), −1.12% (95% CI −1.21% to −1.03%) per year (from 24.4 per 100 000 in 1990 to 18.0 per 100 000 in 2019), and −1.15% (95% CI −1.25% to −1.05%) per year (from 766.1 per 100 000 in 1990 to 560.4 per 100 000 in 2019), respectively. These results indicated that whereas the decrease in cirrhosis incidence cases was marginal, deaths and DALYs cases significantly decreased during 1990–2019 (Tables 1–3). Both ASIR and ASDR of cirrhosis varied widely globally. Observed ASIR was highest in Moldova (80 per 100 000 in 2019), followed by Mongolia (79.6 per 100 000 in 2019) and Egypt (78.7 per 100 000 in 2019). In addition, Egypt had the highest observed ASDR (126.7 per 100 000 in 2019), followed by Mongolia (72.9 per 100 000 in 2019) and Cambodia (67.4 per 100 000 in 2019), as shown in Figure 1. ASIR increased the most in the United Arab Emirates (820%) and decreased the most in South Korea (−43.9%). In contrast, ASDR increased the most in the United Arab Emirates (2725.9%) and decreased the most in Niue (−55.1%) (Supporting Information: Figure S1). The map of EAPC change is shown in Supporting Information: Figure S2.
| Location | 1990 | 2019 | 1990–2019 | ||
|---|---|---|---|---|---|
| Incident cases | ASIR per 100 000 | Incident cases | ASIR per 100 000 | EAPC | |
| n × 103 (95% UI) | n (95% UI) | n × 103 (95% UI) | n (95% UI) | n (95% CI) | |
| Global | 1274.0 (1027.2–1548.5) | 25.7 (20.2–31.6) | 2051.6 (1661.4–2478.1) | 25.3 (20.8–30.4) | −0.05 (−0.07 to −0.02) |
| High-income Asia Pacific | 77.5 (63.5–92.1) | 39.1 (32.4–45.9) | 50.8 (41.6–60.1) | 25.1 (21.3–29.2) | –1.63 (−1.7 to –1.57) |
| High-income North America | 72.0 (56.1–89.2) | 23.5 (18.3–29.2) | 98.6 (81.1–116.8) | 25.6 (21.5–30.1) | 0.16 (0.07–0.25) |
| High-middle SDI | 311.2 (249.4–377.5) | 27 (21.6–32.9) | 421.1 (340.3–507.3) | 24.9 (20.5–29.9) | −0.25 (−0.28 to −0.21) |
| High SDI | 250.6 (213.2–289.3) | 28 (23.9–32.3) | 268.4 (231.1–305.7) | 23.9 (20.8–27.1) | −0.67 (−0.72 to −0.62) |
| Low-middle SDI | 216.4 (170.8–269.3) | 21.2 (15.8–27.1) | 439.5 (343.0–544.1) | 25.3 (19.7–31.4) | 0.7 (0.65–0.75) |
| Low SDI | 95.8 (75.2–118.2) | 21.2 (15.5–27.4) | 233.1 (186.1–284.3) | 24.1 (18.5–30.2) | 0.47 (0.41–0.53) |
| Middle SDI | 399.4 (303.2–502.1) | 26.3 (19.6–33.5) | 688.6 (544.1–840.9) | 25.8 (20.6–31.3) | −0.04 (−0.08 to −0.01) |
| Andean Latin America | 7.2 (6.3–8.2) | 23.2 (19.9–26.8) | 20.0 (17.6–22.7) | 32.2 (28.4–36.7) | 1.06 (0.87–1.24) |
| Australasia | 2.4 (2.1–2.7) | 11.1 (9.5–12.5) | 3.3 (2.8–3.8) | 10.3 (8.8–11.7) | −0.31 (−0.37 to −0.24) |
| Caribbean | 6.3 (5.5–7.1) | 20.2 (17.4–23) | 10.8 (9.2–12.6) | 21.9 (18.6–25.1) | 0.05 (−0.03 to 0.13) |
| Central Asia | 19.5 (17.3–21.9) | 32.8 (28.8–36.9) | 58.2 (51.5–64.8) | 59.1 (52.3–66) | 2.24 (2.09–2.39) |
| Central Europe | 42.9 (38.6–47.1) | 32.9 (29.5–36.1) | 37.6 (33.2–41.7) | 29.1 (25.8–32.4) | −0.44 (−0.6 to −0.28) |
| Central Latin America | 55.7 (43.8–68.6) | 42.7 (33.1–53.2) | 106.2 (85.0–127.9) | 40.8 (32.7–49) | −0.06 (−0.1 to −0.01) |
| Central Sub-Saharan Africa | 10.9 (8.9–13.0) | 22.7 (17.2–28.6) | 30.8 (25.7–36.8) | 27 (21.5–33.1) | 0.52 (0.44–0.61) |
| East Asia | 324.5 (238.8–415.4) | 27.3 (19.6–35.3) | 424.4 (321.4–529.8) | 22.5 (17.7–27.6) | −0.57 (−0.65 to −0.48) |
| Eastern Europe | 46.0 (35.1–58.5) | 19.9 (15.4–24.8) | 66.9 (47.7–90.0) | 31.3 (23.4–41.1) | 1.9 (1.62–2.17) |
| Eastern Sub-Saharan Africa | 35.8 (27.9–44.4) | 25.4 (18–33.9) | 86.0 (67.8–105.5) | 27.1 (19.8–35.4) | 0.18 (0.13–0.24) |
| North Africa and Middle East | 60.8 (51.0–72.6) | 23.8 (19–29.2) | 160.1 (133.5–190.7) | 28.7 (23.6–34.9) | 0.55 (0.53–0.58) |
| Oceania | 0.6 (0.5–0.7) | 9.8 (7.9–11.7) | 1.1 (0.9–1.3) | 8.5 (7–10) | −0.68 (−0.74 to −0.62) |
| South Asia | 185.7 (140.3-240.7) | 17.3 (12.1-23.1) | 414.0 (299.6-539.3) | 23 (16.7-29.9) | 1.19 (1.11-1.28) |
| Southeast Asia | 105.7 (80.4–133.4) | 26.3 (19.1–34.2) | 181.5 (142.6–219.7) | 24.8 (19.5–30) | −0.43 (−0.52 to −0.35) |
| Southern Latin America | 12.2 (10.7–13.7) | 25.9 (22.7–29.2) | 22.2 (19.6–24.8) | 30.5 (27.1–34.1) | 0.38 (0.3–0.47) |
| Southern Sub-Saharan Africa | 9.1 (7.0–11.3) | 19.3 (13.9–25) | 12.2 (9.2–15.4) | 15.6 (11.7–19.9) | −0.96 (−1.1 to –0.82) |
| Tropical Latin America | 35.9 (26.2–46.5) | 26.1 (18.5–34) | 50.2 (36.2–65.5) | 19.8 (14.4–25.6) | −1.26 (−1.38 to −1.14) |
| Western Europe | 124.6 (110.5–138.4) | 30.3 (26.9–33.5) | 116.2 (102.9–129.2) | 24.5 (21.9–26.9) | −0.86 (−0.97 to −0.75) |
| Western Sub-Saharan Africa | 38.8 (30.8–47.7) | 23.9 (17–31.6) | 100.5 (80.8–121.9) | 26.2 (19.9–33.2) | 0.24 (0.17–0.31) |
- Abbreviations: ASIR, age standardized incidence rate; CI, confidence interval; EAPC, estimated annual percentage change; UI, uncertainty interval.
| Location | 1990 | 2019 | 1990–2019 | ||
|---|---|---|---|---|---|
| Deaths cases | ASDR per 100 000 | Deaths cases | ASDR per 100 000 | EAPC | |
| n × 103 (95% UI) | n (95% UI) | n × 103 (95% UI) | n (95% UI) | n (95% CI) | |
| Global | 1013.0 (948.9–1073.9) | 24.4 (22.9–25.7) | 1472.0 (1374.6–1578.7) | 18 (16.8–19.3) | −1.12 (−1.21 to −1.03) |
| High-income Asia Pacific | 41.4 (39.7–42.4) | 20.8 (19.9–21.4) | 36.9 (32.2–41.1) | 8.7 (7.9–9.4) | −3.24 (−3.38 to −3.1) |
| High-income North America | 40.5 (39.0–41.4) | 12.2 (11.8–12.5) | 72.7 (69.3–75.5) | 12.7 (12.2–13.1) | 0.31 (0.24–0.37) |
| High-middle SDI | 198.1 (188.5–207.3) | 18.4 (17.5–19.2) | 251.9 (236.1–269.3) | 12.8 (12–13.7) | −1.35 (−1.59 to −1.11) |
| High SDI | 150.7 (145.3–154.0) | 15.2 (14.6–15.5) | 185.5 (173.7–196.1) | 10.8 (10.2–11.3) | −1.3 (−1.34 −1.25) |
| Low-middle SDI | 229.6 (208.5–256.5) | 34 (30.6–37.8) | 376.2 (342.1–416.6) | 26.2 (23.9–29) | −0.92 (−1.01 to −0.83) |
| Low SDI | 119.0 (103.3–138.3) | 45.5 (39.3–52.2) | 187.9 (163.8–215.2) | 32.8 (28.9–37.1) | −1.19 (−1.32 to −1.06) |
| Middle SDI | 315.0 (292.7–335.8) | 29.6 (27.2–31.9) | 469.6 (427.8–516.8) | 19.2 (17.5–21.2) | −1.54 (−1.6 to −1.49) |
| Andean Latin America | 7.0 (6.1–8.2) | 31.4 (27.2–36.5) | 14.1 (11.2–17.3) | 25.1 (20.1–30.9) | −0.8 (−0.87 to −0.74) |
| Australasia | 1.6 (1.5–1.7) | 7 (6.7–7.3) | 2.5 (2.3–2.7) | 5.5 (5–5.9) | −0.72 (−0.88 to −0.56) |
| Caribbean | 6.2 (5.5–6.8) | 23.1 (20.6–25.1) | 9.5 (7.8–11.4) | 18.5 (15–22.2) | −1.01 (−1.26 to −0.75) |
| Central Asia | 13.6 (13.2–14.2) | 27.7 (26.7–28.8) | 33.9 (30.5–37.7) | 42.9 (38.5–47.5) | 1.37 (0.94–1.79) |
| Central Europe | 32.7 (32.0–33.4) | 22.4 (21.9–22.9) | 33.6 (29.3–37.9) | 17.7 (15.4–20) | −1.27 (−1.49 to −1.05) |
| Central Latin America | 33.7 (32.9–34.4) | 36.2 (35.1–37) | 68.1 (58.6–78.3) | 28.3 (24.5–32.6) | −1.15 (−1.26 to −1.03) |
| Central Sub-Saharan Africa | 13.2 (10.9–15.9) | 51.9 (43.2–61.8) | 22.8 (17.1–29.1) | 37 (28–47.4) | −1.2 (−1.25 to −1.14) |
| East Asia | 177.0 (153.9–201.3) | 19.3 (17–21.8) | 164.7 (140.1–191.7) | 8.2 (7–9.5) | −3.08 (−3.25 to −2.9) |
| Eastern Europe | 29.2 (28.2–30.6) | 10.6 (10.2–11.1) | 72.7 (65.0–81.0) | 24.3 (21.7–27) | 2.97 (2.16–3.8) |
| Eastern Sub-Saharan Africa | 47.9 (38.2–57.4) | 59.2 (48.7–70.2) | 77.0 (66.3–91.4) | 44.1 (38.5–51.9) | −1.11 (−1.21 to −1.02) |
| North Africa and Middle East | 68.4 (62.6–73.4) | 43.9 (38.8–48.7) | 109.7 (81.4–135.2) | 27.7 (21.1–33.9) | −1.56 (−1.63 to −1.49) |
| Oceania | 0.6 (0.5–0.7) | 16.6 (13.8–20.1) | 1.2 (0.9–1.4) | 13.2 (10.6–16.4) | −0.79 (−0.85 to −0.74) |
| South Asia | 202.4 (181.6–236.9) | 30.9 (27.3–35.6) | 348.4 (306.9–404.8) | 23.5 (20.7–27.1) | −0.98 (−1.15 to −0.81) |
| Southeast Asia | 119.3 (107.5–130.4) | 41.6 (36.9–46.4) | 186.2 (165.4–207.7) | 30.2 (26.9–33.5) | −1.18 (−1.21 to −1.15) |
| Southern Latin America | 10.4 (10.0–10.7) | 22.5 (21.7–23.2) | 14.2 (13.2–15.1) | 17.3 (16.2–18.5) | −0.77 (−0.88 to −0.65) |
| Southern Sub-Saharan Africa | 7.0 (5.8–8.6) | 22.6 (18.5–28.3) | 9.2 (8.2–10.3) | 15.4 (13.8–17.2) | −1.54 (−1.89 to −1.18) |
| Tropical Latin America | 24.7 (23.8–25.5) | 24.2 (23.1–25) | 38.8 (36.5–41.3) | 15.7 (14.8–16.8) | −1.46 (−1.5 to −1.42) |
| Western Europe | 85.8 (82.5–87.7) | 16 (15.4–16.4) | 77.2 (72.0–82.5) | 9.4 (8.9–10) | −2.1 (−2.19 to −2.01) |
| Western Sub-Saharan Africa | 50.2 (39.5–62.9) | 53.2 (41.7–67) | 78.7 (61.7–99.8) | 37.5 (30.3–46.5) | −1.13 (−1.26 to −1.01) |
- Abbreviation: ASDR, age-standardized death rate; CI, confidence interval; EAPC, estimated annual percentage change; UI, uncertainty interval.
| Location | 1990 | 2019 | 1990-2019 | ||
|---|---|---|---|---|---|
| Cases | ASR per 100 000 | Cases | ASR per 100 000 | EAPC | |
| n × 103 (95% UI) | n (95% UI) | n × 103 (95% UI) | n (95% UI) | n (95% CI) | |
| Global | 34727.7 (32383.0–37132.8) | 766.1 (718.3–813.1) | 46189.4 (43027.1–49551.3) | 560.4 (521.9–602) | −1.15 (−1.25 to −1.05) |
| High-income Asia Pacific | 1227.1 (1159.5–1258.9) | 599.6 (566.1–615.2) | 776.9 (717.3–824.3) | 230.2 (216–242.9) | −3.59 (−3.72 to −3.46) |
| High-income North America | 1179.4 (1145.7–1199.5) | 372 (361.8–378.3) | 1955.3 (1892.7–2014.3) | 371.4 (360.2–381.8) | 0.21 (0.13–0.28) |
| High-middle SDI | 6270.4 (5945.8–6601.5) | 557.8 (529.4–586.5) | 7639.4 (7168.7–8166.8) | 401.8 (377.6–429.5) | −1.22 (−1.5 to −0.93) |
| High SDI | 4396.3 (4269.3–4477.1) | 459.1 (445.6–467.5) | 4702.4 (4508.8–4882.1) | 307.8 (296.1–318.9) | −1.5 (−1.55 to −1.46) |
| Low-middle SDI | 8800.7 (7951.7–9997.3) | 1076.9 (982.6–1204) | 12974.5 (11742.2–14369.5) | 817.9 (739.8–907.8) | −0.95 (−1.04 to −0.86) |
| Low SDI | 4369.9 (3667.2–5227.8) | 1334.9 (1159.2–1550.7) | 6773.0 (5867.0–7846.2) | 949.5 (826.9–1088.9) | −1.21 (−1.35 to −1.08) |
| Middle SDI | 10871.9 (10119.9–11572.1) | 850.5 (792.8–903.8) | 14074.0 (12898.5–15406.2) | 538.9 (494.3–589.7) | −1.66 (−1.71 to −1.61) |
| Andean Latin America | 245.9 (210.6–294.2) | 928 (802.8–1088.4) | 373.6 (294.1–465.1) | 639.1 (506.1–795.5) | −1.4 (−1.47 to −1.32) |
| Australasia | 45.5 (43.6–47.2) | 203.9 (195.3–211.7) | 63.3 (58.7–67.8) | 153.4 (142.8–164.1) | −0.77 (−0.93 to −0.6) |
| Caribbean | 203.0 (172.7–231.7) | 701.5 (606.1–785.3) | 278.3 (219.6–341.1) | 545.7 (429.1–672.6) | −1.09 (−1.37 to −0.81) |
| Central Asia | 461.7 (446.7–482.6) | 848.3 (821.9–885.7) | 1173.1 (1054.3–1309.1) | 1318.2 (1187–1467.1) | 1.31 (0.83–1.78) |
| Central Europe | 1016.9 (996.5–1039.6) | 707.5 (693.5–723.2) | 964.5 (839.8–1091.7) | 554.8 (483.1–627.7) | −1.36 (−1.61 to −1.11) |
| Central Latin America | 1196.2 (1169.8–1223.3) | 1109.2 (1085.5–1131.5) | 2031.9 (1740.9–2343.6) | 816.6 (700.8–940.4) | −1.4 (−1.55 to −1.25) |
| Central Sub-Saharan Africa | 483.9 (394.4–583.5) | 1515.6 (1243.1–1819.2) | 843.4 (629.4–1107.0) | 1089.7 (821–1392.7) | −1.15 (−1.2 to −1.09) |
| East Asia | 5996.9 (5177.7–6848.2) | 579.2 (501.3–659.5) | 4698.6 (3982.1–5513.2) | 227.9 (193.8–266.2) | −3.38 (−3.53 to −3.23) |
| Eastern Europe | 914.5 (883.7–957.8) | 338.8 (327.1–355.4) | 2575.3 (2298.8–2874.7) | 919.5 (820.7–1026.8) | 3.54 (2.59–4.5) |
| Eastern Sub-Saharan Africa | 1649.4 (1277.0–2008.6) | 1642.7 (1308.1–1973.6) | 2608.1 (2191.3–3151.2) | 1178.4 (1015.5–1402.5) | −1.25 (−1.35 to −1.16) |
| North Africa and Middle East | 1969.1 (1742.3–2168.2) | 978.9 (897.8–1040.9) | 2878.4 (2126.4–3565.0) | 616.8 (457.7–761.6) | −1.56 (−1.62 to −1.51) |
| Oceania | 24.8 (20.5–29.8) | 557.3 (460.4–673.7) | 46.0 (36.0–57.7) | 440.8 (347.9–550.5) | −0.81 (−0.86 to −0.77) |
| South Asia | 8112.0 (7291.6–9582.7) | 993.9 (892.9–1159.3) | 12436.3 (10954.3–14414.1) | 750.6 (662.2–867.9) | −0.97 (−1.13 to −0.8) |
| Southeast Asia | 4431.8 (3925.4–4858.7) | 1296.8 (1173.8–1409) | 5915.3 (5207.2–6642.9) | 867 (765.1–970.7) | −1.5 (−1.55--1.46) |
| Southern Latin America | 314.9 (305.4–324.2) | 670.7 (650.4–690.3) | 372.4 (351.8–394.7) | 471.6 (446.4–499.4) | -1.05 (-1.21 to −0.89) |
| Southern Sub-Saharan Africa | 258.1 (220.6–306.8) | 715.5 (602.1–863.6) | 310.8 (272.9–353.5) | 458.7 (405.3–516.6) | −1.73 (−2.13 to −1.34) |
| Tropical Latin America | 918.2 (886.3–945.0) | 782.3 (753.5–804.9) | 1193.3 (1134.4–1261.3) | 473.9 (449.6–501.1) | −1.75 (−1.79 to −1.71) |
| Western Europe | 2328.7 (2264.0–2371.5) | 467.9 (455.6–476.1) | 1833.0 (1752.1–1930.4) | 260.9 (250.6–273.6) | −2.32 (−2.41 to −2.22) |
| Western Sub-Saharan Africa | 1749.7 (1391.8–2152.7) | 1494.1 (1171–1880.2) | 2862.3 (2196.4–3688.9) | 1052.2 (823.2–1337.6) | −1.14 (−1.25 to −1.03) |
- Abbreviations: ASR, age-standardized rate; CI, confidence interval; DALYs, disability-adjusted life-years; EAPC, estimated annual percentage change; UI, uncertainty interval.
Hierarchical cluster analysis was performed using the R package “factoextra” to group countries and territories into four categories based on their EAPC data on morbidity and mortality. Fifteen countries (or territories) were classified as the “significant increase” group, including the UK, India, and Ireland. Additionally, six countries (or territories) were classified as the “significant decrease” group, which included Ukraine, Russian Federation, and Armenia. Ten countries (or territories) were classified as the “minor decrease” group, including Singapore, Italy, and Republic of Korea. Lastly, 173 countries (or territories) were classified as the “minor increase” group, including Egypt, the US, Japan, and China. These results are shown in Supporting Information: Figure S3. The observed versus expected regional SDI levels for individual locations were also explored. According to ASIR results, Southern Latin America, North Africa, and the Middle East approximately followed expected trends over the study period. In contrast, in Asian regions, the observed patterns varied widely, as shown in Supporting Information: Figure S4. Additionally, ASDR results indicated that the Andean Latin American and Western Europe approximately followed expected trends over the study period. Similar trends were also observed at the national level, as shown in Supporting Information: Figure S5.
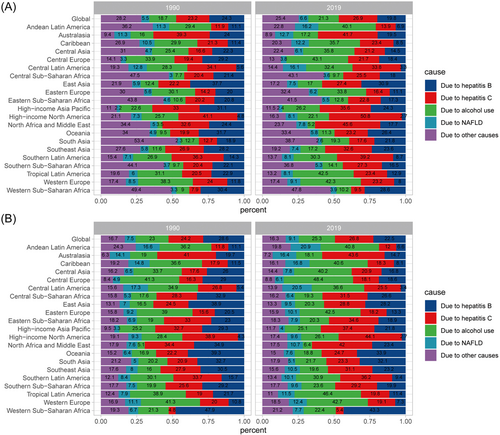
Hepatitis virus was the most important cirrhosis mortality risk factor. Globally, hepatitis virus infection (HBV + HCV) accounted for more than 45% of the incidence of cirrhosis cases and about 50% of cirrhosis deaths. Importantly, from 1990 to 2019, the proportion of cirrhosis incidence due to HBV decreased from 24.3% to 19.8%, whereas that due to alcohol use increased from 18.7% to 21.3%. Additionally, the proportion of NAFLD-induced cirrhosis incidence increased from 5.5% to 6.6% over the same period (Figure 2A). The proportion of cirrhosis deaths caused by specific etiologies also demonstrated similar trends (Figure 2B). Temporal trends of incidence and deaths by specific etiologies of cirrhosis are shown in Figure 3 and Supporting Information: Figure S6, respectively.
3.2 Global cirrhosis burden by subgroup
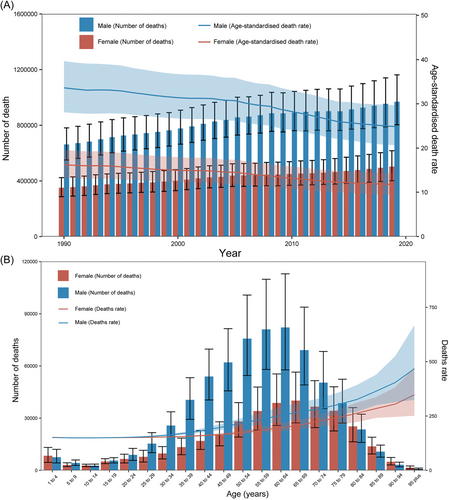
The cirrhosis incidence counts of different age groups and sex at the global level in 1990 and 2019 are shown in Supporting Information: Figure S7. The incidence counts peaked in individuals aged 40–44 years for both males and females. Notably, there were more new cases in females aged >55 years in 2019. Importantly, the incidence of cirrhosis was higher in younger patients (<39 years) than in elderly cases. The death count was highest in males aged 55–64 years and females aged 65–69 years. Overall, deaths were highest in females aged >80 years in 2019 (Figure 4). Notably, the incidence and death cases were higher in males than in females over the past 30 years (Figure 4A and Supporting Information: Figure S7A). Globally, a considerable percentage of DALYs was attributable to three risk factors for which GBD estimates were accessible. Specifically, in 2019, 48.7% of the DALYs (95% UI 38.7–59.1) were attributable to alcohol use, 14.7% (95% UI 12.2–17.6) to drug use, and 55.8% (95% UI 48.3–63.6) to behavioral risks. Moreover, 47.9% (95% UI 38.3–58.1) of the deaths were attributable to alcohol use, 13.9% (95% UI 11.6–16.6) to drug use, and 54.8% (95% UI 47.6–62.5) to behavioral risks in 2019 (Supporting Information: Figure S8). Overall, the DALYs and deaths attributable to alcohol use, drug use, and behavioral risks increased during 1990–2019 globally. Significant changes in the proportion of risk occurred in some regions. For instance, in the high-income Asia Pacific, the proportion of deaths due to alcohol use decreased from 67% in 1990 to 63.9% in 2016, whereas that due to drug use increased from 8.6% to 14.2% during the same period. Finally, the DALYs and deaths attributable to alcohol use and drug use lifestyle patterns in cirrhosis due to HBV and HCV risk factors are shown in Supporting Information: Figure S9.
3.3 Joinpoint regression analysis
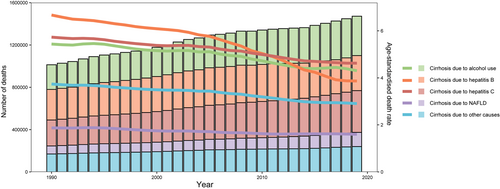
Globally, the cirrhosis incidence decreased most significantly during 1990–1993, with APC of −0.47% per year (/year) (95% CI: −0.76% to −0.17%). It plateaued during 1993–2005 and then increased in the 2005–2011 period, with an APC of 0.06% (95% CI: −0.04% to 0.17%). From 2011 to 2014, this incidence significantly decreased and then significantly increased from 2014 to 2019, with an APC of −0.52% (95% CI: −0.97% to −0.07%) and 0.48% (95% CI: 0.37% to 0.59%), respectively (Figure 5A and Supporting Information: Tables S1 and S2). In contrast, cirrhosis deaths exhibited a gradual downward trend with an AAPC of −1.04% (95% CI: −1.19% to −0.89%) during 1990–2019 (Figure 5B and Supporting Information: Tables S3 and S4). Globally, for cirrhosis due to HBV, cirrhosis incidence and deaths significantly decreased with an AAPC of −0.93% (95% CI: −1.01% to −0.87%), −1.87% (95% CI: −2.00% to −1.74%), respectively, during 1990–2019 (Supporting Information: Figure S10 and Tables S5–S8). Globally, for cirrhosis due to HCV, the cirrhosis incidence remained stable in the period before 2006 and then significantly increased during 2006–2019 with an APC of 0.94% (95% CI: 0.89%–0.98%) during 2015–2019. On the other hand, cirrhosis mortality significantly decreased during 1990–2019, with an AAPC of −0.74% (95% CI: −0.84% to −0.65%) (Supporting information: Figure S10 and Tables S9–S12). Comparatively, for cirrhosis due to NAFLD, cirrhosis incidence remained relatively stable globally in the period before 2005 and then significantly increased during 2005–2019 with an APC of 1.57% (95% CI: 1.29%–1.86%) during 2014–2017. By comparison, the cirrhosis deaths decreased from 1990 to 2019 with an AAPC of −0.54% (95% CI: −0.67% to −0.41%) (Supporting Information: Figure S10 and Tables S13–S16). Globally, the incidence of cirrhosis due to alcohol use significantly decreased with an APC of −0.17% (95% CI: −0.19% to −0.16%) during 1990–2005 and then increased with 0.47% (95% CI: 0.37%–0.58%) from 2005 to 2010. But this incidence significantly decreased with an APC of −0.28% (95% CI: −0.44% to −0.12%) from 2010 to 2014 and then significantly increased with an APC of 2.05% (95% CI: 1.70%–2.39%) from 2014 to 2017. Finally, from 2017 to 2019, it significantly decreased with an APC of −0.91% (95% CI: −1.28% to −0.54%). In contrast, the cirrhosis deaths decreased with an AAPC of −0.79% (95% CI: −0.99% to −0.59%) during 1990–2019 (Figure S10 and Tables S17–S20).

3.4 Joinpoint regression analysis by sex and age subgroup
Globally, in male patients, the cirrhosis incidence decreased most significantly during 1990–1994 with an APC of −0.41% (95% CI: −0.61% to −0.21%). From 1994 to 2011, it significantly increased and then significantly decreased from 2011 to 2014 with an APC of 0.04% (95% CI: 0.02%–0.06%) and −0.57% (95% CI: −1.08% to −0.06%), respectively. Finally, from 2014 to 2019, the incidence significantly increased with an APC of 0.34% (95% CI: 0.21%–0.46%). Contrarily, in female patients, the cirrhosis incidence showed a downward trend before 2004 and a significant increase from 2015 to 2019 with an APC of 0.82% (95% CI: 0.73%–0.90%). In addition, cirrhosis deaths significantly decreased with an AAPC of −1.03% (95% CI: −1.14% to −0.92%) and −1.11% (95% CI: −1.20% to −1.02%) in male and female patients during 1990–2019 (Supporting Information: Figure S11). Moreover, the APC of cirrhosis incidence and death change among 0–9, 10–19, 20–54, and 55–89 years subgroup is shown in Supporting Information: Figure S12.
3.5 Global age-period-cohort analyses
At the global level, DALYs were attributable to drug use, alcohol use, and behavioral risks. Therefore, we performed age-period-cohort analyses to explore the mortality attributed to these three risk factors. Net Drift was used to represent the overall temporal trend in mortality/morbidity and reflect trends attributable to period and cohort factors. For cirrhosis due to HBV, the overall Net Drift was −1.41%/year (95% CI: −1.47% to −1.36%/year). The mortality rate exhibited a decreasing trend over the study period based on local drift values (Supporting Information: Figure S13A). Age-effect analyses of mortality showed that in the same birth cohort, the mortality rate increased gradually by 5.42%/year (95% CI: 5.33%–5.52%/year) until age 47.5 and then began to decline based on the longitudinal age curves values (Supporting Information: Figure S13B). For period effects based on the period rate ratio (RR) values, RR revealed a decreasing effect on cirrhosis deaths. The relative risk of mortality/morbidity was higher before 2002 than in the reference period (Supporting Information: Figure S13C). Cohort-effect analyses based on cohort RR values indicated that the mortality risk of cirrhosis decreased among all birth cohorts. Additionally, the relative risk of mortality/morbidity was higher before 1950 than in the reference birth cohorts (Supporting Information: Figure S13D). For cirrhosis due to HCV, the overall Net Drift was −0.47%/year (95% CI: −0.54% to −0.40%/year), and the mortality rate under the age of 77.5 showed a decreasing trend over the study period. The age effect analyses of mortality showed that in the same birth cohort, the mortality rate increased. For the period effect, RR revealed a decreasing effect on cirrhosis deaths whereas the cohort effect analyses showed that the mortality risk of cirrhosis decreased after 1930 (Supporting Information: Figure S14A). Similar trends were also observed for cirrhosis due to alcohol use (Supporting Information: Figure S14B).
4 DISCUSSION
In the current study, we comprehensively explored the temporal trends in cirrhosis incidence and mortality at the global, regional, and national levels based on the GBD 2019 study and described major causes of the cirrhosis burden. In general, cirrhosis steadily decreased in incidence and mortality from 1990 to 2019 globally. Importantly, the four major causes of cirrhosis mortality and morbidity were heterogeneous across countries. Meanwhile, the incidence cases of cirrhosis primarily attributed to HCV, alcohol consumption, and NAFLD. Our results can help design targeted strategies for cirrhosis prevention in individual countries.
In our study, more than 45% of cirrhosis incidence and about 50% of deaths in cirrhosis cases were caused by HBV and HCV infections globally. East Asia (mainly China) and Western Sub-Sahara Africa are considered high HBV endemic regions.26 The ASDR of cirrhosis has been declining in these areas over the past few decades. However, the number of cirrhosis cases is increasing in Western Sub-Sahara Africa. Nevertheless, this is compensated by the decreasing ASDR. The decline might be mainly attributed to the availability of effective antiviral drugs and the adoption of preventive public health measures, such as vaccination. As of 2012, 183 countries had adopted hepatitis B vaccine in their national infant immunization plans, with an estimated 79% of the 2008 birth cohort receiving three doses of the hepatitis B vaccine.27 The Chinese government has listed HBV vaccination as a public health priority, contributing to a significant decline in the prevalence of HBsAg.28 These efforts have led to a significant reduction in the incidence and death rate of cirrhosis in the general population. In some South Asia countries, such as India, although the incidence of cirrhosis cases due to AAPC in HBV infection is still high, AAPC deaths are decreasing (Supporting Information: Tables S5–S8). The elderly are more likely to suffer from vaccine-preventable infections due to the age-related decline in their immune responses. Although the vaccination recommendations for elderly patients with chronic liver disease or cirrhosis are similar to those for healthy individuals, it is highly recommended that they be vaccinated against hepatitis B and C as soon as possible after the diagnosis of chronic liver disease. An assessment of postvaccination titers can also be useful in identifying patients who would benefit from receiving additional doses of the vaccine.29 Thus, we suggest that these countries adjust their HBV prevention strategies to promote utilization of maternal healthcare services and adopt universal hepatitis B vaccination programs in childhood.30 Notably, the decline in cirrhosis deaths also reflects the efficacy of efforts to reduce the disease burden over the past few decades.
Globally, for cirrhosis due to HCV, the cirrhosis incidence remained stable in the period before 2006 and then significantly increased during 2006–2019. In high-income regions, including North America, Eastern Europe, South Asia, and Central Asia, the incidence of cirrhosis cases due to HCV infection is still high (Supporting Information: Table S9). Globally, two out of three people who inject drugs have HCV infection, with rates of between 14% and 84% in the European Union countries.31 The primary mode of HCV transmission is sharing of injection equipment; thus, many of the new cases are reported in active injectors who share.32 Therefore, sharing of injection instruments undoubtedly increases the risk of cirrhosis in this group. During the past 10 years, direct-acting antivirals (DAA) have been approved for HCV with over 90% efficacy. While about 10%–15% of patients infected with HCV were coinfected with HBV in the United States, coinfection was found to accelerate the progression of liver disease to cirrhosis.33 Therefore, it is recommended that patients receiving newly developed DAA for HCV should receive HBV screening and careful monitoring or preventive treatment.34 Thus, cirrhosis due to HCV might be prevented by curbing the spread of HCV through sterile needle and syringe plans, coinfection screening, and HCV vaccine development.
Similarly, for cirrhosis due to NAFLD, the incidence plateaued in the period before 2005 and then significantly increased during 2005–2019 globally and in many countries. Among them, Kazakhstan had the highest AAPC value (Tables S13 and S14). In recent years, with the increasing incidence rate of obesity and metabolic syndrome, NAFLD has become an important risk factor for cirrhosis in some developed countries.35 Cirrhosis is an essential factor for liver-related morbidity and mortality in NAFLD patients.36 In a study that analyzed data from 33 379 patients with liver cirrhosis at 58 hospitals in Japan, 2.1% of all cases of cirrhosis were due to NAFLD.37 Currently, there is no pharmacotherapy approved for NAFLD; hence, lifestyle modification through diet and exercise is indicated for the treatment of NAFLD. Weight loss of more than 10% may be associated with fibrosis regression in 45% of patients.38 Another study also revealed that first-degree relatives of probands with NAFLD-cirrhosis have a 12 times higher risk of advanced fibrosis.39 Therefore, early identification of NAFLD patients is recommended to provide intensive lifestyle modification.
The incidence of cirrhosis due to alcohol use has significantly increased in the past 2 years (Supporting Information: Table S17). In 2010, alcohol-induced liver cirrhosis was responsible for 47.9% of all liver cirrhosis deaths.40 Regardless of sex, drinking more than 40 g of pure alcohol was associated with a more than a nine-fold increase in the risk of liver cirrhosis compared with abstainers.41 The pattern of drinking may confer a higher risk for cirrhosis. European countries mainly consume spirits as opposed to wine and beer.42 Binge drinking or daily drinking is also related to a higher burden of alcoholic cirrhosis.43 Therefore, different countries should develop individual strategies to reduce the burden of alcoholic cirrhosis based on population drinking patterns, underlying risk factors for cirrhosis, and level of economic development. Importantly, considering that cirrhosis deaths due to alcohol use were significantly higher, targeted strategies should limit daily heavy drinking to reduce the burden of cirrhosis. Studies also utilized the age-period-cohort approach to evaluate the independent correlation between cohort and the incidence of cirrhosis. Flemming et al.44, 45 employed age-period-cohort modeling and used the median birth year as the reference. They discovered that the incidence of cirrhosis was higher in individuals born in 1980 and predicted that the incidence of cirrhosis would continue to increase for the next two decades using age-period-cohort modeling and joinpoint regression analyses. In our research, we found that the relative risk of mortality/morbidity was higher before 1950 than in the reference birth cohorts. Thus, age-period-cohort modeling and joinpoint regression analyses are better tools for evaluating overall and etiology-specific cirrhosis trends from age and sex standardized incidence rates. Some limitations of our study must be acknowledged. First, we could not estimate the burden of common risk factors (such as BMI) and dietary patterns on cirrhosis.46 Second, the specific factors influencing the cirrhosis burden in different regions were difficult to study. Finally, GBD data were produced by a combination of estimation corrections made by various models, and therefore we may not fully account for the offset caused by the source of the original data.
5 CONCLUSION
In summary, cirrhosis remains a major public health concern globally. Government policy intervention should prioritize early identification of NAFLD patients for intensive lifestyle modification interventions. Additionally, universal hepatitis B and hepatitis C vaccination in childhood should be strengthened. Different countries should develop individual strategies to reduce the burden of alcoholic cirrhosis based on population drinking patterns. Our findings on the global disease burden of cirrhosis provide a valuable resource for developing targeted prevention strategies.
AUTHOR CONTRIBUTIONS
Zenghong Wu designed and analyzed the research study; Kun Zhang, Mengke Fan, and Weijun Wang collected the data; Zenghong Wu and Rong Lin wrote and revised the manuscript. All authors have read and approved the manuscript.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grant numbers 82170571 and 81974068) and Natural Science Foundation of Hubei Province (nos. 2022CFA009).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated for this study are available on request to the corresponding author.