Transcranial magnetic stimulation in assessment of vocal cord paralysis due to post-viral (COVID-19) vagal neuropathy
Coronavirus disease (COVID-19), caused by SARS-CoV-2, may be asymptomatic or could cause mild to severe symptoms affecting the respiratory system. Approximately 22%–27% of patients present with laryngeal-related symptoms, including dysphonia or aphonia, hoarseness, voice fatigue,1, 2 vocal fold paralysis, and paresis.3 Vocal cord paralysis, probably caused by postviral vagal neuropathy resulting in dysfunctional vocal cord mobility, has been confirmed by laryngeal electromyography (EMG) in patients with no history of intubation recovering from COVID-19.3
Many individuals suffering from COVID-19 have reported persistent symptoms and/or complications lasting beyond 4–12 and >12 weeks, which is now called ongoing symptomatic COVID-19, long COVID-19 or post-COVID-19 syndrome.4, 5
The primary motor cortex for laryngeal motor representation (M1) plays an important role in human voice and speech production. To date, methodologies for mapping the M1 with transcranial magnetic stimulation (TMS) have been previously developed with motor evoked potentials (MEPs) recorded from laryngeal muscles in healthy subjects, neurosurgical patients, and patients with laryngeal dystonia.6-8 Assessment of MEP response represents the final pathway of spatial and temporal summation of several descending volleys activating the α-motoneurons, thus reliably reflecting the excitation state of the M1, cortico-bulbar cells for the vagal nerve, the corticobulbar tract, the peripheral recurrent laryngeal nerve, and the target laryngeal muscle.8, 9
The present study shows a case of a 39-year-old female who has recovered from COVID-19 twice, in whom paralysis of the left vocal cord occurred after the second COVID-19. Video laryngoscopy and stroboscopic examination revealed paralysis of the left vocal cord with an intermediate (cadaveric) position (Figure 1A). Acoustic voice analysis showed pathological voice findings for jitter (1.15%), shimmer (1.182 dB), and noise-to-harmonic ratio (NHR) (8 dB). Three weeks before the hospital admittance and examination, upper respiratory tract infection symptoms were reported, followed by headache, burning sensation in the throat, and nasal secretion. Laryngeal symptoms (dysphonia) was developed on the third day of the second COVID-19. The patient had no known history of surgery, chronic disease, or trauma. Neurological examination and brain magnetic resonance imaging revealed no signs of neurological deficits or other cranial/peripheral nerve involvement. A CT scan of the neck and chest showed no lesions or masses. Left vocal cord paralysis was still evident 5 months after the first examination. Eleven months after the first examination, laryngoscopy and stroboscopic examination showed symmetric and consistent vocal cords mobility with complete adduction during phonation (Figure 1B). In addition, the voice analysis measures were normal for jitter (0.428%), shimmer (0.251 dB), and NHR (19.882 dB).

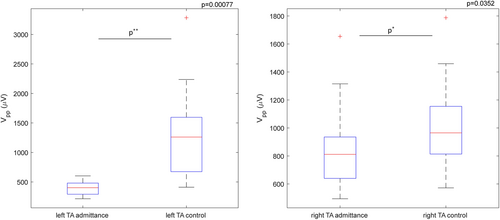
E-field navigated TMS (Nexstim Plc.) was used as a nonstandard neurophysiologic technique to evaluate the excitability of the corticobulbar tract by recording the MEP from thyroarytenoid (TA) muscle and estimating the MEP peak-top-peak amplitude.6-8 MEP amplitude measures a nerve's signal strength pathway response (detected on a TA muscle) after applying TMS (an excitation) on the M1 region. The TMS was applied over the left and right M1 for TA muscle representation, and contralateral MEPs were recorded, first at the hospital admittance and then eleven months after. The disposable paired hook-wire electrodes (type 003-400160-6, SGM d.o.o.) were inserted in the TA muscle using a transcutaneous approach via a cricothyroid membrane.6, 7 A single-pulse TMS was applied with a figure-of-eight coil, and 5 out of 10–20 repeatable MEPs were collected.6, 7 The magnetic stimulation intensity was 160%, regarding the resting motor threshold for the upper extremity muscle.8, 9 The results of MEP amplitude estimation showed that the right hemispheric M1 stimulation elicited MEPs, from the left TA, with peak-to-peak MEP amplitude significantly (t(32) = −3.71, p < 0.001) lower at the patient's hospital admittance (mean: 396.02 µV) compared to the control examination (mean: 1298.36 µV) (Figure 2). Further, the left hemispheric M1 stimulation for TA muscle representation elicited MEPs in the right TA muscle significantly (t(53) = −2.16, p < 0.03) lower at the patient's hospital admittance (mean: 843.99 µV) compared to the control examination (mean: 1002.28 µV) (Figure 2).
Therefore, to our knowledge, the present case report shows the first application of TMS methodology to assess corticobulbar excitability in TA muscles6-8 in a single patient with vocal cord paralysis after suffering COVID-19. Additionally, the radiological absence of CNS, neck, and thorax processes and no history of endotracheal intubation with positive antibody testing indicate that unilateral left vocal cord paralysis was due to postviral (COVID-19) vagal neuropathy.
To summarize, noninvasive e-field navigated TMS has real-time features: coil position, magnetic field orientation, and point on the cortex where the stimulus was delivered. In the presented case report, the stimulated spots from the first examinations were repeated on the control examination to evaluate the corticobulbar tract excitability. Navigated TMS may well be suited in laryngological research and clinical settings for evaluating COVID-19-related symptoms due to: (a) the precision of the system in visualizing individual brain M1 anatomy, (b) the possibility of investigating functional cortical maps for single muscle, (c) the possibility to stimulate and repeat the positive hot spot for the monitoring purposes.10 Future studies can use the navigated TMS technology8 with presented methodology for assessing corticobulbar excitability by recording MEPs6-8 on a larger number of patients with vocal cord paralysis after post-COVID-19 to prove its clinical utility. Finally, navigated TMS methodology8 can be compared to laryngeal EMG11 in predicting the vocal function recovery.
AUTHOR CONTRIBUTIONS
Braco Bošković: conception, and design, conduct, interpretation of results, critically revising and approving all aspects of the final manuscript. Irena Bilić: interpretation of results, conduct, critically revising, and approving all aspects of the final manuscript. Joško Šoda: conduct, analysis, drafting manuscript, critically revising and approving all aspects of the final manuscript. Ivana Kero: drafting the manuscript and conduct. Maja Rogić Vidaković: conception, and design, conduct, analysis, drafting the manuscript, approved all aspects of the final manuscript.
ACKNOWLEDGMENTS
We thank the patient for granting permission to publish this information.
CONFLICT OF INTEREST STATEMENT
The author declare no conflicts of interest.
ETHICS STATEMENT
All the procedures performed in the studies that involved human participants followed the ethical standards of the Ethics Committee of the University of Split, School of Medicine (Class: 003-08/22-03/0003, No.: 2181-198-03-04-22-005) the Ethical Committee of the University Hospital of Split (Class: 500-03/21-01/184, No.: 2181-147/01/06/M.S.−21-02), and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The subject gave written consent by her signature to participate in the study.
Open Research
DATA AVAILABILITY STATEMENT
All data generated during this study may be available on request.