NFX1-123: A potential therapeutic target in cervical cancer
Abstract
NFX1-123 is a splice variant isoform of the NFX1 gene. It is highly expressed in cervical cancers caused by HPV, and NFX1-123 is a protein partner with the HPV oncoprotein E6. Together, NFX1-123 and E6 affect cellular growth, longevity, and differentiation. The expression status of NFX1-123 in cancers beyond cervical and head and neck cancers, and its potential as therapeutic target, have not been investigated. TSVdb of TCGA was used to quantify NFX1-123 expression in 24 cancers compared with normal tissues. The NFX1-123 protein structure was predicted and then submitted to retrieve suitable drug molecules. The top four compounds, found to bind in silico to NFX1-123, were tested experimentally to determine their effects on NFX1-123-related cellular growth, survival, and migration. 46% of cancers (11 of 24 had significant differences in NFX1-123 expression, with nine having had greater NFX1-123 expression, when compared with adjacent normal tissues. Bioinformatics and proteomic predictive analysis modeled the three-dimensional structure of NFX1-123, and drug libraries were screened for high-binding affinity compounds using this modeled structure. Seventeen drugs with binding energies ranging from −1.3 to −10 Kcal/mol were identified. The top four compounds were used to treat HPV- and HPV+ cervical cancer cell lines, three of which (Ropitoin, R428 and Ketoconazole) reduced NFX1-123 protein levels, inhibited cellular growth, survival, and migration, and enhanced the cytotoxicity of Cisplatin. These findings highlight cancers expressing high levels of NFX1-123, and drugs that target it, may reduce cellular growth, survival, and migration, making NFX1-123 a potential novel therapeutic target.
1 INTRODUCTION
NFX1 and its homologs across different species have been known to disrupt drug interactions, affect cellular growth, and protect cells from stress.1 The longer splice variant isoform of NFX1, NFX1-123, is an endogenous cytoplasmic protein that binds to the high-risk HPV (HR-HPV) type 16 oncoprotein E6 (16E6). Previous studies have determined that 16E6 and NFX1-123 together shifted the normal localization of signaling proteins involved in the innate immune response's activation,2 a critical step in immune evasion by HPV. Additionally, 16E6 utilized NFX1-123 to affect cellular growth, differentiation, and immortalization genes and pathways through posttranscriptional gene regulation,1, 3-8 affecting multiple pathways important in HPV-associated oncogenesis.
While the shorter splice variant isoform of NFX1, NFX1-91, is not (Ref 54 Xu M 2010), NFX1-123 is overexpressed in HPV-associated cancers of the cervix8, 9 and the head and neck.10 These findings point to the importance of NFX1-123 in cancer cell growth, survival, and differentiation, and it is inferred from its high expression in HPV-associated cancers. The potential for NFX1-123 to be a cancer therapy target, however, has not been investigated. Specifically, the expression levels of NFX1-123 in other non-HPV associated cancers, the predicted three-dimensional structure of NFX1-123, or what compounds could bind to NFX1-123, reducing its amount or functionality in cells, have not been determined.
In this study, we utilized the publicly available Transcript Splice Variant database (TSVdb) of The Cancer Genome Atlas Data (TCGA) to quantify the expression of NFX1-123 in 24 cancers relative to normal tissues, modeled the three-dimensional structure of NFX1-123, identified drug compounds in silico that bound to that protein structure, and tested those compounds to determine their effects on the NFX1-123 protein in cervical cancer cell lines. We found three compounds that affected NFX1-123 also independently reduced cellular growth, survival, and migration, and synergized the cytotoxic effect of Cisplatin when combined.
2 METHODS
2.1 NFX1-123 expression analysis in cancer
The specific messenger RNA (mRNA) expression of NFX1-123, as a splice variant isoform of the NFX1 gene, was analyzed using TCGA splice variant database TSVdb11 as described recently.10 The TSVdb contains splice variant mRNA expression data for 33 different anatomical sites of cancer tissues. Nine cancers were excluded as they did not have comparative adjacent normal tissue expression for NFX1-123. The list of 24 cancers with data from primary tumors and their respective normal tissues is provided in the table below. Message RNA expression data were downloaded from each type of cancer and its adjacent normal tissue, and the expression data of the NFX1-123 isoform (isoform_uc003zsq) in “primary solid tumor” and “solid tissue normal” was separated to quantify differential expression.
| Cancer type | |
|---|---|
| 1 | BLCA, Bladder Urothelial carcinoma |
| 2 | BRCA, Breast invasive carcinoma |
| 3 | CESC, Cervical squamous cell carcinoma and endocervical adenocarcinoma |
| 4 | CHOL, Cholangiocarcinoma |
| 5 | COAD, Colon adenocarcinoma |
| 6 | ESCA, Esophageal carcinoma |
| 7 | GBM, Glioblastoma multiforme |
| 8 | HNSC, Head and Neck squamous cell carcinoma |
| 9 | KICH, Kidney Chromophobe |
| 10 | KIRC, Kidney renal clear cell carcinoma |
| 11 | KIRP, Kidney renal papillary cell carcinoma |
| 12 | LIHC, Liver hepatocellular carcinoma |
| 13 | LUAD, Lung adenocarcinoma |
| 14 | LUSC, Lung squamous cell carcinoma |
| 15 | PAAD, Pancreatic adenocarcinoma |
| 16 | PCPG, Pheochromocytoma and paraganglioma |
| 17 | PRAD, Prostate adenocarcinoma |
| 18 | READ, Rectum adenocarcinoma |
| 19 | SARC, Sarcoma |
| 20 | SKCM, Skin Cutaneous Melanoma |
| 21 | STAD, Stomach adenocarcinoma |
| 22 | THYM, Thymoma |
| 23 | THCA, Thyroid carcinoma |
| 24 | UCEC, Uterine Corpus Endometrial Carcinoma |
2.2 Bioinformatics analysis to identify NFX1-123 binding compounds
The following tools and databases were used to predict the protein structure of NFX1-123 and to screen small chemical compounds predicted to bind that NFX1-123 structure.
2.3 I-TASSER: Iterative Threading ASSEmbly Refinement
I-TASSER used the hierarchical approach to predict the protein structure and function. Using this tool first identified the structural templates from the PDB by multiple threading approach LOMETS with full-length automatic models constructed by iterative template-based fragment assembly simulation.12-14
2.4 ZINC database
ZINC database, a free database used for virtual screening of commercially available compounds. This database contained over 35 million purchasable compounds in ready-to-dock, 3D formats. ZINC was provided by the Irwin and Shoichet Laboratories in the Department of Pharmaceutical Chemistry at the University of California, San Francisco (UCSF).15
2.5 MTi-OpenScreen
MTi-OpenScreen is a web-server used for virtual screening of small compounds,16 and it uses AutoDock Vina to screen the drug molecules. The drug molecules were characterized into five types: (1) Diverse-lib: Diverse chemical compound collection; (2) iPPI-lib: Focused chemical compound collection to target protein–protein interactions; (3) NP-lib: Natural product compound collection; (4) Drugs-lib: collection of purchasable approved drugs (ZINC database); and (5) Food-lib: food constitute compound collection.
2.6 PyRx interphase
PyRx is a virtual screening software for computational drug discovery to screen libraries of compounds against potential drug targets. PyRx is enabled to run virtual screening from any platform and aids users with data preparation, job submission, and analysis of results. PyRx includes a docking wizard with an easy-to-use user interface which made it as a valuable tool for Computer-Aided Drug Design.17
2.7 AutoDock Vina
AutoDock Vina is an open-source program for molecular docking. It was designed and implemented by Dr. Oleg Trott in the Molecular Graphics Lab at The Scripps Research Institute.18
2.8 Cell culture and drug compound studies
One HPV-negative cervical cancer cell line (C33A), three HPV-positive cervical cancer cell lines (CaSki, SiHa, and HeLa), two HPV-positive cervical cancer cell lines (SiHa and CaSki) cell lines with single guide RNA (sgRNA) to knock out (KO) NFX1-123 or a control gRNA, and a noncancerous 293T cell line were used to validate compounds that were modeled to bind NFX1-123. Cells were cultured in complete DMEM medium with sodium pyruvate, 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin. The following compounds were used in drug studies: R428 (Bemcentinib, catalog #21523; Cayman Chemicals); Ketoconazole (R41400) (catalog #S1353; Selleckchem); Perospirone Hydrochloride (catalog #S4889; Selleckchem); Ropitoin (TR 2985) (catalog #HY-U00274; MedChemExpress), and Cisplatin (catalog #23120; Sigma-Aldrich Inc). Stock solutions of R428, Ketoconazole, Perospirone Hydrochloride, and Ropitoin were prepared in DMSO. Cisplatin was prepared in sterile water. All were used to treat cells for western immunoblot studies, MTT assays, colony formation assays, and cell migration assays (see below).
2.9 NFX1-123 KO cells using the CRISPR/Cas9 system
A sgRNA that aligns with NFX1-123 at exon 16 was designed (5’-AGATATCAACCAGGTGACAG-3’) was used to disrupt NFX1-123 expression. Exon 16 is the first exon unique to this longer splice variant isoform of NFX1. A control sgRNA, a gift from Dr. Paddison's laboratory at the Fred Hutchinson Cancer Research Center which does not target any functional mammalian gene, was used. These sgRNA sequences were cloned into the ZLCv2-neo vector, also a gift from Dr. Paddison's laboratory, at the BsmB1 restriction site. Forward primer 5’ CACCgAGATATCAACCAGGTGACAG 3’ with BsmBI cut site+prep G Oligo template and reverse primer 5’-AAACCTGTCACCTGGTTGATATCTC-3’ with BsmBI cut site overhang End C were used, and PCR was performed to determine the presence of sgRNA in ZLCv2 neo vector. The control sgRNA and NFX1-123 targeted sgRNA containing plasmid vectors were packaged in second-generation lentivirus with MD2.G and psPAX, also gifts from Dr. Paddison's laboratory, and lentivirus was concentrated by ultracentrifugation, following standard protocols.4
300 × 103 cells of SiHa and CaSki cells were seeded in six-well plates and allowed to grow overnight. Medium was removed, 1 mL of control sgRNA or NFX1-123 targeted sgRNA lentivirus suspension in DMEM medium was mixed with polybrene (10 µg/mL) and added to cells, and cells were incubated overnight at 37°C. The medium was changed in the morning, and cells were allowed to grow for an additional 48 h. The transduced cells were replated into new six-well plates and then selected for using the G418 for four-to-five passages. After selection and passaging, the NFX1-123 KO efficacy was compared with control sgRNA (Cont gRNA) by Western immunoblot, as described below.
2.10 Western immunoblot
NFX1-123 protein expression was determined by western immunoblot as described previously.9 Briefly, cervical cancer cell lines were seeded in six-well or 10 cm plates. Twenty-four hours later, they were treated with Ropitoin (5–20 µM), R428 (0.5–5 µM), Ketoconazole (10–40 µM), and Perospirone Hydrochloride (10–20 µM) and pelleted for protein at 72 h. DMSO-treated cells were pelleted as controls. Cell pellets were lysed with lysis buffer (50 mM Tris HCl pH 7.5, 150 mM NaCl and 0.01% Triton X-100) and cup sonicated in an ice-bath. A total of 20–40 µg of protein was loaded on a 4%–20% gradient gel (BioRad) and transferred to PVDF membrane using the Trans Blot Turbo System (BioRad). Membranes were blocked with 2% blocking agent (Cytiva Lifesciences) for 1 h at room temperature. Primary antibodies for NFX1-123 1:500 (Novus Biologicals) or GAPDH (1:50,000; Abcam) and HRP conjugated secondary antibodies of anti-rabbit (1:5000 dilution) and anti-mouse (1:50,000) were used.
2.11 MTT assay
To evaluate and quantify cellular growth and survival effects of drug compounds on cervical cancer cell lines and 293T cells, the Vybrant MTT Cell Proliferation Assay Kit was used (Molecular Probes). Briefly, 1–2 × 103 C33A, CaSki, SiHa, HeLa, 293T, and SiHa and CaSki NFX1-123 KO and control sgRNA cells were seeded in 96 well plates with phenol red-free DMEM medium, 10% FBS, and 1% penicillin and streptomycin. Twenty-four hours later cells were treated with Ropitoin (1.875–15 µM), R428 (0.25–2 µM), Ketoconazole (2.5–50 µM), or a vehicle control. After 72 h of treatment, the media was removed, replaced with 100 µL of fresh media and 10 µL of 12 mM MTT stock solution for 4 h. One hundred microliters of freshly prepared SDS-HCl solution was then added to each well's media/MTT stock solution and mixed thoroughly. After 4 h of incubation at 37°C, the absorbance of each well at 570 nm was calculated using a BioTek Synergy H1 plate reader (Agilent). Growth inhibition was calculated as a percent compared with the vehicle control growth.
To evaluate and quantify the effect of drug compounds in combination with Cisplatin in cervical cancer cell lines, 0.3125–10 µM of Cisplatin was used in combination with Ropitoin, R428, and Ketoconazole in the same manner as the single drug compounds alone. Growth inhibition was calculated as a percent compared with the vehicle control growth as well as to the single drug compound.
To determine the effect of NFX1-123 KO on growth, control sgRNA (Cont gRNA) and NFX1-123 KO SiHa and CaSki cells were seeded as specified above and allowed to grow for 72 h. The cell growth, as absorbance (OD) of MTT, was then quantified. To determine the drug effects on NFX1-123 KO and Control gRNA SiHa cells, cells were treated with R428 (1 µM), KTZ (10 µM) and Cisplatin (5 µM) for 72 h, and the MTT assay was performed, with the percent growth quantified.
The cytotoxic effects of Ropitoin, R428, and KTZ were assessed by MTT, comparing the noncancerous 293T cell line with CaSki cervical cancer cell line. In this assay, two different sets of plated cells were treated with Ropitoin (1.875, 3.75, and 7.5 µM), R428 (0.25 and 0.5 µM), and KTZ (12.5 and 25 µM) for 72 h. One set of cells were processed at 72 h to determine the percent growth inhibition, normalized to an untreated control. The second set of cells had the drug compounds removed and rinsed at 72 h, and then fresh medium was added. Cells were allowed to recover for 5 days. The percent recovered growth was calculated, normalized to untreated controls as well as to the number of cells at the time the drug compounds were removed. For both cell lines, each drug compound and dosage was conducted in quadruplicate.
2.12 Clonogenic assay
To determine the survival and colony formation of cancer cells after drug treatment, clonogenic assays were performed. Cervical cancer cell lines were plated onto six-well plates at densities of 1–3 × 103 cells per well. Twenty-four hours later, cells were treated with Ropitoin (5–10 µM), R428 (0.2–1 μM), Ketoconazole (10–40 μM), or a vehicle control. After 72 h of treatment, media was removed, cells were washed using DPBS, and fresh media was replaced. Cells were incubated at 37°C for 5–7 days. Cells were fixed and stained using 0.5% crystal violet (Sigma-Aldrich) and imaged using a flat-bed scanner. To quantify colony growth relative to the vehicle treatment control, 0.5% SDS was added to each plate to dissolve stained colonies. The solution from each well was diluted 1:10, and the absorbance was read at 540 nm using a Bio-Tek plate reader. Percent colony growth was calculated as a percentage of the vehicle control colony growth (set to 100%).
2.13 Scratch assay
Scratch assays were performed to determine the wound healing abilities of cervical cancer cells after treatment with Ropitoin, R428, or Ketoconazole. Cervical cancer cell lines were plated onto six-well plates at a density of 2 × 105 cells per well. Twenty-four hours later, the plate was scratched in a single, straight line using a 200 µL micropipette tip. Cells were rinsed using DPBS and were treated with Ropitoin (5–10 μM), R428 (0.5–1 μM), Ketoconazole (20–40 μM), or a vehicle control. Images of the scratch were taken at 0, 24, 48, and 72 h using a REVOLVE-Discover ECHO microscope (ECHO BICO Company). Scratch width was calculated using ImageJ software, and percent wound closure was calculated as a percentage of scratch width at 0 h.
2.14 Cell migration assay
CaSki and SiHa cell migration was evaluated using the Cyto Select 24-Well Cell Migration Assay (8 µM Colorometric Format) Kit (Cell Bioloabs, Inc.) as per the manufacturer's protocol. CaSki and SiHa cells were prepared in a cell suspension containing 1.0 × 106 cells in serum-free DMEM medium with 1% sodium pyruvate and 1% penicillin and streptomycin. Cells were treated with either Ropitoin (20 or 30 μM), R428 (3 or 5 μM), Ketoconazole (60 or 80 μM), or a vehicle control. DMEM medium containing 10% FBS, 1% sodium pyruvate, and 1% penicillin and streptomycin was added to the lower well of the migration plate. 3 × 105 cells were added to the interior well of the migration plate insert, and cells were incubated at 37°C with 5% CO2 for 24 h. Media was removed from the insert, and nonmigratory cells were removed from inside of the insert by gently wiping it with a wet cotton swab several times. The insert was stained for 10 min at room temperature using the Cell Stain Solution before being rinsed gently in tap water and air dried. Migratory cells were imaged using a REVOLVE-Discover ECHO microscope (ECHO BICO Company) at ×10 magnification. Each insert was transferred to an empty well with the Extraction Solution and was incubated for 10 min on an orbital shaker. The absorbance was read at 560 nm on a Bio-Tek plate reader.
3 RESULTS
3.1 NFX1-123 is highly expressed in cancers
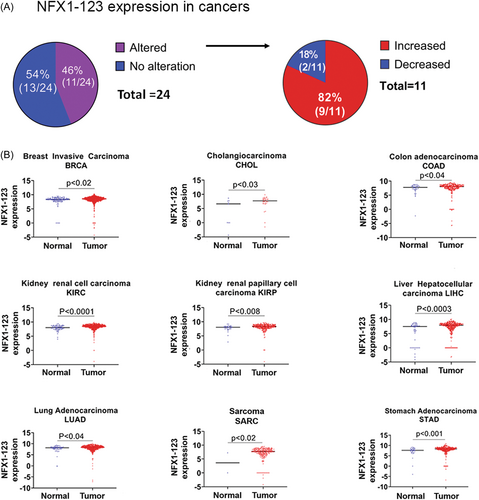
NFX1-123 has been established as a critical regulator of several cellular and signaling pathways in HPV-associated oncogenesis,2, 4-8, 19 so it was important to determine the expression levels of NFX1-123 in other non-HPV-associated cancers. The publicly available TSVdb of TCGA11 was used for this purpose. This database parses all mRNA splice variants of genes within the TCGA to identify which isoform of a gene has differential expression in a cancer when compared with its normal control. All NFX1 splice variant isoform expression data were extracted, and the NFX1-123 isoform was quantified in primary solid tumors when compared with their normal solid tissue controls. In the TSVdb, 24 different cancers with paired normal controls contained data on expression of the NFX1-123 isoform. 11 (46%) exhibited altered NFX1-123 expression when compared with normal solid tissue (Figure 1A). Among the 46% of tumors that had differential expression of NFX1-123, only 18% (2 out of 11) had reduced while 82% (9 out of 11) had increased NFX1-123 mRNA (Figure 1A). Lung squamous cell carcinoma (LUSC) and pheochromocytoma and paraganglioma (PCPG) had reduced NFX1-123 mRNA (data not shown), whereas breast invasive carcinoma (BRCA), cholangiocarcinoma (CHOL), colon adenocarcinoma (COAD), kidney renal cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), liver hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), sarcoma (SARC), and stomach adenocarcinoma (STAD) all had greater NFX1-123 mRNA (Figure 1B). This suggested that NFX1-123 has a broader function in oncogenesis.
3.2 Determination of 3D structure of NFX1-123 and identification of NFX1-123-binding compounds
The three-dimension structure of the NFX1-123 protein is not known, so the sequence of NFX1-123 was submitted to I-TASSER webserver to predict its protein structure. Five model structures were generated, and these structures were submitted to the MTi-Openscreen Virtual screening webserver to retrieve suitable, commercially available drug compounds from the Drug-lib database. Among the five models of NFX1-123, the structure of Model 2 (Figure 2A) resulted in the highest number of drug compound hits. Thus, we choose Model 2 for further analysis.
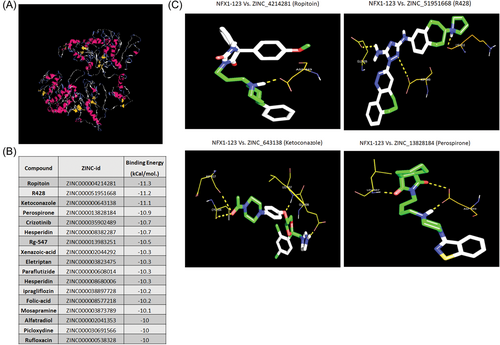
Eight hundred and ninety-five of the top 1500 drug compounds from MTI-Openscreen had three-dimensional structures available in the ZINC database. Those drug compound structures were converted to our desired docking format using PyRx interface. AutoDock-Vina, embedded in PyRx interface, was used to dock the compounds against the Model 2 of NFX1-123, resulting in the best docked compounds and binding energies for each compound. Seventeen drug compounds had binding energy scores that were more than 10 Kcal/mol. (Figure 2B).
Of the 17 drug compounds noted above, the top 10 drugs modeled to bind NFX1-123 were further analyzed to identify their binding sites on NFX1-123, using the autodock docking tool kit followed by the pymol visualization tool, shown in Figure 3B. The binding sites and modeled amino acid-drug positions of the top four drug compounds with NFX1-123 were visualized (Figure 2C). Of note, the binding amino acids positions ASP-849 of Ropitoin, ASP-849 and LYS-853 of R428, and ASP-849 of Perospirone are unique to the NFX1-123 splice variant as they are not present in the shorter NFX1 isoform, NFX1-91.1, 3 These drug compounds were then tested in cell-based assays to determine if they could bind to, and affect, the NFX1-123 protein itself or affect its targeted function.
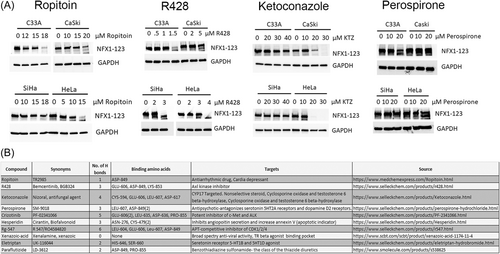
3.3 Drug compounds reduced NFX1-123 protein levels
Because prior work had demonstrated NFX1-123's role in cervical cancer cell line growth,8 and high NFX1-123 protein expression is seen in cervical cancer cell lines19 and in HPV 16+ archived cervical cancer samples,9 cervical cancer cells C33A (HPV-), CaSki (HPV 16+), SiHa (HPV 16+), and HeLa (HPV 18+) were treated with Ropitoin, R428, Ketoconazole (KTZ), and Perospirone to determine their effect on NFX1-123 protein. All four compounds, except Perospirone, decreased NFX1-123 protein levels, regardless of HPV status (Figure 3A). The top 10 NFX1-123 binding compounds, their synonyms, their binding amino acid positions, and their known targets are shown (Figure 3B) This confirmed the in silico modeling and drug compound screening of three of the top four compounds, as they decreased NFX1-123 protein levels in cervical cancer cell lines.
3.4 Drug compound treatment of cervical cancer cell lines reduced survival and growth and enhanced cisplatin effects
As drug treatment by Ropitoin, R428, and Ketoconazole led to decreased NFX1-123 protein, it was important to quantify if there also were concomitant changes to cellular growth with treatment, and if Cisplatin, a first line drug therapy for cervical cancer, could gain synergistic cytotoxicity when combined with a drug compound. C33A, CaSki, SiHa, and HeLa cells were treated with increasing amounts of Ropitoin (Figure 4A), R428 (Figure 4B), or Ketoconazole (Figure 4C), and cellular growth was reduced in a dose-dependent manner across all cell lines (Figure 4, blue lines). Cisplatin cytotoxic effects were enhanced in combination with Ropitoin in CaSki cells, and with R428 and Ketoconazole across all cell lines (Figure 4A–C, red lines Cisplatin alone, green lines in combination). Taken together, R428 and Ketoconazole not only reduced NFX1-123 protein levels but also slowed cellular growth and enhanced the cytotoxic effects of Cisplatin.
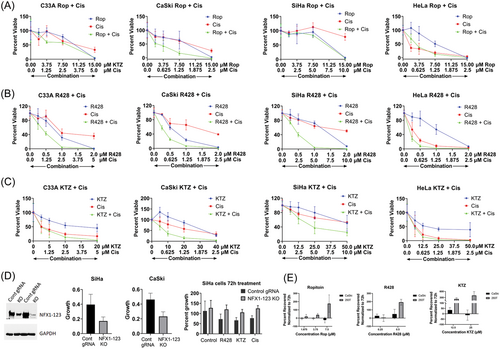
To determine the role of NFX1-123 on cellular growth in HPV 16 positive cervical cancer cell lines, CRISPR/Cas9 was utilized to KO NFX1-123 in SiHa and CaSki cell lines (Figure 4D), and their growth was quantified compared with control cells. SiHa and CaSki cells with decreased NFX1-123 expression had slowed growth when compared with control KO cells (Figure 4D). This demonstrated that the presence of NFX1-123 itself was important to cellular growth in HPV-positive cancer cell lines, and it is supported by our previously published data in SiHa cells with knock down of NFX1-123.8
Finally, when compared with 293T cells, an immortalized but noncancerous cell line, CaSki cells were more sensitive to a 72 h treatment with all three drug compounds after being allowed to recover for 5 days (Figure 4E), indicating that they were more susceptible to drug treatment than a non-HPV and noncancerous cell line.
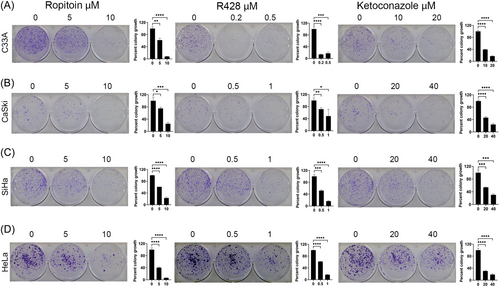
3.5 Drug compounds decreased the colony formation of cervical cancer cells
The colony formation abilities of cervical cancer cell lines, when treated with Ketoconazole, R428, or Ropitoin, were quantified using clonogenic assays. C33A (Figure 5A), CaSki (Figure 5B), SiHa (Figure 5C), and HeLa (Figure 5D) cells were seeded and treated with two concentrations of Ropitoin, R428, Ketoconazole, or a vector control for 72 h, after which the drug was removed, and cells were allowed to form colonies. Treatment with all three drug compounds significantly decreased colony formation a dose-dependent manner (Figure 5) in parallel with the reduction in NFX1-123 protein levels.
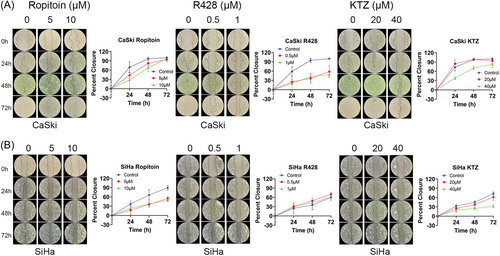
3.6 Cervical cancer cell wound healing and migration was inhibited by drug compounds
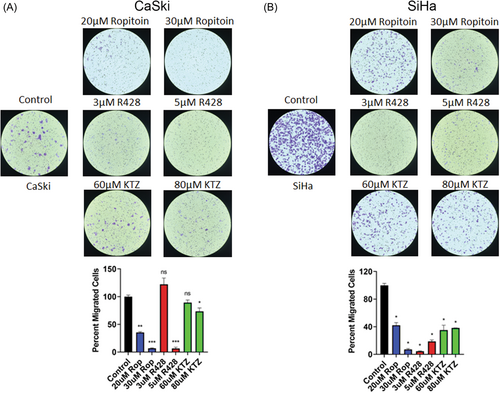
Cervical cancer cell line wound healing responses were determined using a scratch assay and their cell migration with the CytoSelect Cell Migration Assay. First, CaSki (Figure 6A) and SiHa (Figure 6B) confluent monolayer cells were scratched using a pipette tip and treated with Ropitoin, R428, or Ketoconazole. After 24, 48, and 72, all three drug compounds significantly decreased the wound healing abilities (Figure 6, red and green lines vs. blue control lines). Cell migration was quantified for CaSki (Figure 7A) and SiHa (Figure 7B) cell lines, with Ropitoin, R428, or Ketoconazole treatment. Like wound healing, all three drug compounds significantly inhibited cell migration in a dose-dependent manner (Figure 7). Taken together, these drug compounds reduced NFX1-123, decreased cellular growth and viability, and diminished cellular wound healing and colony formation in cervical cancer cell lines with high endogenous expression of NFX1-123.
4 DISCUSSION
The importance of NFX1-123, in cooperation with 16E6, on cellular growth, immortalization, survival, and differentiation has been shown,4, 5, 7, 8, 19, 20 and NFX1-123 is highly expression in cervical cancer cell lines19 and primary tumors, and in HPV positive head and neck cancers.9, 10 NFX1-123 itself contains a PHD/RING domain, which functions as an E3 ubiquitin ligase, and the deubiquitinase USP9X, which protects the proteins from proteasome-mediated degradation, binds to NFX1-123.4 Greater expression of USP9X is seen in several tumor types,21 and 16E6 increases USP9X expression, thus indirectly affecting NFX1-123 levels as well.21 All these studies point to high expression of NFX1-123 being important in tumor cell growth and survival, specifically in HPV-associated cancers.
There are six known types of cancer that are caused by HPV: cervical, vulvar, vaginal, anal, penile, and head and neck cancers. NFX1-123 appears to play a foundational and functional role in the cellular dysregulation required by HPV at the very least in cervical cancer.7-9, 19 The role NFX1-123 has in cancers more broadly, whether HPV-associated or not, has not been well studied. Using the TSVdb. we found that 46% (11 out of 24) of cancers had altered NFX1-123 expression, 9 of which were increased (Figure 1A), further emphasizing the potential role of NFX1-123 in cancers with and without HPV.
When considering treatments for diseases, such as cancers, conventional target identification and drug screening strategies can be very laborious, expensive, and time intensive.22 Structure-based drug screening, with the incorporation of bioinformatics to analyze protein targets and perform virtual screens to identify compounds which bind a target, is gaining prominence because it is inexpensive and fast.23-26 In this study, we leveraged this in silico work to identify drug compounds that may bind to, and affect, NFX1-123.
First, the predicted three-dimensional structure of NFX1-123 was determined (Figure 2A) and then utilized as a model to screen ligand drug compounds. A total of 894 were identified whose three-dimensional structures were available in ZINC database and which had strong drug: NFX1-123 binding energies. The top four drug compounds were evaluated to determine their effects on NFX1-123 and its functions. Three of the four drug compounds identified in our in silico screen (Ropitoin, R428, and Ketoconazole) reduced the protein level of NFX1-123 within 72 h (Figure 3A). Interestingly, while Ropitoin is not, R428 and Ketoconazole have been in use, or are in clinical trials to repurpose drugs for other diseases including cancer, and their greater effect on cancer cells compared with normal cells is been demonstrated.27, 28 While we cannot rule out the possibility of other known targets of these drugs associated with the observed inhibition of growth, survival, and migration, the NFX1-123 KO SiHa cells were less responsive to KTZ and R428, indicating the drug effects were associated with the relative expression of NFX1-123 protein.
Ropitoin (TR 2985) is an antiarrhythmic drug that depresses the maximum upstroke velocity of the action potential in a voltage-dependent fashion.29 While the voltage-gated sodium and potassium channels have been shown as potential therapeutic targets and biomarkers in cancers,30-32 and specifically in cervical cancer,33-35 the direct effect of Ropitoin on sodium or potassium channels, let alone NFX1-123, has not been investigated. Our findings make Ropitoin an interesting drug for further study in cancers and as a voltage-gated channel regulator.
R428 (Bencentinib) is an inhibitor of Axl, the receptor tyrosine kinase. It is currently under evaluation as a drug therapy in several cancer clinical trials.36 Axl has been demonstrated to be a therapeutic target in head and neck cancers37 and cervical cancers,38 and Axl has a role in cancer therapeutic resistance.39-41 Axl is a known target of R428, but R428 also affects several biological processes including apoptosis, lysosomal acidification and recycling, and the immune microenvironment. Because R428 has already been shown to affect HPV-associated cancers,37-39 our data adds to those findings.
Ketoconazole (KTZ) is a FDA-approved antifungal drug. Interestingly, studies have identified Ketoconazole's anticancer activities,42, 43 and clinical trials are ongoing to test its efficacy against cancer (NCT00895310, NCT03796273). Ketoconazole inhibits adrenal testosterone synthesis through the inhibition of cytochrome P450 14α-demethylase.44 This implies that Ketoconazole could function in the treatment of metastatic, castration-resistant prostate cancer, leading to improved survival.45 In renal cancer, which has increased expression of NFX1-123 (Figure 1), Ketoconazole has been shown to be an inhibitor of exosome production, and as an adjunct therapy, Ketoconazole potentiates the effectiveness of Sunitinib by decreasing tumor cell proliferation, and the clonogenic potential of renal cancer cell lines.46
All three of these drug compounds decreased NFX1-123 protein in cervical cancer cell lines and were associated, in a dose-dependent manner, with the downregulation of key indicators of cellular growth and metastatic potential; however, the final drug compound modeled to bind to NFX1-123, Perospirone Hydrochloride, did not reduce NFX1-123 levels. This emphasizes the importance of validation studies, and that most, but not all, of these drug compounds did appear to target the NFX1-123 protein, and its effects on cellular growth, in cervical cancer cell lines.
In conclusion, we interrogated publicly available databases to identify nine cancers, beyond HPV-associated cervical and head and neck cancers, that had higher expression of NFX1-123 relative to their normal tissues. Through in silico modeling, we also found three drug compounds that were predicted to bind to NFX1-123. These drug compounds reduced NFX1-123 protein and inhibited cervical cancer cell line growth, survival, and migration, singularly or in combination with Cisplatin. These findings suggest that NFX1-123 may be a potential therapeutic target in cancer treatment.
AUTHOR CONTRIBUTIONS
Sreenivasulu Chintala: Conception; experimental design; data generation; analysis; writing. Maura A. Dankoski: Data generation; analysis; writing. Anand Anbarasu: Bioinformatics analysis design; data generation analysis; writing. Sudha Ramaiah: Bioinformatics data generation; analysis, writing. Sravan Kumar Miryala: Bioinformatics data generation; analysis; writing. Rachel A. Katzenellenbogen: Conception; data analysis; writing.
ACKNOWLEDGMENTS
This research was funded by NIH R01 CA172742 to Rachel A. Katzenellenbogen. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data sets used or analyzed from this study will be available upon reasonable request.