Antiviral effect of Favipiravir against Chandipura virus in vitro and in vivo
Abstract
Chandipura virus (CHPV) is an emerging encephalitic virus with outbreak potential in a pediatric population. It causes acute encephalitis, with clinical symptoms leading to death within 48–72 h and an alarmingly high case fatality rate up to 55%–78%. Despite the high mortality rate in children, no vaccines or antivirals are currently available; thus, repurposing licensed drugs seems to be one of the attractive therapeutic approaches. Among the various options available, Favipiravir emerged as a promising candidate, and its unique characteristics and clinical efficacy have garnered significant attention and demonstrated considerable potential in the fight against viral diseases. In the current study, we have evaluated the antiviral effect of Favipiravir against CHPV by Plaque reduction assay and viral growth kinetics assay in Vero cells and in vivo effect of drug treatment against lethal viral challenge was analysed in 10-day-old CD1 mice. A dose-dependent reduction in CHPV plaque size and number was observed in Vero cells treated with Favipiravir, with an EC50 of 92.26 μM. Complete inhibition of CHPV replication was observed at 320 μM drug concentration and 50% cytotoxicity (CC50) at 4774 μM, indicating a high selectivity index 51.24. In vivo, studies in mice showed 100% survival with 300 mg/kg/day of Favipiravir given orally till seventh-day postinfection. The study provides evidence of the antiviral activity of Favipiravir against CHPV infection, and further clinical evaluation may alleviate the associated mortality.
1 INTRODUCTION
Chandipura virus (CHPV) is a single-stranded negative-sense RNA virus belonging to the Vesiculovirus genus of the Rhabdoviridae family. The human case of CHPV was first identified in Maharashtra, India, in 1965 while investigating the Dengue and Chikungunya virus outbreak.1 In 2003, it emerged as a cause of an acute encephalitis outbreak in the paediatric age group documented from Andhra Pradesh, currently Telangana.2 Subsequently, in 2004, CHPV was identified as the cause of the focal encephalitis outbreak in Gujarat state, with a high case fatality rate approaching 78%.3 Furthermore, CHPV continued to cause recurring sporadic cases in Telangana, Maharashtra and Gujarat states4-7 and also reported from eastern India.8 The prevalence of CHPV-neutralizing antibodies in humans, domestic animals and Rhesus monkeys suggests its wide circulation.1, 6 The anti-CHPV antibodies have also been detected in wild macaques from Sri Lanka, and the virus has been isolated from phlebotomine sand flies in Senegal and hedgehogs from Nigeria, West Africa, underscoring the global circulation of the virus beyond India.9-11
The disease runs an acute course characterized by sudden onset of fever persisting for 2–3 days, headache, altered sensorium, seizures, diarrhoea, and vomiting. Most deaths occurred within 24–48 h of the onset of illness, and no sequelae were observed in survivors.3, 5 Treatment of CHPV encephalitis is mainly supportive, and vector control, specifically targeting sand flies, remains the only option to prevent the disease to some extent. Prolonging the disease course with some therapeutic intervention may allow the host system to develop a robust immune response. Thus, availability of safe therapeutic measures will help curtail the high mortality associated with CHPV infection.
Now a day's repurposing broad-spectrum antivirals is a rapidly evolving and promising strategy for developing effective treatments against viral diseases. Among these antivirals, Favipiravir holds significant potential. It is a prodrug that gets converted to an active nucleoside analogue within the human system. Through its binding to and inhibition of viral RdRp, it effectively hinders viral transcription and replication, as demonstrated in earlier studies.12, 13 It has shown broad-spectrum antiviral activity against lethal RNA viruses in experimental studies and has been used to treat human infections such as Ebola, Lassa fever, rabies etc., showcasing its efficacy.14-17 Studying its effectiveness against neurotropic viral infections is promising. Considering the rapid progress of the Chandipura disease to a fatal outcome and the lack of effective prophylactic or treatment protocols, this study evaluated the antiviral potential of Favipiravir against CHPV infection in mammalian cells and an experimental murine model.
2 MATERIALS AND METHODS
2.1 Cells and virus
Vero cell line (African green monkey kidney cell line, ATCC-CCL-81), maintained in minimal essential medium (MEM; Gibco) supplemented with 2.2 g/L NaHCO3, 0.292 g/L l-glutamine, 100 units/mL penicillin and 100 μg/mL streptomycin (Sigma Aldrich), and 10% foetal bovine serum (Gibco), i.e., growth medium, was used for the cell line passaging, and maintenance. CHPV strain (NIV ID: CHPV-1511584, Human, India, 2015) isolated from the serum of a CHPV encephalitis case, maintained at the lowest passage level, was used for all in vitro and in vivo studies.
2.2 Reagents and chemicals
Favipiravir drug substance was purchased from MedChemExpress, (NJ). For drug dilution, dimethyl sulfoxide (DMSO) (Sigma Aldrich) was used; for different in vitro assays, subsequent desired drug dilutions were done in MEM (Sigma) while ensuring that the DMSO percentage remained ≤1%. For oral dosing of mice during in vivo studies, Favipiravir was diluted in 0.4% carboxymethylcellulose (CMC). 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) (Sigma Aldrich) was used for assessment for Favipiravir cytotoxicity in Vero cells.
2.3 Animals
After acclimatization, for 7 days, 10-day-old CD1 mice were transferred to the experimental area, where they were placed in polycarbonate cages. The experimental animals were housed per standard laboratory conditions of temperature, relative humidity and 12 h light and dark cycle with the ad libitum food and water supply.
2.4 Assessment of cytotoxicity
The toxicity of Favipiravir in Vero cells was determined by an MTT assay, as described earlier,18 using different drug concentrations (1, 5, 10, 50, 100, 500, 750, and 1000 µg/mL) in quadruplicate as three independent experiments. The absorbance values were expressed as percentages of untreated and drug-treated cells, and 50% cell cytotoxicity (CC50) values were calculated.
2.5 In vitro activity of Favipiravir against CHPV
2.5.1 Virus propagation
As described earlier, the CHPV was propagated in Vero cells to prepare reference virus stock for different in vivo and in vitro assays.19 The reference stock was aliquoted as 0.5 mL each and stored at −70°C till further use.
2.5.2 Plaque assay for quantitation of virus stock
The reference virus stock of CHPV was quantitated using plaque assay in Vero cells as described earlier.19 On 36 h postinfection (PI), the assay was terminated, and plaques were counted manually. The virus titre was expressed as plaque forming unit (PFU)/mL of virus stock.
2.5.3 In vitro activity of Favipiravir using plaque reduction assay
In a preliminary experiment, the effect of Favipiravir on the growth of CHPV in Vero cells was analyzed using a plaque reduction assay. Briefly, Vero cells were seeded in the 24-well tissue culture plate at a concentration of 0.25 × 106 cell/mL in growth medium. After 24 h, when the confluent monolayer was formed, the wells were infected with 50 PFU of CHPV/100 µL, diluted in assay diluent (MEM + 2% fetal bovine serum [FBS]). The virus was allowed to adsorb for 1 h at 37°C in a CO2 incubator, with intermittent plate rotation. The 100 µL of assay diluent was added to the drug and cell control wells. Subsequently, the inoculum was removed, and the wells were overlaid with 1 mL of overlay medium. The drug treatment wells (virus-infected followed by drug overlay) received the overlay with different drug concentrations (1, 5, 10, 20, 50, and 100 μg/mL), while the drug control wells received 50 and 100 μg/mL in duplicate. The virus and cell control wells received a plain overlay medium. The plates were incubated for 36 h at 37°C in a CO2 incubator. After incubation, the assay was terminated, and plaques were counted, as described earlier in the plaque assay method. The EC50 of Favipiravir was calculated using GraphPad Prism software Version 8.0.1. Three independent assays were performed to ensure the statistical significance of the observed data.
The effect of a drug on CHPV plaque size was measured by estimating the plaque radius using NIS-Elements-BR software, version 4.0 image analysis software, using Nikon Eclipse TS100 inverted microscope at ×4 magnification. To evaluate the impact of the drug on CHPV plaque size, the diameter of 20 individual plaques from the untreated virus control wells (in duplicate) and the plaques from wells treated with various drug concentrations followed by virus infection (in duplicate) were analyzed and compared. For wells where the number of plaques formed was less than 20, the size of all plaques was estimated.
2.5.4 Effect of Favipiravir on growth kinetics of CHPV in Vero cells
The growth kinetics of CHPV in the presence of different drug concentrations (10, 20, 40, 80, 160, and 320 µM) was carried out in Vero cells. Initially, Vero cells were infected with 0.01 moi of CHPV for 1 h at 37°C in a CO2 incubator. The control cells were treated with MEM. After infection, cells were washed thrice with MEM and seeded in quadruplicate in a 24-well plate, with a culture medium containing MEM + 2% FBS at a concentration of 0.25 × 106 cells per well. The cells were then further incubated for 48 h. The well designated for drug treatment received the medium with the desired drug concentration, while the virus and cell control wells received the medium without the drug. At different time points (0, 4, 12, 18, 24, and 48 h PI), the culture supernatant from respective wells was harvested and stored at −80°C until used for virus quantitation by 50% tissue culture infective dose (TCID50) assay. For the TCID50 assay, 4 × 104 cells/well/100 µL in growth medium were seeded in 96-well tissue culture plates and incubated at 37°C for 24 h in a CO2 incubator to form a confluent monolayer. The culture supernatant was removed, and 10-fold serial dilutions of previously collected culture supernatant were added to the respective wells in quadruplicate. The cell control wells received only MEM + 2% FBS. The plates were incubated for 36 h at 37°C in a CO2 incubator, followed by termination of the assay as described earlier in the plaque assay protocol. The wells were scored for % cytopathic effects, and TCID50 titer was calculated using Reed and Muench method.20
2.6 Antiviral effect of Favipiravir against CHPV in experimental animals
Experimental infection of CHPV in mice shows age and route of inoculation-dependent susceptibility. In infant mice, CHPV infection through intravenous (iv) or intraperitoneal (ip) routes results in prominent neurological clinical symptoms followed by death within 4–5 days. However, adult mice are susceptible to infection only through the intracerebral (ic) route.21, 22
2.6.1 Experimental animal model and lethal viral dose selection
To evaluate the antiviral activity of Favipiravir against CHPV infection, an experimental model was established by inoculating CD1 infant mice of various ages with different routes and doses of CHPV. The lethal dose of CHPV in the infant mouse model was determined by inoculating 10- and 12-day-old CD1 mice with 1000 and 10 000 PFU via the intraperitoneal (IP) or subcutaneous (SC) route. The mice were closely observed for the CHPV associated neurological symptoms and mortality.
Based on the results, the combination of animal age and virus dose that resulted in 100% mortality was selected for further experiments to assess the antiviral efficacy.
2.6.2 In vivo antiviral activity
The experimental design for analysis of the antiviral activity of Favipiravir included six groups of 10-day-old CD1 mice (n = 8 per group), which is mentioned below.
Group A: virus control: (infected with 10 000 PFU of CHPV through ip route) on day 1 of the experiment plus 30 μL of 0.4% CMC, given orally from the second to the seventh day of the experiment.
Group B: infected with 10 000 PFU of CHPV through ip route on day 1, plus Favipiravir at 100 mg/kg/day given orally from the second to the seventh day of the experiment.
Group C: infected with 10 000 PFU of CHPV through ip route on day 1, plus Favipiravir at 300 mg/kg/day given orally in 30 μL volume from the second to the seventh day of the experiment.
Group D: drug control: 50 μL of Saline solution through ip route on day 1, plus Favipiravir at 100 mg/kg/day given orally in 30 μL volume from the second to the seventh day of the experiment.
Group E: drug control: 50 μL saline solution through ip route on day 1, plus Favipiravir at 300 mg/kg/day given orally from the second to the seventh day of the experiment.
Group F: vehicle control: 50 μL saline solution through ip route on day 1, plus 30 μL of 0.4% CMC, given orally from the second to the seventh day of the experiment.
Animals were observed twice daily till day 21 PI for the CHPV-associated overt clinical signs and mortality. Sera collected from survived animals from Groups B and C at the end of the experiment were analyzed for the presence of anti-CHPV neutralizing antibodies using plaque reduction neutralization test (PRNT) as described earlier19 with slight modification in assay termination time limited to 36 h PI with CHPV.
2.6.3 In vivo growth kinetics of CHPV
To study the effect of a protective dose of Favipiravir on CHPV replication, the animals were challenged with 10 000 PFU of CHPV. Subsequently, the animals received drug treatment from the second to the seventh day of PI. On days 4, 8, and 21, PI, brain and other vital organs were harvested from four mice per group. Except in Group I, where all animals succumbed to infection by day 6, the organs were collected on day 4, and terminally ill animals were included on day 6. The experimental setup included four groups of 10-day-old CD1 mice, each comprising 16 mice per group, as mentioned below.
Group I: virus control: (infected with 10 000 PFU of CHPV through ip route) on day 1 of the experiment plus 30 μL of 0.4% CMC, given orally from the second to seventh day of the experiment.
Group II: infected with 10 000 PFU of CHPV through ip route on day 1, plus Favipiravir at 300 mg/kg/day given orally from the second to the seventh day of the experiment.
Group III: drug control: 50 μL of Saline solution through ip route on day 1, plus Favipiravir at 300 mg/kg/day given orally in 30 μL volume from the second day to seventh day of the experiment.
Group IV: vehicle control: 50 μL of Saline solution through ip route on day 1, plus 30 μL of 0.4% CMC, given orally from the second to the seventh day of the experiment.
The collected organs were stored at −70°C till further use. The 10% wt/vol organ suspension was made in MEM by trituration in a chilled mortar pestle. Tissue suspension was centrifuged at 3000g for 10 min at 4°C, the supernatant was used to quantify CHPV by TCID50 assay in quadruplicate as described earlier.7
2.7 Statistical analysis
All statistical analysis was carried out using GraphPad Prism V.8.0.1 software. The significance of drug treatment on virus titer and plaque size was analysed using a two-tailed paired t-test. The dose-response curve and EC50 calculations were carried out using nonlinear regression analysis.
3 RESULTS
3.1 Virus titration
The titer of reference virus stock was estimated as 4 × 108 PFU/mL, which was further used to calculate the moi for the growth kinetics assay and calculation of challenge dose during in vivo experiments.
3.2 Cytotoxicity of Favipiravir in Vero cells
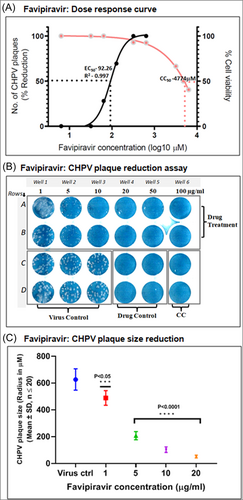
The results of percent viability of Vero cells treated with different concentrations of Favipiravir showed no toxicity up to 10 µg/mL as estimated by MTT assay. Compared to the nontreated control cells, 8% cytotoxicity was recorded in cells treated with 100 µg/mL, while 50% cytotoxicity (CC50) concentration was around 750 µg/mL, corresponding to 4774 μM concentration of Favipiravir (Figure 1A).
3.3 Antiviral effect of Favipiravir in Vero cells
The antiviral effect of Favipiravir was evaluated by plaque reduction assay on CHPV infected cells exposed to different drug concentrations. The potential effect of Favipiravir treatment on CHPV plaque formation was evident even at the lower concentrations (5–10 µg/mL corresponding to 31.83–63.65 µM) in the form of a gradual reduction on average plaque size. However, at lower drug concentrations, CHPV, the number of plaques formed at 5–10 µg/mL of the drug remained the same as that of virus control and 1 µg/mL treated wells. The 20 µg/mL (127.3 µM) drug concentration was found to reduce the plaque number by 70%, while complete inhibition of CHPV plaque formation was evident in cells exposed to 50 µg/mL (318.3 µM) and 100 µg/mL (636.5 µM) Favipiravir (Figure 1B). The estimation of EC50 using the nonlinear regression method using GraphPad Prism 8.0.1 showed 50% of the maximal response of the drug at 92.26 µM concentration (Figure 1A).
A significant effect on CHPV plaque number and size was observed with the increasing concentration of drug treatment in infected Vero cells (Figure 1B,C). As compared to the average plaque size (radius in micrometer) (627 ± 79.64 µm: mean ± SD) in virus control wells, a 67% average size reduction was observed in cells treated with 5 µg/mL (31.83 µM) drug, which further reduced to 91% (51.75 ± 10.54 µm: mean ± SD) in cells treated with 20 µg/mL (127.3 µM). The reduction in plaque size from 5 to 20 µg/mL was highly significant with a p < 0.00001, and the reduction in 1 µg/mL was significant with a p < 0.05.
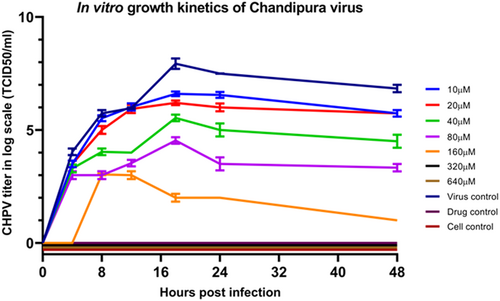
Growth kinetics of CHPV in Vero cells at 0.01 moi, when treated with different concentrations of Favipiravir, showed a substantial reduction to complete inhibition of virus growth in the drug dose-dependent manner (Figure 2). At 80 µM (12.56 µg/mL) drug concentration, a 5 log reduction in CHPV titer at 24 h PI was evident, which yielded virus titer of 103.5TCID50/mL as against the peak titer of 107TCID50/mL measured in the virus control. Complete inhibition of CHPV multiplication was observed at a concentration ≥320 µM (50.27 µg/mL) (Figure 2).
3.4 Therapeutic efficacy of Favipiravir in mice model
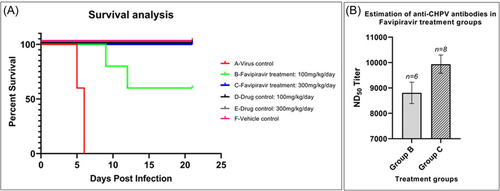
The antiviral activity of Favipiravir against CHPV infection was assessed in experimental animals. Twelve-day-old CD1 mice were inoculated with 1000 PFU of CHPV via the sc route, resulting in no mortality until day 21 postinoculation. In contrast, mice inoculated intraperitoneally showed 90% mortality by days 7–8 postinoculation. However, when 10-day-old CD1 mice were inoculated with 1000 PFU of CHPV via the sc or ip routes, mortality rates of 90% and 100%, respectively, were observed by day 6 postinoculation. To further evaluate the efficacy of the drug, 10-day-old mice were challenged with a 10X higher dose of CHPV (10 000 PFU) via the ip route. Treatment with Favipiravir from the second to seventh day PI, at doses of 100 mg/kg/day (Group B) and 300 mg/kg/day (Group C), resulted in 75% and 100% survival, respectively. The virus control group (Group A) showed 100% mortality by day 6 after the challenge. In contrast, the drug control group (Groups D and E) and the vehicle control group (Group F) did not exhibit any mortality (Figure 3A). Sera collected from surviving mice in Groups B and C showed significant anti-CHPV neutralizing antibodies (ND50) titers of 8807 ± 421 and 9936 ± 361.3, respectively, as measured by PRNT (Figure 3B). The presence of these neutralizing antibodies in blood collected 21 days after the challenge indicates the development of an immune response against CHPV, leading to protection in the animals.
The effect of a protective dose (300 mg/kg/day) of Favipiravir on in vivo CHPV replication showed the absence of the virus in the brain and other organs on days 4, 8, and 21 PI, suggesting the clearance of infectious virus within the first 3 days of treatment (Group II). However, untreated mice (Group I) showed the presence of high virus titer: 107 ± 0.4, 104 ± 0.36, and 103 ± 0.7 TCID50/mL titer (mean ± SD) in the brain, spleen and serum, respectively, on day 4 PI and 108.5TCID50/mL titer in the brain at day 6 PI (terminally ill animals). Since all the mice in the virus control group died by day 6, it was not possible to evaluate the quantitative distribution of CHPV in different body organs at later time points.
4 DISCUSSION
Chandipura encephalitis is a re-emerging viral disease primarily affecting the paediatric population and has been associated with significant mortality rates, reaching as high as 78%.13 The propensity of the CHPV to cause explosive outbreaks and rapidly progress to a fatal outcome emphasizes the urgent need for research to develop effective vaccines or appropriate antiviral treatments.6 CHPV carries a non-segmented, negative sense, single-stranded RNA genome, which undergoes transcription and replication using viral RNA-dependent RNA polymerase (RdRp). RdRp is a conserved enzyme in different RNA viruses. Thus, antivirals with known inhibitory activity against RdRpare being extensively studied for repurposing against fatal viral infections. A clinical trial of Favipiravir against various RNA virus infections, including COVID-19, suggests its potent antiviral action with significantly fewer adverse reactions than other RdRp inhibitors.13, 23 The CC50 evaluation of Favipiravir in Vero cells indicates that it falls within the acceptable range of toxicity (750 µg/mL). Similar findings have been documented in various mammalian cell lines, including Vero, MDCK, HEL, A549, HeLa, HEp-2, and Caco-2 cells.24 CHPV is a rapidly replicating virus, and in mammalian cells, the CPE becomes noticeable within 4–8 h PI, and complete CPE is typically observed within 16–24 h PI.25 Hence, the in vitro antiviral effect of CHPV infection in Vero cells was assessed for 48 h following drug treatment. Dose-dependent inhibition of CHPV replication was observed in Vero cells. The effective concentration of Favipiravir, resulting in 50% CHPV plaque reduction (EC50), was estimated to be 92.26 µM. In addition, 100% inhibition of CHPV plaque formation was evident at 318.3 µM as established by plaque reduction assay (Figure 1A,B) and viral growth kinetics performed in the presence of different drug concentrations (Figure 2). Differential range of in vitro antiviral activity of Favipiravir against different RNA viruses has been established with the EC50 in different cell types ranging from 0.45 to 335 μM.24, 26-32 As evident from our data, the EC50 concentration against CHPV was found to be in the noncytotoxic range. The results obtained in our primary screening by applying the quantitative assay like plaque reduction assay showed its potential in substantially reducing the virus replication. A drug with an selectivity index (SI) value of >10 is considered a good candidate.33 In the current study, the SI of Favipiravir, i.e., the ratio of CC50/EC50 (4774 μM/92.26 µM), was found to be 51.74, indicating a wide therapeutic safety margin. In addition to reducing the number of CHPV plaques formed, we also documented the steep reduction in the plaque size with increasing drug concentrations. As compared to the size of plaques formed (627 ± 79.64 µm: mean ± SD µm) in virus control wells, we documented >91% reduction in plaque size at 127.3 µM concentration of Favipiravir (Figure 1C). The size of plaques formed under the methylcellulose overlay is influenced by how quickly the virus multiplies and spreads between neighboring cells in the monolayer. Besides inhibiting virus multiplication, Favipiravir's antiviral activity can also limit virus spread and infection under the CMC overlay. Therefore, when evaluating antiviral activity, a reduction in the number and size of plaques can serve as a valuable indicator of antiviral effectiveness.34 The high SI values obtained in the plaque reduction assay prompted us to evaluate the quantitative effect on the CHPV replication. The in vitro growth kinetics assay performed in Vero cells using 0.01 moi of CHPV showed dose-dependent antiviral activity with complete inhibition of virus replication ≥320 μM concentration (Figure 2).
In vivo, assessment of the antiviral activity provides better insights into the efficacy and information on drug dosing and any adverse side effects caused in animals. CHPV is a neurotropic virus that causes age-dependent acute infection in mice upon inoculation by different routes. Clinical symptoms in CHPV infected mice are characterized by ruffled fur, hunched posture, ataxia, hyperesthesia, convulsions and quadriplegia, leading to death within 4–5 days of inoculation.6, 21, 22, 35 While establishing the animal model necessary for evaluating the inhibitory activity of Favipiravir against actively multiplying CHPV, we documented the age and route of virus inoculation dependent susceptibility. Independent of age, mice inoculated by the subcutaneous route resulted in partial mortality compared to the intraperitoneal route of infection. Twelve-day-old CD1 mice were refractory for CHPV infection by the sc route and showed delayed and partial mortality upon inoculation by the intraperitoneal route. The present study established that the 10-day-old CD1 mice inoculated with 1000 PFU of CHPV by ip route showed 100% mortality by days 5–6 postchallenge. For in vivo efficacy study, we have used 10 LD100 as a challenge dose of CHPV by ip route. Starting from day 2 PI, the groups of mice infected with CHPV were treated with oral doses of 100 and 300 mg/kg/day (equivalent to the recommended dose for COVID-19 cases) until day 7.36 Mice groups treated with 300 mg/kg/day dose survived till the end of the experiment, while mice groups treated with 100 mg/kg/day per os showed partial protection. The presence of anti-CHPV neutralizing antibodies in mice that survived till day 21 posttreatment suggests that the Favipiravir treatment in CHPV-challenged mice allowed the generation of antiviral immunity along with its RdRP inhibitory effect resulting in 100% protection, particularly with 300 mg/kg/day dosage group.
A similar study published recently using an immunodeficient mouse model: severe combined immunodeficiency (SCID) also revealed the potent antiviral activity of Favipiravir against CHPV infection.37 However, the SCID mice lacks the compounding effect of host immune response, which results in the death of animals in the treatment group upon drug discontinuation. In the current study, an infant mouse model was used to evaluate the efficacy of Favipiravir using a prime-type strain of CHPV (a genetic variant of CHPV) isolated from fatal case (during 2015). The treatment of the infant mice with 300 mg/kg/day of Favipiravir until day 7 PI resulted in the protection of treated animals until the end of the experimental period, i.e., 21 days PI. The challenge dose of 10 000 PFU via the intraperitoneal route mimicked the natural way of infection. Evaluation of anti-CHPV antibodies in the serum of survived animals from treatment groups also revealed the development of neutralizing immune response against CHPV, which will further help protect the infected population. In natural infection, the innate and adaptive immune response plays a crucial role in outcome of the disease. Our earlier studies also indicated the role of B-cells and the monocyte population in the primary replication of CHPV.38 Thus, using a non-immuno-compromised mouse model with a peripheral route of infection mimics the natural infection aptly. To summarize, the infant mouse model proves to be a valuable approach for studying antiviral efficacy against CHPV. Nevertheless, the accurate dosing of infant mice requires skilled technical expertise.
The in vivo viral growth kinetics study, using a protective drug dose, i.e. 300 mg/kg/day (Group II), revealed the prevention of neuro-invasion by CHPV. This was evidenced by the absence of the virus in the brains of drug-treated mice at days 4, 8, and 21 PI, in contrast to the high viral load detected in the brains of the virus control group (Group I) on days 4 and 6. Similarly, the virus was not detected in any of the organs of drug-treated mice, but in untreated mice, as compared to the brain, low titre virus was detected in the spleen and serum. In conclusion, this study provides evidence of the potential antiviral activity of Favipiravir in in vitro and preclinical infant mouse models. Clinicians can further utilize these findings to evaluate the potential of Favipiravir during CHPV infection.
AUTHOR CONTRIBUTIONS
Vijay P. Bondre: performed study conception and experiment supervision. Daya V. Pavitrakar: wrote first draft of the manuscript and performed experimental design, material preparation, data collection, and analysis. All authors commented on previous versions. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Priya Abraham, Director, ICMR-NIV, Pune, for her encouragement and meaningful insights. Thanks to Mr. Kunal Sakhare for technical support and Dr. Chandhu Balachandran for the critical evaluation of the manuscript. This work was supported by the Indian Council of Medical Research (ICMR) through NIV Project ID: ENC2002.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
All experimental work was carried out with prior approval from Institutional Biosafety Committee. The animal experiments were conducted as per guidelines and approved by the Institutional Animal Ethics Committee under project ID–ENC2002.
Open Research
DATA AVAILABILITY STATEMENT
The manuscript includes all the data generated or analyzed during this study.