Profiling the acute phase antibody response against mpox virus in patients infected during the 2022 outbreak
Abstract
Information on the immune response during the mpox virus (MPXV) infection is still scarce or limited to past studies when cross-reactive immunity from smallpox vaccination was predominant. Here, we describe the short-term kinetics of the antibody response in patients with acute MPXV infection during the 2022 multicountry outbreak. A total of 64 samples from 18 MPXV-positive patients were longitudinally collected from the day of symptom onset (DSO) up to 20 days after and tested for anti-MPXV immunoglobulin G (IgG), IgM, IgA, and neutralizing antibodies (nAb) using the whole-live virus isolated in May 2022. IgG, IgM, and IgA were detected as early as 4 DSO (median time of seroconversion 7.5 DSO for IgG, 8 DSO for IgM and IgA). Anti-MPXV nAb were detectable in samples collected as early as 1 week after symptoms, with stable levels up to 20 DSO. After 2 weeks, IgG and nAb reached high titers. No significant differences were observed regardless of status of smallpox vaccination, human immunodeficiency virus positivity, or disease severity. Significant lower levels of IgM and IgG were observed in the patients treated with antivirals. These results contribute to extending the knowledge of the MPXV infection and the antibody response in a population with no historic smallpox vaccination.
1 INTRODUCTION
Mpox virus (MPXV) causes a smallpox-like disease with the potential for significant clinical impact and has been classified as a neglected zoonotic pathogen with limited interhuman transmission.1 Until the 2022 outbreak involving multiple countries worldwide, MPXV was confined to Africa, with sporadic cases reported in other continents (i.e., England, United States, Singapore, and Israel), the majority associated with travelers returning from endemic countries, following nosocomial exposure, or contacts with infected imported rodents.2, 3 The unexpected spread of the MPXV outbreak in multiple countries outside of Africa in 2022 raised concerns about a possible shift in transmission and pathogenic characteristics. Intense research is ongoing, and novel data are available with a better understanding of the clinical features of the disease, the pathobiology of the virus, and its tropism and transmission. Information on the immune response during the infection is still scarce or mainly limited to past studies when cross-reactive immunity from smallpox vaccination was predominant.4-6 immunoglobulin M (IgM) and IgG were detected after 5 and 8 days from the symptoms' onset, respectively.7 In addition, IgM response has been shown to be a possible biomarker for disease severity, with high IgG titers associated with mild illness: in a cohort of 200 patients infected with MPXV who were recruited between March 2007 and August 2011 in the Democratic Republic of Congo, individuals with both IgM and IgG responses were 5.09 times more likely to develop severe lesions compared with individuals who had an IgG-only response6; similarly, in a 2003 MPXV outbreak in the United States, patients with moderate/severe disease had an overall higher titer of antiorthopoxvirus IgM as compared with those with mild disease.4 Few data are available for IgA response, which could play a role in the effective host response as the human-to-human transmission of MPXV can occur through respiratory droplets in prolonged face-to-face contact with evidence of virus detection in mucosal and respiratory tract samples.8-12 We have recently reported the antibody kinetics in three mpox patients infected during the recent outbreak showing that IgM, IgA, and IgG antibodies were detected early within 2 weeks postsymptoms onset, with levels increased during the second week of illness and IgG reaching high titers.9 Even though serology has limited diagnostic utility and can be misleading due to immunological cross-reactivity between human-pathogenic orthopoxviruses and for smallpox vaccination,13 its profiling can help with diagnosis and expand understanding of MPXV pathogenesis and host response.
In this study, we enlarged the cohort and dissected the dynamics of the antibody response to 18 MPXV patients infected during the recent outbreak, evaluating specific IgM, IgA, IgG, and neutralizing antibodies (nAb) on longitudinal samples collected along the infection and investigating the humoral response in relation to patients' characteristics and disease course.
2 METHODS
2.1 Study group
Eighteen patients who tested positive for MPXV DNA at the National Institute for Infectious Diseases “Lazzaro Spallanzani” (INMI) in Rome, between May and July 2022 were randomly enrolled. Demographic and epidemiologic data, including clinical information, are shown in Table 1. All patients were male and self-reported as men who have sex with men (MSM), the median age was 38 years old (interquartile range [IQR]: 32.5–46). Two patients reported prior smallpox vaccination. Fourteen (77.8%) patients were human immunodeficiency virus (HIV) positive with a CD4+ T-cell lymphocyte median count at last observation of 686 cells/mm3 (IQR: 604.5–807.5); of these, seven (38.1%) patients reported other concurrent sexually transmitted infections (STI), including gonorrhea, syphilis, mycoplasma, and ureaplasma. All patients had a rash, and 17 (94.4%) had one or more systemic symptoms (i.e., fever, headache, sore throat, lymphadenopathy, myalgia, and diarrhea). Eleven patients were diagnosed with a severe infection, defined as having more than 20 skin lesions and/or hospitalization for complicated lesions localization (rectum, oropharynx, eye). Clinical recovery is defined by the date of the last scab fall. All patients recovered from MPXV infection after a median of 20 days (15.25–24) from symptom onset. Fourteen (77.8%) patients were hospitalized mainly for the management of pain, isolation purposes, or complications including secondary skin infections and affections such as pharyngotonsillitis, proctitis, and ocular involvement; six of them received treatment for MPXV infection, including tecovirimat (5/6) and cidofovir (1/6), and no adverse effects were reported.
| Characteristic | MPXV cases |
|---|---|
| Age (median, IQR) | 38 (46–32.5) |
| Gender (n, % male) | 18 (100) |
| Sexual orientation (n, % homosexual) | 18 (100) |
| Prior smallpox vaccination (n, % yes/no) | 2 (11.1)/16 (88.9) |
| Concomitant STIs (n, %) | |
| HIV | 14 (77.8) |
| Gonorrhea | 1 (6) |
| Syphilis | 2 (11.1) |
| Mycoplasma | 3 (16.7) |
| Ureaplasma | 1 (6) |
| Systemic MPXV (n, % yes/no) | 17 (94.4)/1 (6) |
| Systemic symptoms (n, %) | |
| Fever | 16 (94.1) |
| Headache | 5 (29.4) |
| Sore throat | 4 (23.5) |
| Lymphadenopathy | 11 (64.7) |
| Myalgia | 5 (29.4) |
| Diarrhea | 4 (23.5) |
| Rash (n, %) | 18 (100) |
| Type of rash (n, %) | |
| Macules | 1 (6) |
| Pustules | 15 (83.3) |
| Single ulcer | 1 (6) |
| Multi ulcer | 1 (6) |
| Number of lesions (n, %) | |
| <5 | 5 (27.8) |
| 5–10 | 2 (11.1) |
| 11–20 | 6 (33.3) |
| >20 | 5 (27.8) |
| Localization of lesions (n, %) | |
| Genital area | 12 (66.7) |
| Anal area | 12 (66.7) |
| Oral/nasal area | 6 (33.3) |
| Ocular area | 1 (6) |
| Hospitalization (n, % yes/no) | 14 (77.8)/4 (22.2) |
| MPXV treatment (n, % yes/no) | 6 (33.3)/12 (66.7) |
| Type of treatment (n, %) | |
| Tecovirimat | 5 (83.3) |
| Cidofovir | 1 (16.7) |
- Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; MPXV, mpox virus; STI, sexually transmitted infections.
Serum samples were longitudinally collected from all patients for a total of 64 samples and at least 2 serial samples from each patient. The time range of collection was from the day of symptom onset (DSO) up to DSO 20.
2.2 Anti-MPXV antibodies detection
Specific IgM, IgA, and IgG in serum were detected using an indirect immunofluorescence assay (IFA) as described elsewhere9 and based on slides prepared in-house with Vero E6 cells infected with an MPXV isolate from the skin lesion of a mpox patient infected during the 2022 outbreak (GenBank: ON745215.1, referred to the clinical sample). To augment sensitivity in detection, samples were assayed starting from a 1:10 dilution and antibody titers were established by limiting dilution up to 1:10 240. The assay protocol was preliminarily set-up using 30 serum samples from healthy donors born after 1970s (in order to avoid cross-reaction by historical smallpox vaccination) as negative controls. For IgG IFA set-up, serum samples from historically smallpox-vaccinated individuals were also useful as controls because of the described residual cross-humoral response.14 All the serum samples collected for this study and here presented were derived from polymerase chain reaction-confirmed mpox patients.
2.3 Plaque reduction neutralization test
nAb were measured in serum samples with either undetectable or low MPXV DNA load (cycle threshold > 35). Specifically, serum samples were heat-inactivated at 56°C for 30 min and titrated in duplicate in 4 four-fold serial dilutions (starting dilution 1:10). Each serum dilution was added to the same volume (1:1) of a solution containing 100 TCID50 MPXV isolate (GenBank: ON745215.1, referred to the clinical sample) from the 2022 outbreak, and incubated at 37°C for 2 h. Subsequently, 96-well tissue culture plates with subconfluent Vero E6 cell (ATCC) monolayers were infected with 120 μL/well of virus/serum mixtures and incubated at 37°C and 5% CO2. After 5 days, the neutralizing titers were estimated by measuring the plaques number reduction as compared to the control virus wells. Specifically, the supernatant of each plate was carefully discarded, and a crystal violet solution (Diapath S.P.A.) containing 10% formaldehyde (Sigma-Aldrich) was added to each well. After 30 min, the fixing solution was removed and the 96-well plates were washed using phosphate-buffered saline (PBS, 1X; Sigma-Aldrich). After drying, the number of plaques was counted using the Cytation 5 reader (Biotek). A sample was considered positive when the average number of plaques was lower than half the number counted in the virus control (at least a 50% reduction). The highest serum dilution showing at least 50% of the plaques number reduction was indicated as the 50% neutralization titer (PRNT50%). Each test included serum control (1:10 dilution of each sample tested without virus), cell control (Vero E6 cells alone), and virus control (100 TCID50 MPXV in sextuplicate). The assay protocol was preliminarily set-up using control samples as described for the IFA protocol.
2.4 Statistical analysis
Demographic and epidemiological characteristics of the patients were described using the median and IQR for continuous parameters and absolute and relative (percentage) frequencies for categorical variables. Antibody titers were described using the Kruskal–Wallis and Dunn tests to compare titers at different time ranges, while the Friedman–Dunn test was used when antibody titers were compared in different populations. Spearman test was performed to evaluate the correlation between the anti-MPXV IgG and nAb PRNT50 titers. Analyses were performed using GraphPad Prism version 9 (GraphPad Software) for Windows statistical software; p < 0.05 was considered statistically significant.
3 RESULTS
The specific humoral response against MPXV was evaluated on serum samples longitudinally collected and analyzed in relation to DSO grouped in different time ranges: 1–3, 4–7, 8–11, 12–15, and 16–20 days.
As shown in Figure 1, no antibody response was detected before 4 DSO. The overall trend showed that seroconversion occurred after 4–7 DSO for IgG and 8–11 DSO for IgM and IgA, with a median time of 7.5 (IQR: 5.3–9) DSO for IgG, 8 DSO for IgM (IQR: 5.5–9), and IgA (IQR: 5.3–10.5). nAb were detectable in samples collected as early as 1 week from symptoms, with stable levels up to 20 DSO.
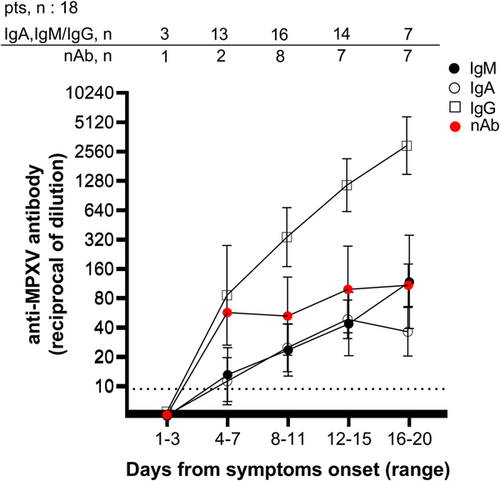
During the first week of the infection, IgG was detected in 83.3% (10/12) of the samples, while half were positive for IgM and IgA (Table 2 and Supporting Information: Figure 1). At 8–11 DSO, the rate of positivity increased for IgM (75.0%; 12/16), IgA (75%; 12/16), IgG (93.8%; 15/16), and nAb (87.5%; 7/8), and a further increase was observed at 12–15 DSO when 100% of the samples were positive for IgA, IgG, and nAb. At 15–20 DSO, 100% of serum tested was positive for all four immune markers (Table 2). Notably, in three patients, no IgM was detected on the samples available (collection time up to 7, 9, or 14 DSO), two of them were negative for IgA (collection time up to 6 DSO), and one showed no nAb (last sample at 6 DSO).
| Days | 1–3 | 4–7 | 8–11 | 12–15 | 16–20 |
|---|---|---|---|---|---|
| IgM GMT (95% CI) | b.d. | 13.5 (7.1–25.2) | 23.8 (12.9–43.8) | 44.2 (20.8–93.5) | 118.9 (39.5–357.7) |
| IgM % reactive (n/tot) | 0 (0/3) | 58.3 (7/12) | 75.0 (12/16) | 92.9 (13/14) | 100 (7/7) |
| IgA GMT (95% CI) | b.d. | 11.2 ( < 10–19.6) | 24.8 (14.1–43.8) | 48.8 (30.9–76.9) | 36.2 (20.4–64.5) |
| IgA % reactive (n/tot) | 0 (0/3) | 50.0 (6/12) | 75.0 (12/16) | 100 (14/14) | 100 (7/7) |
| IgG GMT (95% CI) | b.d. | 80.0 (24.4–262.4) | 320.0 (158.8–644.9) | 1103.0 (586.8–2075.0) | 2826.0 (1424–5609) |
| IgG % reactive (n/tot) | 0 (0/3) | 83.3 (10/12) | 93.8 (15/16) | 100 (14/14) | 100 (7/7) |
| nAb GMT (95% CI) | b.d. | n.a. | 51.9 (20.6–131) | 97.5 (34.9–272.6) | 107.7 (65.0–178.3) |
| nAb % reactive (n/tot) | 0 (0/1) | 50 (1/2) | 87.5 (7/8) | 100 (14/14) | 100 (7/7) |
- Note: Value of 5 was used for the samples resulted negative at the starting serum dilution 1:10.
- Abbreviation: 95% CI, 95% confidence interval; b.d., all samples below the detection limit (serum dilution = 1:10); GMT, geometric mean titers; IgM, immunoglobulin G; MPXV, mpox virus; n.a., not available; nAb, neutralizing antibodies.
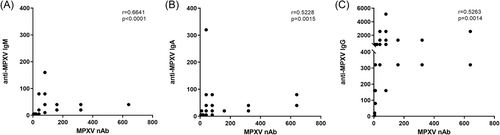
The antibody levels steadily increased following the onset of symptoms, with geometric mean titers (GMT) significantly higher during the second and third weeks, and IgG detected at higher levels than IgM and IgA (Figure 1). A significant correlation was observed between the anti-MPXV Ig and nAb PRNT50 titers (IgG, r = 0.5263, p = 0.0014; IgA, r = 0.5228, p = 0.0015; IgM, r = 0.6368, p ≤ 0.0001; Spearman correlation test) (Figure 2).
When analyzing factors potentially affecting the kinetics and strength of the humoral response, we did not observe differences associated with prior smallpox vaccination (2 vaccinated patients) or HIV status (14 HIV-positive out of 18 patients). Of note, one of the two smallpox-vaccinated patients showed no residual immunity from vaccination at the first examination on 4 DSO (all antibody classes were negative) (Supporting Information: Figure 1).
The antibody response was then assessed in relation to disease severity, which was divided into mild (n = 7) or severe (n = 11) cases (based on >20 lesions and/or hospitalization for complicated lesions localization, i.e., rectum, oropharynx, eye). Patients presenting severe symptoms showed a trend toward lower titers and rates of seroconversion after the symptoms onset, mainly in the very early phase of the disease (up to 8–11 DSO); however, no significant differences were observed as compared to the mild cases (Figure 3).
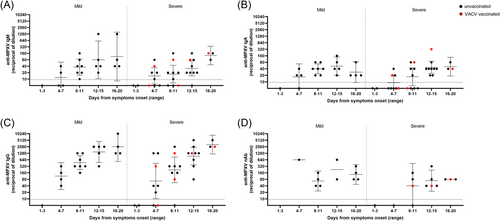
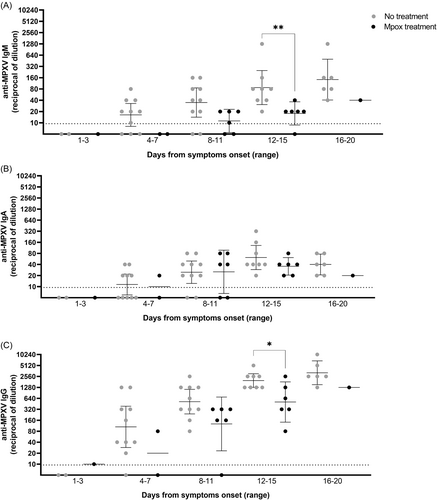
Finally, we analyzed the humoral markers in the six patients who received antivirals (cidofovir, n = 1, and tecovirimat, n = 5) after a median DSO of 11.5 (9.5–15.7) as compared to untreated patients. IgM and IgG showed significantly higher titers in untreated patients with respect to the treated patients in the time range after the therapy administration (12–15 DSO) (Figure 4). No differences were observed for IgA.
4 DISCUSSION
The recent mpox multicountry outbreak represented an important opportunity to study the human immune response against this virus in the era far from a smallpox vaccination campaign. We have previously described the kinetics of cellular immunity, the inflammatory profile, and pox-specific T-cell induction during the acute phase of infection in patients with laboratory-confirmed monkeypox, showing a prompt and powerful T-cell response regardless of HIV coinfection.15
The present study enlarges the findings we previously reported for three MPXV cases on the follow-up of the natural humoral response against MPXV,9 describing 18 mpox-infected patients, including the evaluation of nAb response, and investigating the antibodies levels in relation to patients' characteristics and disease course. Here, we report that MPXV infection leads to an effective functional antibody response during the acute phase of the disease, characterized by early seroconversion and production of IgG and nAb as early as 1 week from symptom onset. Antibody levels increased significantly during the second week of illness, with anti-MPXV IgG and nAb reaching highest titers during the third week from symptoms onset. The humoral response observed in our cohort of patients during the acute phase of infection seems not being influenced by HIV status and prior smallpox vaccination as reported in other studies.7, 16
IgM and IgG detection is considered helpful for the diagnosis of the MPXV infection, as the development of specific IgM or evidence of seroconversion in paired samples indicates recent exposure to orthopoxviruses (World Health Organization, 2022)17 and can be used to distinguish a natural response from vaccination.8, 18 Furthermore, we looked at the IgA response and, as we previously described,9 observed that kinetics and levels were comparable to IgM, but with a higher positivity rate during the acute phase of disease. Therefore, according to other recent data on MPXV8 and previous studies on other viruses such as SARS-coronaviruses,19, 20 IgA might represent a useful marker to be integrated in the mpox testing algorithms to assess recent infection in combination with IgM and IgG, especially in high-risk contacts of mpox cases to assess asymptomatic infections.
Previous data showed a correlation between the vaccinia virus-reactive IgG antibodies and MPXV nAb in infected patients and that MPXV infection boosted antibody levels in previously vaccinated individuals.14 In our study population, nAb titers significantly correlated to those of the specific IgG, IgM, and IgA, and MPXV infection induced specific antibody response regardless of previous smallpox vaccination. In fact, smallpox unvaccinated MPXV patients rapidly mounted nAb comparable to those of the two vaccinated patients, for whom no clear boosting effect of MPXV infection on antibody response was observed.
During the recent worldwide outbreak, tecovirimat and cidofovir have been used for severe cases of mpox, showing good tolerability and a possible favorable impact on the symptoms' improvement and viral load decline over the course of treatment,21-24 even though controlled evidence of antivirals effectiveness are still lacking. Interestingly, our analysis revealed that treated mpox patients showed a reduced production of specific antibodies as compared to the untreated patients. Although antiviral drugs can influence the antibody development25 and given the uncontrolled assumption of clinical benefit of tecovirimat and cidofovir on the mpox severity,21-24 our observation needs to be furtherly confirmed and investigated, as both drugs target viral replication with different mechanisms and play no direct role in the host immune response. In addition, these results might be independent from the therapy and might reflect the disease severity of the treated patients supporting the observation in Figure 2, showing reduced antibody levels in severe cases as compared to mild. While disease severity has been shown positively associated with cytokine storm occurring during human mpox disease, it remains unclear which humoral marker can be associated to disease outcome.26
The present study presents some limitations that should be acknowledged. First, the earlier detection and higher titers of specific IgG compared to IgM and IgA might be related to the presence of low levels of the latter classes of antibodies, with titers near to the detection limit of the method as suggested in other studies.8, 19, 20 Therefore, antibody kinetics should be confirmed using alternate methods of detection (e.g., enzyme-linked immunosorbent assay), which are nevertheless poorly available. The development of an international standard for MPXV serology will be beneficial to set up new methods and together with collaborative studies, will help to harmonize in-house assays that measure anti-MPXV antibodies. Second, the present was an observational study that included a small number of unbalanced patients, which limited the statistics and comparisons between different populations, precluding conclusions about the influence on the natural antibody response of the historical smallpox vaccination, HIV status, disease severity, and antiviral treatment. Furthermore, this study focused on the short-term immune response and follow-up of antibody kinetics over 3 weeks postsymptom onset is missing.
Further studies are crucial to replicate our observations on larger cohorts of MPXV patients and better define the specific humoral response in MPXV-infected patients.
In conclusion, these results contribute to extending the knowledge of the MPXV infection and the natural antibody response in a naïve population with no historic smallpox vaccination, thus supporting decision-making and the design of integrated diagnostic and surveillance algorithms for MPXV case management.
AUTHOR CONTRIBUTIONS
Francesca Colavita, Giulia Matusali, Silvia Meschi, Daniele Lapa, Aurora Bettini, Massimo Francalancia, Licia Bordi, Klizia Mizzoni, and Sabrina Coen were directly involved in the laboratory activities and performed the serological testing. Valentina Mazzotta and Carmela Pinnetti were directly involved in the patient care and collected clinical data. Francesca Colavita wrote the original draft. Francesca Colavita, Fabrizio Maggi, and Giulia Matusali reviewed and edited the manuscript. Fabrizio Maggi, Emanuele Nicastri, and Andrea Antinori supervised the activities. Francesco Vaia and Enrico Girardi acquired funding. All authors have read and agreed to the published version of the manuscript. All members of the “INMI mpox Study Group” were involved in patient care and microbiological experiments.
ACKNOWLEDGMENTS
We gratefully acknowledge the personnel of the Laboratory of Virology and the medical and nursing staff of the Infectious Diseases Clinical Unit. We acknowledge the “INMI mpox Study Group”: Isabella Abbate, Alessandro Agresta, Alessandra Amendola, Andrea Antinori, Francesco Baldini, Tommaso Ascoli Bartoli, Alessia Beccacece, Rita Bellagamba, Giulia Berno, Aurora Bettini, Nazario Bevilacqua, Licia Bordi, Marta Camici, Fabrizio Carletti, Angela Corpolongo, Stefania Cicalini, Francesca Colavita, Alessandra D'Abramo, Gabriella De Carli, Patrizia De Marco, Federico De Zottis, Lavinia Fabeni, Francesca Faraglia, Federica Forbici, Massimo Francalancia, Concetta Maria Fusco, Roberta Gagliardini, Anna Rosa Garbuglia, Saba Gebremeskel, Maria Letizia Giancola, Emanuela Giombini, Enrico Girardi, Giulia Gramigna, Elisabetta Grilli, Susanna Grisetti, Cesare Ernesto Maria Gruber, Eleonora Lalle, Simone Lanini, Daniele Lapa, Gaetano Maffongelli, Fabrizio Maggi, Alessandra Marani, Andrea Mariano, Davide Mariotti, Ilaria Mastrorosa, Giulia Matusali, Silvia Meschi, Valentina Mazzotta, Claudia Minosse, Klizia Mizzoni, Martina Moccione, Annalisa Mondi, Vanessa Mondillo, Nicoletta Orchi, Sandrine Ottou, Carmela Pinnetti, Silvia Pittalis, Vincenzo Puro, Silvia Rosati, Gabriella Rozera, Martina Rueca, Giuseppe Sberna, Laura Scorzolini, Eliana Specchiarello, Francesco Vaia, Francesco Vairo, Beatrice Valli, Alessandra Vergori, Serena Vita. This work was supported by the Italian Ministry of Health (Ricerca Corrente—lines 1 and 2), the European Commission–Horizon 2020 (European-Virus-Archive-GLOBAL—871029), and ISIDORe (European Union's Horizon Europe research and innovation programme under grant agreement number 101046133). Open access funding provided by BIBLIOSAN.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was included in the MonkeyCohort protocol: “Studio di coorte osservazionale monocentrica su soggetti che afferiscono per sospetto clinico o epidemiologico di malattia del vaiolo delle scimmie (Monkeypox)”; approval number 40z, Register of Non-Covid Trials 2022). The patients provided written informed consent to participate in this study.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the findings are available, only for sections noninfringing personal information, from the corresponding author upon reasonable request.