Comparison of the immunogenicity of nasal-spray rVSV vector, adenovirus vector, and inactivated COVID-19-based vaccines in rodent models
Yuhang Zhang, Jiandong Liu, Hongyue Li, and Fei Yuan contributed equally to this study.
Abstract
Intranasal (i.n.) vaccines can induce mucosal and systemic immunity against respiratory pathogens. Previously, we demonstrated that the recombinant vesicular stomatitis virus (rVSV)-based COVID-19 vaccine rVSV-SARS-CoV-2, with poor immunogenicity via the intramuscular route (i.m.), is more suitable for i.n. administration in mice and nonhuman primates. Here, we found that the rVSV-SARS-CoV-2 Beta variant was more immunogenic than the wild-type strain and other variants of concern (VOCs) in golden Syrian hamsters. Furthermore, the immune responses elicited by rVSV-based vaccine candidates via the i.n. route were significantly higher than those of two licensed vaccines: the inactivated vaccine KCONVAC delivered via the i.m. route and the adenovirus-based Vaxzevria delivered i.n. or i.m. We next assessed the booster efficacy of rVSV following two i.m. doses of KCONVAC. Twenty-eight days after receiving two i.m. doses of KCONVAC, hamsters were boosted with a third dose of KCONVAC (i.m.), Vaxzevria (i.m. or i.n.), or rVSVs (i.n.). Consistent with other heterologous booster studies, Vaxzevria and rVSV elicited significantly higher humoral immunity than the homogenous KCONVAC. In summary, our results confirmed that two i.n. doses of rVSV-Beta elicited significantly higher humoral immune responses than commercial inactivated and adeno-based COVID vaccines in hamsters. As a heterologous booster dose, rVSV-Beta induced potent, persistent, and broad-spectrum humoral and mucosal neutralizing responses against all VOCs, highlighting its potential to be developed into a nasal-spray vaccine.
1 INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the ongoing Coronavirus disease 2019 (COVID-19) global pandemic that began in 2019.1 The seroprevalence of SARS-CoV-2 was estimated to be 94% in the United States on November 9, 2022.2 The dynamic zero-COVID policy in China has been terminated, and most of the global population has been infected at least once. As of February 17, 2023, over 6.8 million deaths have been reported worldwide (https://covid19.who.int/), and the COVID-19 pandemic is not over. As of December 2022, the number of weekly deaths from COVID-19 in the United States remains above 2000 without obvious seasonal dependence (https://covid.cdc.gov/).
SARS-CoV-2 accumulates genomic mutations over time. A series of SARS-CoV-2 variants have emerged since the end of 2020. Of these, five (Alpha, Beta, Gamma, Delta, and Omicron) were previously classified as variants of concern (VOCs; VOCs here and below represent previously or presently circulating variants unless noted otherwise.) due to evidence of increased transmissibility, disease severity, and a significant reduction in neutralizing antibodies (NAbs) and vaccine effectiveness. All these VOCs share the N501Y mutation in the receptor-binding domain (RBD), which increased virus transmission by 40%–70%.3 Two additional mutations appeared in the RBD of Beta, Gamma, and Omicron: K417 (K417T in Gamma; K417N in Beta and Omicron) and E484 (E484K in Beta and Gamma; E484A in Omicron), which are potentially responsible for immune escape from antibodies raised by infection or vaccination with the prototype vaccines.4-6
Omicron is continuously evolving, leading to many new subvariants. The BA.1, BA.2, and BA.5 subvariants have demonstrated strong neutralizing escape of existing humoral immunity established by vaccine and infection.7, 8 New subvariants continue to emerge, including BA.2.75, BA.4.6, BF.7, BA.2.3.20, BA2.75.2, BQ.1.1, and XBB, carrying RBD mutations such as R346, K356, K444, V445, G446, N450, L452, N460, F486, F490, R493, and S494.9 Most of these mutants are known to evade antibodies.10, 11 Thus, there is a critical need for new vaccines and boosters with alternative administration routes.
Currently, 11 vaccines on the Emergency Use List are approved by the World Health Organization (www.who.int), including inactivated, adenovirus-based, mRNA, and subunit vaccines. However, all these vaccines rely on intramuscular (i.m.) injection and fail to induce mucosal immunity, which is essential for neutralizing SARS-CoV-2 in the upper respiratory tract.12 Vaccines inhaled through the nose or mouth could induce a strong mucosal immune response and might block virus transmission.13
Mucosal-associated lymphoid tissue mainly includes nasal-associated lymphoid tissue, gut-associated lymphoid tissue, tonsils, the nasopharynx, the larynx, ocular tissue, the upper airway, and salivary glands. The mucosal epithelium contains macrophages, lymphocytes, plasma cells, dendritic cells, and mucin-producing glandular cells, producing secretory immunoglobin A (sIgA) and various cytokines and chemokines.14 Humans synthesize approximately twice the amount of IgA as IgG, ~40 mg/kg of their body weight per day.15 Intranasal (i.n.) or oral immunization can activate robust mucosal immunity responses, thereby preventing the virus from spreading into the lower respiratory tract.12, 13, 16, 17 SARS-CoV-2 primarily infects humans via mucosal surfaces, including the eyes, nose, and mouth, then invades the underlying epithelial layers.18 Developing mucosal vaccines for pathogens that infect via mucosal surfaces is crucial.
Around 20 mucosal COVID-19 vaccines have reached the clinical trial stage, and at least 4 are being evaluated in or have been evaluated in phase III clinical trials. Two of these vaccines are approved for emergency usage in China.19 One is a live-attenuated influenza virus vector-based COVID-19 vaccine (CA4-dNS1-nCoV-RBD) administered by nasal spray,20 and the other is an adenovirus 5 vector-based vaccine (Ad5-nCoV-S) administered by oral inhalation.21
Recombinant vesicular stomatitis virus (rVSV)-based COVID-19 vaccines have been extensively investigated; however, no rVSV-based vaccine has been approved to date. Merck & Co discontinued the development of rVSV-based V590 after a phase 1 clinical trial because almost no immune response was elicited after one i.m. dose, even though the vaccine was well-tolerated (NCT04569786). IIBR-100, another i.m. rVSV vector-based vaccine generated by the Israel Institute for Biological Research (IIBR) using a similar strategy, was evaluated in a phase 2b/3 trial beginning in October 2020 (NCT04990466). However, only a low SARS-CoV-2–specific immune response was observed in the volunteers.22 Our previous results suggest that rVSV-SARS-CoV-2 could elicit strong humoral and mucosal immunity in rodents and nonhuman primates when delivered i.n.23, 24 Here, we systematically compared the immune responses elicited by rVSV-SARS-CoV-2 with those resulting from immunization with two licensed vaccines, KCONVAC (an inactivated vaccine from Shenzhen Kangtai Biological Products Co., Ltd.) and Vaxzevria (an adenovirus-based vaccine from AstraZeneca) as a primary or booster dose in golden Syrian hamsters. Our results indicate that rVSV-Beta is a promising mucosal vaccine for primary and booster immunization against SARS-CoV-2 variants.
2 RESULTS
2.1 Construction and characterization of rVSV-SARS-CoV-2 VOCs
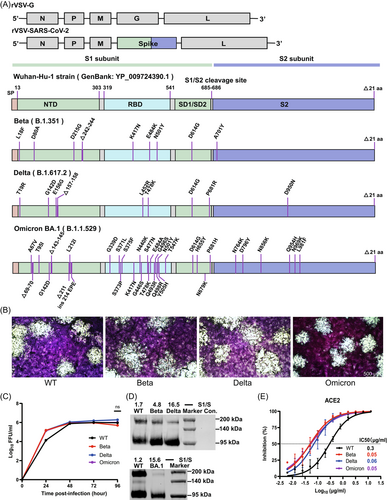
To investigate the immunogenicity of SARS-CoV-2 spike (S) variants expressed by the rVSV vector, we generated rVSVs expressing the S protein of VOCs, including Beta (B.1.351), Delta (B.1.617.2), and Omicron BA.1 (B.1.1.529), designated rVSV-Beta, rVSV-Delta, and rVSV-BA.1, respectively. All the rVSV-SARS-CoV-2 variants were constructed by replacing the original G glycoprotein of rVSV with the SARS-CoV-2 wild-type (WT, Wuhan-Hu-1 strain) or variant S proteins (Figure 1A).24, 25 WT and variant rVSV-SARS-CoV-2 demonstrated similar plaque sizes and growth kinetics in Vero cells, and the peak titers were 106–6.5 FFU/mL, 72 h post-infection (Figure 1B,C and Supporting Information: Figure S1). Expression levels of the full-length S (180 KD) and S1 (110 KD) proteins on viral particles purified from the supernatants were determined by western blot analysis. rVSV-Delta showed a significantly higher S1/S ratio (16.5) than WT (1.7) and rVSV-Beta (4.8) using anti-WT RBD polyclonal antibodies, while rVSV-BA.1 also showed a higher S1/S ratio (15.6) than WT (1.2) using anti-Omicron RBD polyclonal antibodies, implying more efficient furin cleavage of Delta and Omicron variants (Figure 1D). However, the anti-RBD polyclonal antibodies, raised using the WT RBD, failed to recognize the S of Omicron BA.1 due to a high mutation rate (Figure 1A). The receptor-binding affinity of variants was gauged by measuring the inhibitory activity of soluble ACE2 against rVSVs.24 The half-maximal inhibitory concentration (IC50) for soluble ACE2 to neutralize Beta, Delta, and Omicron BA.1 was about fivefold lower than the WT, suggesting stronger receptor-binding activity (Figure 1E).
2.2 rVSV-Beta induces strong humoral, mucosal, and cellular immune responses via the i.n. route
Our previous results reveal that rVSV-SARS-CoV-2 is suitable for i.n. immunization, which can elicit robust humoral and cellular immunity with the advantage of mucosal responses.23 Here, we first investigated the immune responses induced by rVSVs in golden Syrian hamsters (Mesocricetus auratus). Groups of hamsters (n = 5) were i.n. inoculated with a single dose (106 FFU) of rVSV-WT, rVSV-Beta, rVSV-Delta, or rVSV-BA.1. Serum-neutralizing activities against rVSV-WT, rVSV-Beta, rVSV-Delta, and rVSV-BA.1 were determined 28 days after immunization (Figure 2A). Each virus elicited the highest titers of NAbs against itself, among which Beta and Delta showed the highest immunogenicity, while BA.1 demonstrating the lowest. rVSV-Beta elicited broad neutralizing activity against all the VOCs tested, including BA.1. Thus, we selected rVSV-Beta for subsequent experiments in WT BALB/c mice and golden Syrian hamsters.
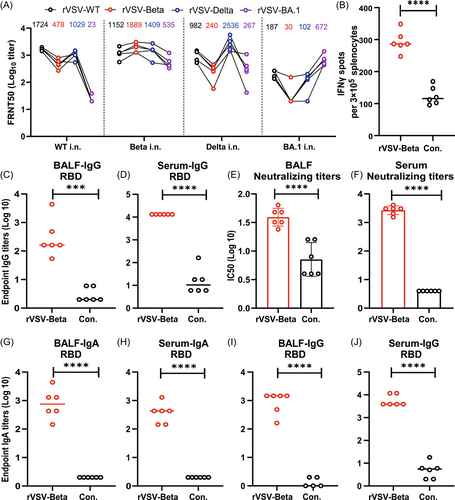
To test the mucosal and cellular immunity elicited by rVSV-SARS-CoV-2, golden Syrian hamsters were i.n. immunized with two doses of rVSV-Beta at an interval of 28 days. Seven days after the booster dose, the cellular immunity level in hamsters was detected by ELISPOT. Our results showed that interferon-γ levels were significantly enhanced in the spleen cells of the hamsters after stimulation with the RBD protein peptide library (Figure 2B).
Bronchoalveolar lavage fluids (BALF) and sera were also collected to assess SARS-CoV-2–specific IgG levels and neutralizing activity. As shown in Figure 2C,D, the levels of RBD-specific IgG were significantly higher in the BALF and sera of the rVSV-Beta group than in the control group samples. In addition to the high levels of IgG detected in hamsters, neutralizing activity against authentic SARS-CoV-2 WT strain was detected in both BALF and sera (Figure 2E,F).
We could not test sIgA levels in the hamsters due to the unavailability of a hamster-IgA–specific secondary antibody. Thus, we determined BALF sIgA levels in BALB/c mice following the immunization protocol described above. The endpoint titers of RBD-specific IgA were 7.6 × 102 in BALF and 3.6 × 102 in the serum (Figure 2G–J), further confirming that rVSV-SARS-CoV-2 could induce effective mucosal immunoglobulins in respiratory airways via the i.n. route. Collectively, these data suggest that rVSV-SARS-CoV-2 can elicit strong humoral, mucosal, and cellular immune responses via the i.n. route.
2.3 Comparison of the humoral immune responses of rVSV-SARS-CoV-2 with those of inactivated vaccine and adenovirus-based vaccine
To further evaluate the immunogenicity of the rVSV vector-based vaccines, we compared the immune responses elicited by rVSV-SARS-CoV-2 in golden Syrian hamsters with those elicited by two licensed vaccines, KCONVAC (an inactivated vaccine from Shenzhen Kangtai Biological Products Co., Ltd.) and Vaxzevria (an adenovirus-based vaccine from AstraZeneca). All the vaccines were generated based on the S protein sequence of WT SARS-CoV-2. The hamster dosages of KCONVAC and Vaxzvria were 1 μg and 1010 VP, respectively, representing one-fifth of the human dosage. A low dose of 2 × 105 FFU and a high dose of 2 × 106 FFU were applied for rVSV-WT on the basis of clinical trial results evaluating rVSV vector-based COVID-19 vaccines. The high dose is 107 FFU for IIBR-100 (ClinicalTrials.gov—NCT04608305)22 and 5.55 × 107 PFU for V590-001 (NCT04569786).26
Groups of hamsters were immunized i.n. with two doses (28-day interval) of rVSV-WT (2 × 105 or 2 × 106 FFU), i.m. with KCONVAC (1 μg), or i.m. or i.n. with Vaxzevria (1010 VP). The serum-neutralizing activity was determined using authentic SARS-CoV-2 by measuring the 50% inhibition of cytopathic effect (CPE) (IC50); the geometric mean titer (GMT) of the IC50 of convalescent COVID-19 patients ranges from 50 to 100.27 All rVSV-immunized groups, regardless of whether they received a low (2 × 105 FFU) or high (2 × 106 FFU) dose, demonstrated significantly higher IC50 titers than the groups that received two-doses of KCONVAC or Vaxzevria; potent and broad neutralization activities against authentic SARS-CoV-2 WT, Beta, Delta, Omicron BA.1, and BA.2 strains were also observed (Figure 3). However, a 10-fold increase in vaccine dosage only slightly enhanced the GMTs, probably due to the replication competency of rVSVs. The GMTs of high doses of rVSV-WT were 6.3-, 6.8-, 6.0-, 2.6-, and 36-fold higher than those of KCONVAC, and 2.4-, 2.1-, 2.0-, 4.7-, and 2.4-fold higher than those of i.n. Vaxzervria against WT, Beta, Delta, Omicron BA.1 and BA.2 strains, respectively, at Day 42. Although the GMTs of NAbs induced by the rVSV-WT vaccine were significantly reduced against Omicron BA.1 and BA.2, the result was still significantly superior to that obtained with inactivated and adenovirus vaccines.
Our previous results reveal that rVSV-Beta can elicit a significantly stronger humoral immune response than the WT and Alpha variant.24 To compare the efficacy of rVSV-Beta with KCONVAC and Vaxzevria as a primary dose, groups of hamsters were immunized with two doses (28-day interval) of rVSV-Beta or rVSV-Delta via i.n. route (105 or 106 FFU), or two-doses KCONVAC (1 μg) and Vaxzevria (1010 VP) via i.m. route (Supporting Information: Figure S2a). All rVSV-immunized groups, no matter with low (105 FFU) or high (106 FFU) dosage, elicited significantly higher IC50 titers than the groups that received two-doses of KCONVAC or Vaxzevria (Supporting Information: Figure S2b). Both rVSV-Beta and rVSV-Delta elicited potent and broad neutralization activity against SARS-CoV-2 WT, Beta, and Delta strains, while rVSV-Beta was more immunogenic than rVSV-Delta. The GMTs of two doses of rVSV-Beta were 2.8-, 4.5-, and 3.7-fold higher than the KCONVAC, and 3.4-, 7.1-, and 2.4-fold higher than Vaxzevria against WT, Beta, and Delta strains, respectively (Supporting Information: Figure S2b).
In conclusion, our results confirmed that two i.n. doses of rVSV-Beta elicited significantly higher humoral immune responses than commercial inactivated and adeno-based COVID vaccines in hamsters.
2.4 Immunogenicity of the rVSV-Bivalent vaccine (rVSV-Beta plus rVSV-BA.1)
New Omicron subvariants are continuously emerging by escaping the immunity induced by previous VOCs. However, Omicron's immunogenicity is extremely low compared with the early VOCs, such as Beta and Delta. Thus, developing a bivalent vaccine with high immunogenicity against the early VOCs and Omicron subvariants is a reasonable vaccine development strategy. Since rVSV-Beta shows strong immunogenicity and a broad spectrum in the above animal experiments, we designed an rVSV-Bivalent vaccine (rVSV-Beta plus rVSV-BA.1).
Groups of golden Syrian hamsters (n = 6) were immunized i.n. with two doses (28-day interval) of rVSV-Beta or rVSV-BA.1 S proteins (106 FFU) or two-doses rVSV-Bivalent (rVSV-Beta 5 × 105 FFU and rVSV-BA.1 5 × 105 FFU) to test the efficacy of the bivalent vaccine. The GMTs induced by rVSV-Bivalent and rVSV-Beta were significantly higher than those induced by rVSV-BA.1 against SARS-CoV-2 WT, Beta, and Delta strains (Figure 4A–C). Additionally, the GMTs raised by rVSV-BA.1 against Omicron BA.1 and Omicron BA.2 were comparable with those induced by rVSV-Bivalent or rVSV-Beta (Figure 4D,E). Overall, the differences in the humoral responses elicited by rVSV-Bivalent and rVSV-Beta were not significant. These results demonstrate that rVSV-BA.1 is less immunogenic and failed to enhance the efficacy of rVSV-Beta as a bivalent vaccine.
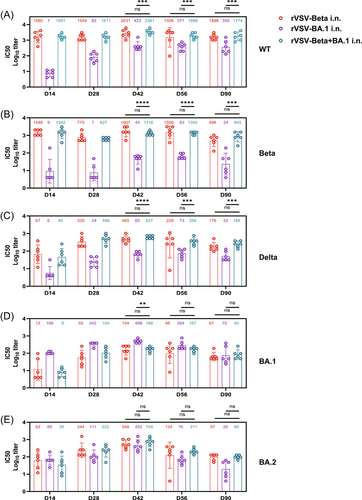
2.5 Efficacy of heterologous boosting with vaxzevria or rVSVs versus homologous boosting following two doses of KCONVAC
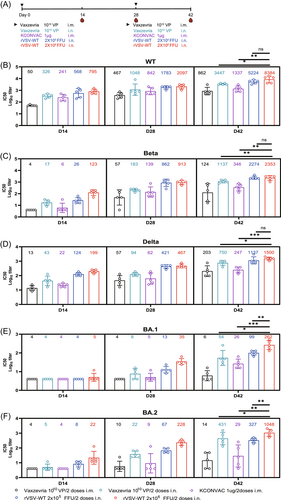
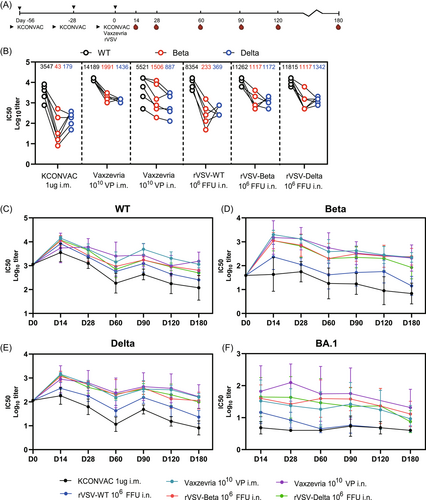
A third dose of homologous or heterologous vaccine has been shown to greatly improve vaccine-induced NAb levels.28-30 We next assessed the booster efficacy of rVSV following two doses of KCONVAC. After receiving two i.m. doses of KCONVAC, the hamsters were boosted with a third dose of KCONVAC (i.m.), Vaxzevria (i.m. or i.n.), or rVSVs (i.n.) at intervals of 28 days (Figure 5A). Consistent with another heterologous booster study,21 Vaxzevria and rVSV elicited significantly higher NAbs than the homogenous KCONVAC. Fourteen days after the last dose, the highest GMTs against WT, Beta, and Delta were observed in the i.m. Vaxzevria-boosted group (14189, 1991, 1436, respectively), followed by the groups that received rVSV-Beta (11262, 1117, 1172, respectively) and rVSV-Delta (11815, 1117, 1342, respectively) (Figure 5B). The serum-neutralizing titers showed a similar decay pattern from 14 to 180 days after the third dose. The GMT peaks in almost all the groups occurred on day 14 after boosting and continuously decreased until 180 days. Notably, even the highest GMTs generated by the homologous vaccination regime against the Omicron variant were consistently lower than those by the heterologous nasal-sprayed rVSV-based and adenovirus-based vaccines boosted within 6 months. Collectively, robust and broad-spectrum neutralizing activity against all tested VOCs was boosted by Vaxzevria, while those by rVSV-Beta or rVSV-Delta were comparable.
3 DISCUSSION
In summary, our results confirmed that two i.n. doses of the rVSV-Beta vaccine could elicit significantly higher humoral immune responses than commercial inactivated and adenovirus-based COVID vaccines in hamsters. As a heterologous booster dose, rVSV- Beta induced potent, persistent, and broad-spectrum humoral and mucosal neutralizing responses against all VOCs, highlighting its potential to be developed into a nasal-spray vaccine. We also found that the addition of rVSV-BA.1 in the bivalent vaccine failed to enhance the neutralizing activity against Omicron strains compared with rVSV-Beta.
Because the first approved viral-vector vaccine (Merck & Co.'s ERVEBO vaccine against Ebola) was developed using an rVSV vector, many groups have pursued rVSV-based COVID-19 vaccines using the same vector construction used here, including Merck & Co. and the IIBR. In phase I clinical trial of Merck's V590, the vaccine was applied i.m. at doses of 5 × 105–5.55 × 107 PFU. V590 was well tolerated, but the immune responses were inferior to those obtained by natural infection or other COVID-19 vaccines. Thus, Merck discontinued V590 and declared that the oral or i.n. route might work better.26 IIBR-100 from the IIBR was also applied i.m at a similar dosage (105–107 PFU).22 The vaccine was also well tolerated in their phase I/II clinical trial, but the immune response was extremely low compared with that obtained with other COVID-19 vaccines. In our previous report, rVSV-SARS-CoV-2 elicited significantly stronger NAbs via the i.n. route than the i.m. route in rodent and primate models. The rVSV vector-based vaccine was constructed by replacing the original glycoprotein G with the S protein of SARS-CoV-2, which is very different from the strategy used for adenovirus-based vaccines. ACE2 mediates rVSV-SARS-CoV-2 uptake, and the tissue distribution of ACE2 in the immunizing route dramatically affects the efficacy of the vaccine.23 rVSV-SARS-CoV-2 is more suitable for i.n. administration because ACE2 levels are higher in the respiratory tract than in the muscle.31
There are safety concerns with rVSV-SARS-CoV-2 as it is a replication-competent viral-vector vaccine. The clinical trials of V590 and IIBR-100 revealed that rVSV-SARS-CoV-2 is safe when administered via the i.m. route. However, administration via the i.n. route raises a new safety issue, especially the potential neuroinvasiveness and neurovirulence of VSV vectors. During the development of the Ebola virus vaccine ERVEBO, the neurovirulence of rVSV vectors was evaluated in nonhuman primates with administration via the intrathalamic route, and no significant lesions were observed in any animals.32 The safety was further demonstrated in pigs by intradermal injection into the apex of the snout, resembling the i.n. route, and no disease was observed.33 In our previous reports, mice, hamsters, and macaques i.n. inoculated with rVSV-SARS-CoV-2 showed no weight loss or obvious diseases. However, a comprehensive evaluation of the safety of rVSV-SARS-CoV-2 via the i.n. route remains necessary. Oral and i.n. vaccines trigger mucosal immunity when in contact with the mucosal surfaces and induce the production of sIgA. sIgA antibodies are delivered across the musical surface to the lumen, where they inhibit the entry of pathogens.34 The oral polio vaccine can efficiently block poliovirus transmission; however, the inactivated vaccine delivered i.m. can only prevent poliomyelitis.35 Nasal immunization of mice, hamsters, or rhesus macaques with multiple COVID-19 adenoviral vector vaccines, including ChAd-SARS-CoV-2-S, ChAd-vectored trivalent COVID-19 vaccines, Ad5-nCoV, and Vaxzevria induced humoral and cellular immunity and also showed strong mucosal immunity in BALF. Results from the clinical trial of Ad5-nCoV by CanSino showed that oral inhalation of aerosolized vaccine with a one-fifth dosage (1010 VP) produced a 10-fold increase in antibody titers compared with the i.m. route (5 × 1010 VP). In this report, i.n. immunized rVSV-SARS-CoV-2 elicited stronger humoral immune responses than Vaxzevria using a chimpanzee adenoviral vector, and high levels of sIgA were detected in BALF, with the ability to neutralize authentic SARS-CoV-2.
Omicron is continuously evolving, leading to many new subvariants, such as BA.1, BA.2, BA.4, and BA.5; these variants can evade existing humoral immunity established by vaccines and infection.7, 8 Bivalent vaccines are a promising design strategy for SARA-CoV-2 vaccines to control new VOCs, and many animal experiments and relevant clinical data have been reported.36-40 However, our bivalent vaccine combining Beta and Omicron BA.1 did not achieve better immune efficacy than the Beta monovalent vaccine; the bivalent did not induce higher NAb titers or increase the spectrum of the antibodies. Therefore, we speculate that Omicron BA.1 strain has poor immunogenicity and is not suitable as an rVSV-vectored vaccine. Consistent with our result, in a clinical human serum sample study, boosting with a bivalent vaccine (D614G + BA.4/5) did not significantly increase the virus-NAbs compared with boosting with the original monovalent vaccines.41 Another study noted no significant difference in CD4+ or CD8+ T-cell responses induced by an mRNA bivalent vaccine (WT + BA.5) in comparison with those induced by a monovalent vaccine.42 In conclusion, we believe that the nasal-spray rVSV-Beta is a promising booster COVID-19 vaccine candidate for future development.
4 MATERIALS AND METHODS
4.1 Cells, antibodies, and viruses
Vero cells (African green monkey kidney cells) were obtained from Shenzhen Kangtai Biological Products Co., Ltd. and maintained in Dulbecco's modified Eagle's medium (DMEM, HyClone) supplemented with 8% fetal bovine serum (FBS), 1% l-glutamine, and 1% penicillin–streptomycin at 37°C in 5% CO2. Rabbit anti-SARS-CoV-2 S RBD polyclonal antibodies were purchased from Sino Biological Inc. (anti-WT, Cat. 40592-T62; anti-Omicron, Cat. 40592-MM117).
The SARS-CoV-2 WT strain, Beta strain, Delta strain, and Omicron (BA.1 and BA.2) strains were provided by the National Institute for Viral Disease Control and Prevention (Chinese Center for Viral Disease Control and Prevention), Guangdong Provincial Center for Disease Control and Prevention, the Third People's Hospital of Shenzhen, and Shenzhen Center for Disease Control and Prevention, respectively.
4.2 Construction, rescue, and characterization of rVSV viruses and other vaccines
The rVSV-SARS-CoV-2 variants (rVSV-WT, rVSV-Beta, rVSV-Delta, and rVSV-BA.1) were designed, synthesized, and constructed as described previously.43-45 The human codon-optimized S protein coding sequence of WT SARS-CoV-2 variants (GenBank accession number: YP_009724390.1), Beta (GISAID: EPI_ISL_678597), Delta (GISAID: EPI_ISL_1911250), or Omicron BA.1 (GISAID: EPI_ISL_6590782.2) were synthesized by Beijing SYKM Gene Biotechnology Co., Ltd. Inactivated vaccine KCONVAC and adenovirus-based Vaxzevria (ChAdOx1-S) were produced by Shenzhen Kangtai Biological Products Co., Ltd.
4.3 Focus-forming assays
A focus-forming assay was used to determine the rVSV viral titer in Vero cells. Vero cells (1.5 × 104/well) were seeded in 96-well plates 24 h before infection. Viruses were serially diluted at 1:10 dilution in DMEM with 2% FBS and incubated with cells for 3 h at 37°C. Then, the cells were washed once and incubated at 28°C with a carboxymethyl cellulose overlay. GFP-positive cells were counted under a fluorescent microscope 20 h postinfection.
4.4 Virus growth curve
Vero cells were infected with rVSV recombinant virus in T75 flasks at a multiplicity of infection (MOI) of 0.01. Supernatants were harvested every 24 h after infection, and viral titers were determined by focus-forming assays in Vero cells.
4.5 Plaque assay
Vero cells were plated in 24-well plates at 100 000 cells per well 24 h before infection. Viral supernatants from infected cells were serially diluted in DMEM with 2% FBS and then added to the Vero cells. The cells were covered with a carboxymethyl cellulose overlay 3 h after infection. Plates were fixed with 4% formaldehyde and stained with crystal violet 6 days postinfection. The plaque size of rVSV-SARS-CoV-2 and variants were measured by Image J.
4.6 Animal experiments
Groups of female golden Syrian hamsters (Mesocricetus auratus) were vaccinated with one or two doses of rVSV-WT, rVSV-Beta, rVSV-Delta, rVSV-Omicron BA.1, rVSV-Bivalent, KCONVAC (1 μg per hamster), or Vaxzevria (1010 VP per hamster) via the i.n. or i.m. route. Serum samples were collected 14, 28, 42, 56, and 90 days after the first dose for evaluating NAb titers against SARS-CoV-2 WT, Beta, Delta, Omicron BA.1, and BA.2 strains.
Groups of BALB/c mice (n = 6) and hamsters (n = 6) were vaccinated with rVSV-Beta (106 FFU per animal) or medium (control) twice at Weeks 0 and 4. Seven days after the booster dose, their spleens were removed, and spleen cells were isolated for cytokine assays; BALF was collected by inserting a syringe into the trachea and flushing the lung three times with 1 mL of PBS (∼800 μL recovered); sera were collected for antibody assays.
In the heterologous boosting experiments, hamsters were primed twice at Weeks 0 and 4 with KCONVAC (1 μg per hamster) and boosted at Week 8 with rVSV-WT (106 FFU), rVSV-Beta (106 FFU), rVSV-Delta (106 FFU), KCONVAC (1 μg), or Vaxzevria (1010 VP) via the i.n. or i.m. route. Serum samples were collected 14, 28, 60, 90, 120, and 180 days postboosting for evaluating NAb titers.
4.7 Neutralization assay
The SARS-CoV-2 (WT, Beta, Delta, and Omicron) neutralizing assay was performed in a certified BSL3 facility. Twofold serial dilutions of heat-inactivated sera or BALFs were mixed with equal volumes of viral solution to a final concentration of 100 TCID50/well and incubated at 37°C for 1 h. The virus-sample mixtures (100 µL/well) were added to Vero cells and incubated at 37°C. The CPE of each well was evaluated under a microscope, and the NAb titers were recorded as the dilution of serum that showed 50% CPE inhibition.
For the FRNT, 1500 FFU of rVSVs were incubated with threefold serially diluted heat-inactivated sera at room temperature for 30 min and then added to a 96-well plate containing Vero cells. GFP-positive cells were counted 20 h postinfection using the Opera Phenix High Content Screening System (PerkinElmer), and NAb titers were calculated as 50% inhibition of viral infection (FRNT50) by the Reed–Muench method.
4.8 ELISA and ELISPOT assay
The SARS-CoV-2 RBD–specific IgA and IgG antibodies in the BALF and sera were measured by ELISA. Plates were coated with 0.1 μg/well SARS-CoV-2 RBD protein (Sino Biological Inc.) and blocked for 2 h at room temperature. The serially diluted BALF and sera samples were incubated in plates for 1 h at 37°C. Plates were washed three times and incubated with Goat anti-Syrian Hamster IgG H&L (HRP) (Abcam), Goat anti-Mouse IgG antibodies (catalog no. SA00001-1), or Goat anti-Mouse IgA antibodies (catalog no. SA00012-7) for 1 h at room temperature. Following three washes, 3,5,3′5′-tetramethylbenzidine (TMB) (TIANGEN Biotech (Beijing) Co., Ltd.) was used as the chromogen in the dark; the reaction was then terminated with 2 M sulfuric acid. Absorbance was measured at 450 nm, and endpoint titers were calculated.
The ELISPOT assay protocol was performed following the manufacturer's instructions (catalog no. 3102-2A; MabTech). Cells were stimulated with a pool of 53 overlapping 15-mer SARS-CoV-2 RBD peptides (jpi: PM-WCPV-S-RBD) or PBS at 37°C for 18 h.
AUTHOR CONTRIBUTIONS
Aihua Zheng, Jiankai Liu and Jiandong Liu conceived and supervised the project. Yuhang Zhang, Jiandong Liu, Hongyue Li, Fei Yuan, Congli Jiang, Tianle Cang, Kelei Li, and Qiang Hu performed the experiments and analyzed the data. Aihua Zheng and Fei Yuan wrote the manuscript with the input of all authors.
ACKNOWLEDGMENTS
This project was funded by the National Key R&D Program of China (2021YFC2300903), Guangdong Provincial Key R&D Program, No. 2022B1111040001.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The rVSV studies were conducted in a biosafety level 2 laboratory (BSL2). Studies with authentic SARS-CoV-2 were performed in a biosafety level 3 (BSL3) facility. The protocols for animal studies were approved by the Committee on the Ethics of Animal Experiments of the Institute of Zoology, Chinese Academy of Sciences, Beijing (Approval number: IOZ-IACUC-2021-158).
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are included in this paper and its supplementary information.




