Oral side effects of COVID-19 vaccines in 32 European countries: Analysis of EudraVigilance reports
Abstract
The recent reports of oral side effects (SEs) following COVID-19 vaccination warrant further investigation into their prevalence, severity, and aetiology. This study was conducted to synthesize the first-ever population-level evidence about oral SEs of COVID-19 vaccines in Europe. The European Union Drug Regulating Authorities Pharmacovigilance (EudraVigilance) database was accessed in August 2022 to extract summary data of all potential oral SEs reported after COVID-19 vaccination. The data were reported descriptively and cross-tabulated to facilitate sub-group analysis per vaccine type, sex, and age group. Dysgeusia was the most commonly reported oral SE (0.381 case per each 100 received reports), followed by oral paraesthesia (0.315%), ageusia (0.296%), lip swelling (0.243%), dry mouth (0.215%), oral hypoaesthesia (0.210%), swollen tongue (0.207%), and taste disorder (0.173%). Females had significantly (Sig. < 0.001) a higher prevalence of all most common (top 20) oral SEs, except for salivary hypersecretion, which was equally prevalent among females and males. The present study revealed a low prevalence of oral SEs, with taste-related, other sensory and anaphylactic SEs being the most common SEs in Europe, similar to what was found earlier among the US population. Future studies should explore the potential risk factors of oral sensory and anaphylactic SEs to verify whether they are causally linked to COVID-19 vaccines.
1 INTRODUCTION
The oral cavity has been widely debated as a potential platform to reflect the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection since the first epidemic wave in 2021.1-3 A wide range of oral and orofacial manifestations such as aphthous stomatitis,4 oral mucositis,5 oral candidiasis,6 acute parotitis,7 and angular cheilitis were reported by coronavirus disease (COVID-19) patients.8 These potential symptoms are of vital importance for dentists and dental team members to be aware of as they may encounter them during their daily practice amid the ongoing pandemic.1
Vaccines are verifiably the most successful public health discovery of all time.9 In the context of COVID-19, vaccines are the only evidence-based intervention to make this pandemic a part of our history through achieving herd immunity.10 Therefore, achieving substantial levels of vaccine coverage is the foremost priority for health systems worldwide nowadays.10 The chronic challenge for mass vaccination strategies is vaccine hesitancy (VH) which is referred to as “delay in acceptance or refusal of vaccination despite availability of vaccination services”.11 According to VH theorists, vaccine safety is a crucial driver of vaccine confidence, frequently targeted by the anti-vaccination movement. Even mild and common side effects (SEs) may undermine public confidence in vaccines; therefore, they should be appropriately addressed by healthcare professionals and authorities.12
Postvaccination SEs are monitored by national surveillance systems such as the Vaccine Adverse Event Reporting System (VAERS) in the United States and the Yellow Card in the United Kingdom during phase IV of clinical trials. Unfortunately, these systems cannot precisely record oral and orofacial SEs because of their passive nature, although they may range from mild (oral paraesthesia) to severe (Bell's palsy) SEs.13, 14 A recent systematic review for the potential oral SEs of COVID-19 vaccines found that mucosal lesions, e.g., erosions, ulcers, vesicles, and papules, were reported in 16 recently vaccinated individuals, all as case reports/series.13 Additionally, several cross-sectional studies revealed variable incidence levels of oral SEs among COVID-19 vaccinees.15-17
Chun et al.18 described nine patients from South Korea who presented with painful oral lesions affecting the posterior palatal region, labial and buccal mucosa, lower gingiva, and tongue, which emerged shortly after receiving BNT162b2 (n = 4) and AZD1222 (n = 5) vaccines. Troeltzsch et al.19 reported the case of a German middle-aged male patient who suffered from oral lichen planus (OLP) after receiving AZD1222. Recently, Caggiano et al.20 reported an Italian middle-aged male patient presented with OLP after BNT162b2 vaccination. A postmarketing (phase IV) cross-sectional study among Czech healthcare workers (HCWs) in early 2021 revealed that up to 13% of BNT162b2 recipients reported various orofacial SEs, including oral blisters (36%), halitosis (25.4%), ulcers (14%), and bleeding gingiva (11.4%).15 Similarly, 12.4% of German HCWs who received messenger RNA (mRNA)-based vaccines reported orofacial SEs such as vesicles (4.6%), oral paraesthesia (2.3%), bleeding gingiva (2.3%), and swollen mucosa (1.7%).16 In Slovakia, only 9.6% of HCWs who received BNT162b2 reported oral SEs without a statistically significant difference between males (5.8%) and females (10.7%).17
The overall aim of this study was to explore the oral SEs following COVID-19 vaccination in Europe passively collected by national regulators. The primary objective was to assess the prevalence of oral SEs, while the secondary objective was to evaluate oral SEs according to vaccine type, sex, and age group.
2 MATERIALS AND METHODS
2.1 Design
Secondary data analysis of the European Union Drug Regulating Authorities Pharmacovigilance (EudraVigilance) database was carried out in August 2022 to evaluate the reports of COVID-19 vaccines suspected SEs.21, 22
2.2 Data sources
EudraVigilance is a passive surveillance system for suspected SEs of medicinal products, including vaccines, and it is managed and maintained by the European Medicines Agency (EMA). The postauthorisation safety reports of EudraVigilance are collected from healthcare professionals and patients in the 32 member states of the European Economic Area (EEA), and they are updated and analysed every 2 or 4 weeks.22
Additionally, the “COVID-19 Vaccine Tracker” database of the European Center for Disease Prevention and Control (ECDC) has been accessed to curate data on the total numbers of COVID-19 vaccine doses administered in EU/EEA countries.23
2.3 Population
As of August 6th, 2022, the EudraVigilance database had suspected SEs reports of the five COVID-19 vaccines that were authorized and administered in the EEA to date; Pfizer-BioNTech (Comirnaty; Tozinameran), Moderna (Spikevax; CX-024414), AstraZeneca (Vaxzevria; ChAdOx1 nCoV-19), Janssen (Jcovden; Ad26.COV2.S), and Novavax (Nuvaxovid; NVX-CoV2373).22
All reports that were received until August 6th, 2022, from COVID-19 vaccinees were included in this study, and these reports were extracted as summary numbers stratified by sex (female, male, and unknown) and age group (0–1 month, 2 months to 2 years, 3–11 years, 12–17 years, 18–64 years, 65–85 years, above 85 years, and unknown).22
2.4 Variables
EudraVigilance uses the Medical Dictionary for Regulatory Activities (MedDRA) methodology in organizing and displaying of suspected SEs reports.24 The MedDRA hierarchy has five levels starting from the “System Organ Class” level such as gastrointestinal disorders until the “Lowest Level Term” level such as aphthous stomatitis.24
First, we developed an anatomo-physiological scheme to search for and extract all potential SEs related to the oral cavity structures and functions from the MedDRA hierarchy.14 Our scheme was explained in detail previously, and it simply divided the oral cavity into six major regions: (a) oral mucosa, (b) tongue, (c) lips, (d) palate, (e) salivary glands, and (f) dentition, and two functions: (a) taste and (b) other sensory disorders.14
Second, an exhaustive list of 310 potential oral SEs was extracted based on our de novo scheme, then reviewed and filtered by a panel of oral surgery specialists. A total of 182 potential SEs were excluded eventually due to being duplicates (n = 43), congenital (n = 16), traumatic injuries (n = 20), iatrogenic (n = 42), chronic or oncologic (n = 52), or biologically irrelevant (n = 9).14
A final list of 128 potential oral SEs was used in this study.
2.5 Analyses
Total frequencies and relative proportions of each suspected SE were extracted and cross-tabulated according to vaccine type, vaccine group, sex, and age group. Two relative proportions were calculated for each side effect: (a) in relation to total suspected SEs and (b) in relation to total administered doses. The age groups were re-organized into three groups: minors (0–17 years old), adults (18–64 years old), and seniors (>65 years old) to facilitate the subsequent analysis.
Chi-squared test (χ2) and Fisher's exact test were used to test for significant differences between vaccine groups, sex, and age groups. All inferential tests were performed following the assumptions of confidence interval 95% and significance level (Sig.) ≤0.05. All statistical tests were performed using GraphPad Prism version 9.3.1 (GraphPad Software Inc.).
3 RESULTS
3.1 Demographic characteristics
A total of 895 572 629 COVID-19 vaccines were administered, and 1 978 116 SEs were reported in the EEA until August 6th, 2022. AstraZeneca had the highest report/dose ratio (748.4 reports per 100 000 doses), while Pfizer-BioNTech had the lowest ratio (164.8 reports per 100 000 doses). Females had most reported SEs (68.9%), while males had only 28.9% of all reported SEs. The adult group (18–64 years old) had the highest proportion of SEs (77.6%) compared to other age groups (Table 1).
| Variable | Outcome | mRNA-based vaccines | Viral vector-based vaccines | Protein subunit | ||
|---|---|---|---|---|---|---|
| PFIZER-BIONTECH (TOZINAMERAN) | MODERNA (CX-024414) | ASTRAZENECA (CHADOX1 NCOV-19) | JANSSEN (AD26.COV2.S) | NOVAVAX (NVX-COV2373) | ||
| Total doses | N | 650 605 721 | 156 325 748 | 68 767 609 | 19 623 460 | 250 091 |
| Received reports | N (ratio) |
1 072 088 (164.8 reports per 100 000 doses) |
323 419 (206.9 reports per 100 000 doses) |
514 655 (748.4 reports per 100 000 doses) |
66 757 (340.2 reports per 100 000 doses) |
1197 (478.6 reports per 100 000 doses) |
| Sexa | Female | 747 145 (69.69%) | 220 908 (68.30%) | 358 050 (69.57%) | 35 608 (53.34%) | 851 (71.09%) |
| Male | 304 040 (28.36%) | 97 836 (30.25%) | 141 499 (27.49%) | 28 506 (42.70%) | 338 (28.24%) | |
| Unknown | 20 903 (1.95%) | 4675 (1.45%) | 15 106 (2.94%) | 2643 (3.96%) | 8 (0.67%) | |
| Age groupa | 0–1 Month | 343 (0.03%) | 98 (0.03%) | 303 (0.06%) | 15 (0.02%) | 0 (0%) |
| 2 Months to 2 Years | 649 (0.06%) | 145 (0.04%) | 342 (0.07%) | 54 (0.08%) | 0 (0%) | |
| 3–11 Years | 4415 (0.41%) | 119 (0.04%) | 289 (0.06%) | 5 (0.01%) | 0 (0%) | |
| 12–17 Years | 28 505 (2.66%) | 2028 (0.63%) | 307 (0.06%) | 117 (0.18%) | 0 (0%) | |
| 18–64 Years | 819 419 (76.43%) | 254 657 (78.74%) | 401 860 (78.08%) | 57 528 (86.18%) | 1061 (88.64%) | |
| 65–85 Years | 136 556 (12.74%) | 48 315 (14.94%) | 75 071 (14.59%) | 4214 (6.31%) | 77 (6.43%) | |
| >85 Years | 24 846 (2.32%) | 5925 (1.83%) | 3131 (0.61%) | 390 (0.58%) | 5 (0.42%) | |
| Unknown | 57 355 (5.35%) | 12 132 (3.75%) | 33 352 (6.48%) | 4434 (6.64%) | 54 (4.51%) | |
- Abbreviation: mRNA, messenger RNA.
- a Of total received reports.
3.2 Crude prevalence of oral SEs
Among dentition-related SEs, toothache was the most common SE (0.104 case per each 100 received reports). Dysgeusia was the most common taste-related SE (0.381%), followed by ageusia (0.296%) and taste disorder (0.173%). Oral paraesthesia (0.315%) and oral hypoesthesia (0.210%) were the most common SEs among other sensations. Dry mouth (0.215%) and salivary hypersecretion (0.025%) were the most common salivary gland-related SEs. The swollen tongue was the most common tongue-related SE (0.207%), followed by glossodynia (0.046%), tongue discomfort (0.036%), and tongue oedema (0.033%) (Table 2).
| Preferred term | mRNA-based vaccines | Viral vector-based vaccines | Protein subunit | mRNA vs. vector | Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFIZER-BIONTECH (TOZINAMERAN) | MODERNA (CX-024414) |
ASTRAZENECA (CHADOX1 NCOV-19) | JANSSEN (AD26.COV2.S) | NOVAVAX (NVX-COV2373) |
Sig. | ||||||||
N (% of SE) |
/100 K doses | N (% of SE) |
/100 K doses | N (% of SE) |
/100 K doses | N (% of SE) |
/100 K doses | N (% of SE) |
/100 K doses | % of SE | /100 K doses | ||
| Dental Discomfort (10054217) | 37 (0.003%) |
0.006 | 21 (0.006%) |
0.013 | 18 (0.003%) |
0.026 | 5 (0.007%) |
0.025 | 0 (0%) | 0 | 0.937 | <0.001 | Dentition-related AEa |
| Dental Paraesthesia (10078276) | 27 (0.003%) |
0.004 | 8 (0.002%) |
0.005 | 13 (0.003%) |
0.019 | 1 (0.001%) |
0.005 | 0 (0%) | 0 | 0.978 | <0.001 | |
| Hyperaesthesia Teeth (10082426) | 85 (0.008%) |
0.013 | 25 (0.008%) |
0.016 | 75 (0.015%) |
0.109 | 5 (0.007%) |
0.025 | 0 (0%) | 0 | <0.001 | <0.001 | |
| Hypoesthesia Teeth (10051780) | 11 (0.001%) |
0.002 | 5 (0.002%) |
0.003 | 2 (<0.001%) |
0.003 | 1 (0.001%) |
0.005 | 0 (0%) | 0 | 0.312 | 0.427 | |
| Toothache (10044055) | 1007 (0.094%) |
0.155 | 320 (0.099%) |
0.205 | 664 (0.129%) |
0.966 | 66 (0.099%) |
0.336 | 2 (0.167%) |
0.800 | <0.001 | <0.001 | |
Ageusia (10001480) |
3048 (0.271%) | 0.381 | 957 (0.282%) |
0.430 | 1893 (0.360%) |
1.210 | 187 (0.270%) |
0.286 | 6 (0.441%) |
0.043 | <0.001 | <0.001 | Taste-related AE* |
| Dysgeusia (10013911) | 4438 (0.395%) | 0.555 | 936 (0.276%) |
0.421 | 2293 (0.436%) |
1.466 | 167 (0.241%) |
0.255 | 12 (0.881%) |
0.085 | <0.001 | <0.001 | |
| Hypergeusia (10029205) | 7 (0.001%) | 0.001 | 2 (0.001%) |
0.001 | 1 (<0.001%) |
0.001 | 0 (0%) | 0 | 0 (0%) | 0 | 0.299 | 1.000 | |
| Hypogeusia (10020989) | 285 (0.025%) | 0.036 | 75 (0.022%) |
0.034 | 95 (0.018%) |
0.061 | 19 (0.027%) |
0.029 | 0 (0%) | 0 | 0.023 | 0.001 | |
| Taste Disorder (10082490) | 1896 (0.169%) | 0.237 | 559 (0.165%) |
0.251 | 1001 (0.190%) |
0.640 | 90 (0.130%) |
0.138 | 9 (0.661%) |
0.064 | 0.015 | <0.001 | |
| Dry Mouth (10013781) | 2003 (0.187%) | 0.308 | 543 (0.168%) |
0.347 | 1603 (0.311%) |
2.331 | 102 (0.153%) |
0.520 | 4 (0.334%) |
1.599 | <0.001 | <0.001 | Salivary Glands-related AEb |
Aptyalism (10003068) |
58 (0.005%) | 0.009 | 10 (0.003%) |
0.006 | 13 (0.003%) |
0.019 | 1 (0.001%) |
0.005 | 1 (0.084%) |
0.400 | 0.020 | 0.045 | |
Saliva Altered (10039379) |
26 (0.002%) | 0.004 | 10 (0.003%) |
0.006 | 14 (0.003%) |
0.020 | 3 (0.004%) |
0.015 | 1 (0.084%) |
0.400 | 0.783 | <0.001 | |
Noninfective Sialoadenitis (10075243) |
52 (0.005%) | 0.008 | 6 (0.002%) |
0.004 | 12 (0.002%) |
0.017 | 1 (0.001%) |
0.005 | 0 (0%) | 0 | 0.055 | <0.001 | |
Saliva Discolouration (10049069) |
3 ( < 0.001%) | <0.001 | 2 (0.001%) |
0.001 | 2 (<0.001%) |
0.003 | 0 (0%) | 0 | 0 (0%) | 0 | 1.000 | 0.147 | |
Salivary Gland Calculus (10039394) |
3 (<0.001%) | <0.001 | 0 (0%) | 0 | 7 (0.001%) |
0.010 | 0 (0%) | 0 | 0 (0%) | 0 | 0.009 | <0.001 | |
Salivary Gland Disorder (10061935) |
13 (0.001%) | 0.002 | 0 (0%) | 0 | 3 (0.001%) |
0.004 | 0 (0%) | 0 | 0 (0%) | 0 | 0.424 | 0.205 | |
Salivary Gland Enlargement (10039408) |
64 (0.006%) | 0.010 | 11 (0.003%) |
0.007 | 14 (0.003%) |
0.020 | 2 (0.003%) |
0.010 | 0 (0%) | 0 | 0.018 | 0.022 | |
Salivary Gland Mass (10057002) |
4 (<0.001%) | 0.001 | 0 (0%) | 0 | 1 (<0.001%) |
0.001 | 0 (0%) | 0 | 0 (0%) | 0 | 1.000 | 0.405 | |
Salivary Gland Pain (10039421) |
50 (0.005%) | 0.008 | 9 (0.003%) |
0.006 | 22 (0.004%) |
0.032 | 0 (0%) | 0 | 0 (0%) | 0 | 0.747 | <0.001 | |
Salivary Hypersecretion (10039424) |
284 (0.026%) | 0.044 | 64 (0.020%) |
0.041 | 127 (0.025%) |
0.185 | 15 (0.022%) |
0.076 | 2 (0.167%) |
0.800 | 0.873 | <0.001 | |
| Salivary Duct Inflammation (10056681) | 3 (<0.001%) | <0.001 | 2 (0.001%) |
0.001 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0.331 | 1.000 | |
| Atrophic Glossitis (10069085) | 4 (<0.001%) |
0.001 | 1 (<0.001%) |
0.001 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0.331 | 0.459 | Tongue-related AEc |
Glossitis (10018386) |
153 (0.014%) |
0.024 | 32 (0.010%) |
0.020 | 47 (0.009%) |
0.068 | 3 (0.004%) |
0.015 | 0 (0%) | 0 | 0.008 | <0.001 | |
| Glossodynia (10018388) | 500 (0.047%) |
0.077 | 118 (0.036%) |
0.075 | 276 (0.054%) |
0.401 | 14 (0.021%) |
0.071 | 1 (0.084%) | 0.400 | 0.102 | <0.001 | |
| Hypertrophy of Tongue Papillae (10020893) | 14 (0.001%) |
0.002 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0.015 | 0.216 | |
| Plicated Tongue (10035630) | 13 (0.001%) |
0.002 | 3 (0.001%) |
0.002 | 10 (0.002%) |
0.015 | 0 (0%) | 0 | 0 (0%) | 0 | 0.425 | <0.001 | |
| Stiff Tongue (10081491) | 30 (0.003%) |
0.005 | 4 (0.001%) |
0.003 | 8 (0.002%) |
0.012 | 2 (0.003%) |
0.010 | 1 (0.084%) | 0.400 | 0.419 | 0.004 | |
| Strawberry Tongue (10051495) | 3 (<0.001%) | <0.001 | 0 (0%) | 0 | 1 (<0.001%) |
0.001 | 0 (0%) | 0 | 0 (0%) | 0 | 1.000 | 0.311 | |
| Swollen Tongue (10042727) | 2351 (0.219%) |
0.361 | 723 (0.224%) |
0.462 | 961 (0.183%) |
0.614 | 66 (0.095%) |
0.101 | 2 (0.167%) | 0.800 | <0.001 | <0.001 | |
| Tongue Blistering (10043942) | 105 (0.010%) |
0.016 | 31 (0.010%) |
0.020 | 52 (0.010%) |
0.076 | 5 (0.007%) |
0.025 | 0 (0%) | 0 | 0.041 | <0.001 | |
| Tongue Coated (10043945) | 98 (0.009%) |
0.015 | 17 (0.005%) |
0.011 | 48 (0.009%) |
0.070 | 8 (0.012%) |
0.041 | 1 (0.084%) | 0.400 | 0.382 | <0.001 | |
| Tongue Discolouration (10043949) | 121 (0.011%) |
0.019 | 36 (0.011%) |
0.023 | 59 (0.011%) |
0.086 | 9 (0.013%) |
0.046 | 1 (0.084%) | 0.400 | 0.846 | <0.001 | |
| Tongue Discomfort (10077855) | 445 (0.042%) |
0.068 | 102 (0.032%) |
0.065 | 144 (0.028%) |
0.209 | 19 (0.028%) |
0.097 | 0 (0%) | 0 | <0.001 | <0.001 | |
| Tongue Disorder (10043951) | 190 (0.018%) |
0.029 | 44 (0.014%) |
0.028 | 51 (0.010%) |
0.074 | 6 (0.009%) |
0.031 | 0 (0%) | 0 | <0.001 | <0.001 | |
| Tongue Dry (10049713) | 71 (0.007%) |
0.011 | 21 (0.006%) |
0.013 | 34 (0.007%) |
0.049 | 2 (0.003%) |
0.010 | 0 (0%) | 0 | 0.824 | <0.001 | |
| Tongue Eruption (10052002) | 59 (0.006%) |
0.009 | 8 (0.002%) |
0.005 | 1 (<0.001%) |
0.001 | 4 (0.006%) |
0.020 | 0 (0%) | 0 | <0.001 | 0.405 | |
| Tongue Erythema (10079075) | 63 (0.006%) |
0.010 | 22 (0.007%) |
0.014 | 23 (0.004%) |
0.033 | 4 (0.006%) |
0.020 | 0 (0%) | 0 | 0.259 | <0.001 | |
| Tongue Exfoliation (10064488) | 9 (0.001%) |
0.001 | 5 (0.002%) |
0.003 | 6 (0.001%) |
0.009 | 0 (0%) | 0 | 0 (0%) | 0 | 0.851 | 0.003 | |
| Tongue Induration (10084548) | 1 (<0.001%) |
<0.001 | 1 (<0.001%) |
0.001 | 1 (<0.001%) |
0.001 | 0 (0%) | 0 | 0 (0%) | 0 | 1.000 | 0.173 | |
| Tongue Movement Disturbance (10043963) | 52 (0.005%) |
0.008 | 16 (0.005%) |
0.010 | 16 (0.003%) |
0.023 | 3 (0.004%) |
0.015 | 0 (0%) | 0 | 0.152 | <0.001 | |
| Tongue Oedema (10043967) | 454 (0.042%) |
0.070 | 76 (0.023%) |
0.049 | 122 (0.024%) |
0.177 | 9 (0.013%) |
0.046 | 1 (0.084%) | 0.400 | <0.001 | <0.001 | |
| Tongue Pigmentation (10069164) | 0 (0%) | 0 | 1 (<0.001%) |
0.001 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 1.000 | 0.741 | |
| Tongue Pruritus (10070072) | 192 (0.018%) |
0.030 | 34 (0.011%) |
0.022 | 26 (0.005%) |
0.038 | 0 (0%) | 0 | 0 (0%) | 0 | <0.001 | 0.813 | |
| Tongue Rough (10043977) | 24 (0.002%) |
0.004 | 5 (0.002%) |
0.003 | 9 (0.002%) |
0.013 | 0 (0%) | 0 | 0 (0%) | 0 | 0.551 | 0.004 | |
| Tongue Spasm (10043981) | 23 (0.002%) |
0.004 | 7 (0.002%) |
0.004 | 6 (0.001%) |
0.009 | 0 (0%) | 0 | 0 (0%) | 0 | 0.135 | 0.172 | |
| Tongue Thrust (10082545) | 1 (<0.001%) |
<0.001 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 1.000 | 0.741 | |
| Tongue Ulceration (10043991) | 92 (0.009%) |
0.014 | 25 (0.008%) |
0.016 | 46 (0.009%) |
0.067 | 7 (0.010%) |
0.036 | 0 (0%) | 0 | 0.674 | <0.001 | |
| Trichoglossia (10080276) | 18 (0.002%) |
0.003 | 1 (<0.001%) |
0.001 | 7 (0.001%) |
0.010 | 2 (0.003%) |
0.010 | 0 (0%) | 0 | 0.912 | <0.001 | |
| Acquired Macroglossia (10058835) | 2 (<0.001%) |
<0.001 | 0 (0%) | 0 | 3 (0.001%) |
0.004 | 0 (0%) | 0 | 0 (0%) | 0 | 0.155 | <0.001 | |
| Ankyloglossia Acquired (10049243) | 1 (<0.001%) |
<0.001 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 1.000 | 0.741 | |
| Atrophy of Tongue Papillae (10003712) | 4 (<0.001%) |
0.001 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0.328 | 0.508 | |
| Angular Cheilitis (10002509) | 29 (0.003%) |
0.004 | 5 (0.002%) |
0.003 | 15 (0.003%) |
0.022 | 3 (0.004%) |
0.015 | 0 (0%) | 0 | 0.502 | <0.001 | Lip-related AE |
Cheilitis (10008417) |
109 (0.010%) |
0.017 | 36 (0.011%) |
0.023 | 59 (0.011%) |
0.086 | 3 (0.004%) |
0.015 | 0 (0%) | 0 | 0.925 | <0.001 | |
| Chapped Lips (10049047) | 53 (0.005%) |
0.008 | 21 (0.006%) |
0.013 | 26 (0.005%) |
0.038 | 4 (0.006%) |
0.020 | 0 (0%) | 0 | 0.985 | <0.001 | |
| Lip Blister (10049307) | 119 (0.011%) |
0.018 | 28 (0.009%) |
0.018 | 43 (0.008%) |
0.063 | 2 (0.003%) |
0.010 | 0 (0%) | 0 | 0.082 | <0.001 | |
| Lip Discolouration (10024549) | 42 (0.004%) |
0.006 | 14 (0.004%) |
0.009 | 14 (0.003%) |
0.020 | 5 (0.007%) |
0.025 | 0 (0%) | 0 | 0.517 | <0.001 | |
| Lip Disorder (10048470) | 52 (0.005%) |
0.008 | 15 (0.005%) |
0.010 | 18 (0.003%) |
0.026 | 1 (0.001%) |
0.005 | 0 (0%) | 0 | 0.170 | 0.001 | |
Lip Dry (10024552) |
129 (0.012%) |
0.020 | 34 (0.011%) |
0.022 | 90 (0.017%) |
0.131 | 7 (0.010%) |
0.036 | 0 (0%) | 0 | 0.006 | <0.001 | |
| Lip Erythema (10080124) | 54 (0.005%) |
0.008 | 12 (0.004%) |
0.008 | 8 (0.002%) |
0.012 | 2 (0.003%) |
0.010 | 0 (0%) | 0 | 0.003 | 0.337 | |
| Lip Exfoliation (10064482) | 16 (0.001%) |
0.002 | 5 (0.002%) |
0.003 | 9 (0.002%) |
0.013 | 2 (0.003%) |
0.010 | 0 (0%) | 0 | 0.673 | <0.001 | |
| Lip Oedema (10024558) | 717 (0.067%) |
0.110 | 141 (0.044%) |
0.090 | 190 (0.037%) |
0.276 | 20 (0.030%) |
0.102 | 1 (0.084%) | 0.400 | <0.001 | <0.001 | |
Lip Pain (10024561) |
172 (0.016%) |
0.026 | 40 (0.012%) |
0.026 | 70 (0.014%) |
0.102 | 6 (0.009%) |
0.031 | 0 (0%) | 0 | 0.289 | <0.001 | |
| Lip Pruritus (10070721) | 175 (0.016%) |
0.027 | 33 (0.010%) |
0.021 | 30 (0.006%) |
0.044 | 3 (0.004%) |
0.015 | 0 (0%) | 0 | <0.001 | 0.047 | |
Lip Scab (10082767) |
2 (<0.001%) |
<0.001 | 1 (<0.001%) |
0.001 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0.560 | 0.567 | |
| Lip Swelling (10024570) | 2512 (0.234%) |
0.386 | 801 (0.248%) |
0.512 | 1414 (0.275%) |
2.056 | 86 (0.129%) |
0.438 | 3 (0.251%) |
1.200 | 0.008 | <0.001 | |
| Lip Ulceration (10024572) | 28 (0.003%) |
0.004 | 12 (0.004%) |
0.008 | 23 (0.004%) |
0.033 | 2 (0.003%) |
0.010 | 0 (0%) | 0 | 0.143 | <0.001 | |
| Lip Erosion (10051992) | 3 (<0.001%) |
<0.001 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 0.560 | 0.567 | |
| Palatal Disorder (10052453) | 23 (0.002%) |
0.004 | 4 (0.001%) |
0.003 | 13 (0.003%) |
0.019 | 4 (0.006%) |
0.020 | 0 (0%) | 0 | 0.239 | <0.001 | Palate-related AEd |
| Palatal Oedema (10056998) | 163 (0.015%) |
0.025 | 24 (0.007%) |
0.015 | 24 (0.005%) |
0.035 | 4 (0.006%) |
0.020 | 0 (0%) | 0 | <0.001 | 0.121 | |
| Palatal Swelling (10074403) | 82 (0.008%) |
0.013 | 25 (0.008%) |
0.016 | 25 (0.005%) |
0.036 | 2 (0.003%) |
0.010 | 0 (0%) | 0 | 0.024 | <0.001 | |
| Palatal Ulcer (10077519) | 3 (<0.001%) |
<0.001 | 1 (<0.001%) |
0.001 | 2 (<0.001%) |
0.003 | 1 (0.001%) |
0.005 | 0 (0%) | 0 | 0.427 | 0.003 | |
| Anaesthesia Oral (10082548) | 91 (0.008%) |
0.014 | 20 (0.006%) |
0.013 | 21 (0.004%) |
0.031 | 1 (0.001%) |
0.005 | 0 (0%) | 0 | 0.002 | 0.015 | Other Sensory AEe |
| Paraesthesia Oral (10057372) | 4027 (0.376%) |
0.619 | 789 (0.244%) |
0.505 | 1300 (0.253%) |
1.890 | 100 (0.150%) |
0.510 | 6 (0.501%) |
2.399 | <0.001 | <0.001 | |
| Hypoaesthesia Oral (10057371) | 2544 (0.237%) |
0.391 | 624 (0.193%) |
0.399 | 887 (0.172%) |
1.290 | 93 (0.139%) |
0.474 | 0 (0%) | 0 | <0.001 | <0.001 | |
| Burning Mouth Syndrome (10068065) | 25 (0.002%) |
0.004 | 3 (0.001%) |
0.002 | 14 (0.003%) |
0.020 | 2 (0.003%) |
0.010 | 0 (0%) | 0 | 0.397 | <0.001 | |
| Oral Dysaesthesia (10050820) | 67 (0.006%) |
0.010 | 14 (0.004%) |
0.009 | 14 (0.003%) |
0.020 | 2 (0.003%) |
0.010 | 0 (0%) | 0 | 0.007 | 0.044 | |
| Aphthous Ulcer (10002959) | 784 (0.073%) |
0.121 | 196 (0.061%) |
0.125 | (0%) | 0 | 45 (0.067%) |
0.229 | 1 (0.084%) | 0.400 | <0.001 | <0.001 | Oral Mucosa-related AEf |
| Coating in Mouth (10075366) | 12 (0.001%) |
0.002 | 6 (0.002%) |
0.004 | 5 (0.001%) |
0.007 | 0 (0%) | 0 | 0 (0%) | 0 | 0.563 | 0.119 | |
| Leukoplakia Oral (10024396) | 8 (0.001%) |
0.001 | 5 (0.002%) |
0.003 | 2 (<0.001%) |
0.003 | 1 (0.001%) |
0.005 | 0 (0%) | 0 | 0.424 | 0.205 | |
| Mouth Swelling (10075203) | 435 (0.041%) |
0.067 | 163 (0.050%) |
0.104 | 193 (0.038%) |
0.281 | 17 (0.025%) |
0.087 | 0 (0%) | 0 | 0.036 | <0.001 | |
| Oedema Mouth (10030110) | 96 (0.009%) |
0.015 | 15 (0.005%) |
0.010 | 31 (0.006%) |
0.045 | 4 (0.006%) |
0.020 | 0 (0%) | 0 | 0.177 | <0.001 | |
| Oral Blood Blister (10076590) | 31 (0.003%) |
0.005 | 19 (0.006%) |
0.012 | 49 (0.010%) |
0.071 | 2 (0.003%) |
0.010 | 0 (0%) | 0 | 0.024 | <0.001 | |
| Oral Discomfort (10030973) | 647 (0.060%) |
0.099 | 167 (0.052%) |
0.107 | 205 (0.040%) |
0.298 | 22 (0.033%) |
0.112 | 1 (0.084%) | 0.400 | <0.001 | <0.001 | |
| Oral Disorder (10067621) | 134 (0.012%) |
0.021 | (0%) | 0 | 39 (0.008%) |
0.057 | 5 (0.007%) |
0.025 | 0 (0%) | 0 | 0.197 | <0.001 | |
| Oral Lichen Planus (10030983) | 51 (0.005%) |
0.008 | 12 (0.004%) |
0.008 | 20 (0.004%) |
0.029 | 1 (0.001%) |
0.005 | 0 (0%) | 0 | 0.443 | <0.001 | |
| Oral Lichenoid Reaction (10083833) | 3 (<0.001%) |
<0.001 | (0%) | 0 | 1 (<0.001%) |
0.001 | 0 (0%) | 0 | 0 (0%) | 0 | 1.000 | 0.311 | |
| Oral Mucosa Erosion (10064594) | 21 (0.002%) |
0.003 | 3 (0.001%) |
0.002 | 6 (0.001%) |
0.009 | 0 (0%) | 0 | 0 (0%) | 0 | 0.352 | 0.063 | |
| Oral Mucosal Blistering (10030995) | 1 (<0.001%) |
<0.001 | 98 (0.030%) |
0.063 | 122 (0.024%) |
0.179 | 11 (0.016%) |
0.056 | 0 (0%) | 0 | <0.001 | <0.001 | |
| Oral Mucosal Discolouration (10030996) | 6 (0.001%) |
0.001 | 2 (0.001%) |
0.001 | 3 (0.001%) |
0.004 | 0 (0%) | 0 | 0 (0%) | 0 | 1.000 | 0.053 | |
| Oral Mucosal Eruption (10030997) | 56 (0.005%) |
0.009 | 24 (0.007%) |
0.015 | 33 (0.006%) |
0.048 | 3 (0.004%) |
0.015 | 0 (0%) | 0 | 0.778 | <0.001 | |
| Oral Mucosal Erythema (10067418) | 83 (0.008%) |
0.013 | 13 (0.004%) |
0.008 | 24 (0.005%) |
0.035 | 0 (0%) | 0 | 0 (0%) | 0 | 0.031 | <0.001 | |
| Oral Mucosal Exfoliation (10064487) | 27 (0.003%) |
0.004 | 6 (0.002%) |
0.004 | 19 (0.004%) |
0.028 | 1 (0.001%) |
0.005 | 0 (0%) | 0 | 0.238 | <0.001 | |
| Oral Mucosal Roughening (10084009) | 9 (0.001%) |
0.001 | 3 (0.001%) |
0.002 | 2 (<0.001%) |
0.003 | 0 (0%) | 0 | 0 (0%) | 0 | 0.377 | 0.580 | |
| Oral Pain (10031009) | 502 (0.047%) |
0.077 | 154 (0.048%) |
0.099 | 399 (0.078%) |
0.580 | 24 (0.036%) |
0.122 | 0 (0%) | 0 | <0.001 | <0.001 | |
| Oral Pigmentation (10077552) | 2 (<0.001%) |
<0.001 | (0%) | 0 | (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 1.000 | 0.640 | |
| Oral Pruritus (10052894) | 288 (0.027%) |
0.044 | 40 (0.012%) |
0.026 | 43 (0.008%) |
0.063 | 3 (0.004%) |
0.015 | 0 (0%) | 0 | <0.001 | 0.116 | |
| Oral Purpura (10083533) | 3 (<0.001%) |
<0.001 | (0%) | 0 | 3 (0.001%) |
0.004 | 0 (0%) | 0 | 0 (0%) | 0 | 0.369 | 0.001 | |
| Stomatitis (10042128) | 479 (0.045%) |
0.074 | 152 (0.047%) |
0.097 | 193 (0.038%) |
0.281 | 24 (0.036%) |
0.122 | 0 (0%) | 0 | 0.016 | <0.001 | |
| Mouth Ulceration (10028034) | 472 (0.044%) |
0.073 | 127 (0.039%) |
0.081 | 607 (0.118%) |
0.883 | 9 (0.013%) |
0.046 | 0 (0%) | 0 | <0.001 | <0.001 | |
| Oral Disorder (10061326) | 134 (0.012%) |
0.021 | (0%) | 0 | (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | <0.001 | 0.001 | |
| Oral Mucosal Hypertrophy (10062956) | 1 (<0.001%) |
<0.001 | (0%) | 0 | (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 1.000 | 0.741 | |
| Oral Mucosal Scab (10082769) | 2 (<0.001%) |
<0.001 | (0%) | 0 | (0%) | 0 | 0 (0%) | 0 | 0 (0%) | 0 | 1.000 | 1.000 | |
| Oral Papule (10031010) | 6 (0.001%) |
0.001 | (0%) | 0 | 1 (<0.001%) |
0.001 | 1 (0.001%) |
0.005 | 0 (0%) | 0 | 1.000 | 0.183 | |
- Note: *Data was updated as of September 17th, 2022. Bold values are significant at p ≤ 0.05
- Abbreviation: mRNA, messenger RNA.
- Chi-squared test (χ2) and Fisher's exact test had been used with a significance level (Sig.) <0.05.
- a The preferred term Sensitivity of Teeth (10040012) was not reported in any vaccine groups.
- b The preferred terms Salivary Duct Stenosis (10039388), Sialoadenitis (10040628), Salivary Duct Obstruction (10039386), and Salivary Gland Induration (10071363) were not reported in any vaccine groups.
- c The preferred terms Macroglossia (10025391), Tongue Fungal Infection (10075845), and Tongue Paralysis (10043972) were not reported in any vaccine groups.
- d The preferred term Palatal Palsy (10072012) was not reported in any vaccine groups.
- e The preferred term Burn Oral Cavity (10075532) was not reported in any vaccine groups.
- f The preferred terms Aphthous Stomatitis (10002958), Circumoral Oedema (10052250), Circumoral Swelling (10081703), Oral Candidiasis (10030963), Oral Fungal Infection (10061324), Oral Herpes (10067152), Oral Pustule (10056674), Oral Viral Infection (10065234), Oropharyngeal Blistering (10067950), Oropharyngeal Plaque (10067721), Perioral Dermatitis (10034541), Buccal Mucosal Roughening (10048479), Mouth Plaque (10028032), and Oral Mucosal Petechiae (10030998) were not reported in any vaccine groups.
Lip swelling was the most common lip-related SE (0.243%), followed by lip oedema (0.054%), lip pain (0.015%), lip dry (0.013%), lip pruritus (0.012%), cheilitis (0.010%), and lip blister (0.010%). Palatal oedema (0.011%) and palatal swelling (0.007%) were the most common palate-related SEs. Among oral mucosa-related SEs, mouth ulceration (0.061%) was the most common SE, followed by oral pain (0.055%), oral discomfort (0.053%), aphthous ulcer (0.052%), stomatitis (0.043%), mouth swelling (0.041%), and oral pruritus (0.019%) (Table 2).
3.3 Vaccine-specific prevalence of oral SEs
Among the top 20 oral SEs, salivary hypersecretion (Sig. = 0.839) and glossodynia (Sig. = 0.102) were not different between mRNA-based and viral vector-based vaccine groups.
Dysgeusia (Sig. < 0.001), ageusia (Sig. < 0.001), lip swelling (Sig. = 0.008), dry mouth (Sig. < 0.001), taste disorder (Sig. = 0.015), toothache (Sig. < 0.001), mouth ulceration (Sig. < 0.001), and oral pain (Sig. < 0.001) were more significantly common in the viral vector-based vaccines group.
On the other hand, oral paraesthesia (Sig. < 0.001), oral hypoesthesia (Sig. < 0.001), swollen tongue (Sig. < 0.001), lip oedema (Sig. < 0.001), oral discomfort (Sig. < 0.001), aphthous ulcer (Sig. < 0.001), mouth swelling (Sig. = 0.036), tongue discomfort (Sig. < 0.001), and tongue oedema (Sig. < 0.001) were significantly more common in the mRNA-based vaccines group (Table 2).
3.4 Sex-specific prevalence of SEs
Dysgeusia (0.438%) was the most common oral SE among females, followed by oral paraesthesia (0.399%), ageusia (0.296%), lip swelling (0.279%), oral hypoesthesia (0.248%), dry mouth (0.242%), swollen tongue (0.191%), and taste disorder (0.183%). Similarly, dysgeusia (0.244%) was the most common oral SE among males, followed by lip swelling (0.162%), ageusia (0.161%), dry mouth (0.152%), taste disorder (0.148%), oral paraesthesia (0.125%), oral hypoesthesia (0.119%), toothache (0.085%), and swollen tongue (0.072%) (Figure 1).
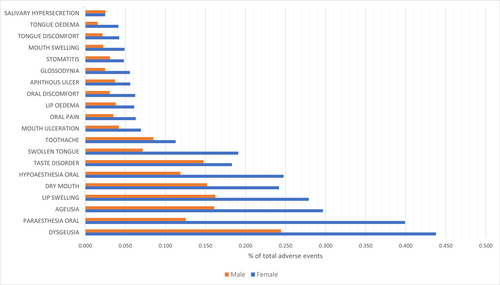
Regarding the top 20 oral SEs, females had significantly (Sig. < 0.001) a higher prevalence of all SEs, except for salivary hypersecretion, which was equally prevalent (Sig. = 0.839) among females (0.025%) and males (0.025%) (Table 3).
| Preferred term | Female | Male | Sig. (F vs. M) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mRNA-based | Viral vector-based | Sig. | Total | mRNA-based | Viral vector-based | Sig. | Total | |||
Dysgeusia (10013911) |
4223 (0.416%) |
1958 (0.487%) |
<0.001 | 6181 (0.438%) |
1040 (0.247%) |
414 (0.238%) |
0.599 | 1454 (0.244%) |
<0.001 | |
Paraesthesia Oral (10057372) |
4205 (0.434%) |
1208 (0.307%) |
<0.001 | 5413 (0.399%) |
551 (0.137%) |
166 (0.098%) |
<0.001 | 717 (0.125%) |
<0.001 | |
Ageusia (10001480) |
2733 (0.269%) |
1458 (0.362%) |
<0.001 | 4191 (0.296%) |
398 (0.094%) |
560 (0.322%) |
<0.001 | 958 (0.161%) |
<0.001 | |
Lip Swelling (10024570) |
2646 (0.273%) |
1142 (0.290%) |
0.092 | 3788 (0.279%) |
618 (0.154%) |
310 (0.182%) |
0.014 | 928 (0.162%) |
<0.001 | |
Dry Mouth (10013781) |
1971 (0.204%) |
1314 (0.334%) |
<0.001 | 3285 (0.242%) |
531 (0.132%) |
338 (0.199%) |
<0.001 | 869 (0.152%) |
<0.001 | |
Hypoaesthesia Oral (10057371) |
2584 (0.267%) |
778 (0.198%) |
<0.001 | 3362 (0.248%) |
509 (0.127%) |
170 (0.100%) |
0.008 | 679 (0.119%) |
<0.001 | |
Taste Disorder (10082490) |
1757 (0.101%) |
832 (0.207%) |
<0.001 | 2589 (0.183%) |
641 (0.152%) |
238 (0.137%) |
0.172 | 879 (0.148%) |
<0.001 | |
Swollen Tongue (10042727) |
2611 (0.047%) |
0 (0%) | <0.001 | 2611 (0.191%) |
412 (0.103%) |
0 (0%) | <0.001 | 412 (0.072%) |
<0.001 | |
Toothache (10044055) |
975 (0.054%) |
559 (0.142%) |
<0.001 | 1534 (0.113%) |
333 (0.083%) |
152 (0.089%) |
0.437 | 485 (0.085%) |
<0.001 | |
Mouth Ulceration (10028034) |
453 (0.047%) |
492 (0.125%) |
<0.001 | 945 (0.069%) |
133 (0.033%) |
106 (0.062%) |
<0.001 | 239 (0.042%) |
<0.001 | |
Oral Pain (10031009) |
521 (0.054%) |
335 (0.085%) |
<0.001 | 856 (0.063%) |
123 (0.031%) |
77 (0.045%) |
0.007 | 200 (0.035%) |
<0.001 | |
Lip Oedema (10024558) |
678 (0.070%) |
151 (0.038%) |
<0.001 | 829 (0.061%) |
163 (0.041%) |
54 (0.032%) |
0.119 | 217 (0.038%) |
<0.001 | |
Oral Discomfort (10030973) |
673 (0.070%) |
173 (0.044%) |
<0.001 | 846 (0.062%) |
123 (0.031%) |
51 (0.030%) |
0.904 | 174 (0.030%) |
<0.001 | |
Aphthous Ulcer (10002959) |
764 (0.079%) |
0 (0%) | <0.001 | 764 (0.056%) |
211 (0.053%) |
0 (0%) | <0.001 | 211 (0.037%) |
<0.001 | |
Glossodynia (10018388) |
512 (0.053%) |
244 (0.062%) |
0.041 | 756 (0.056%) |
101 (0.025%) |
40 (0.024%) |
0.724 | 141 (0.025%) |
<0.001 | |
Stomatitis (10042128) |
493 (0.051%) |
160 (0.041%) |
0.013 | 653 (0.048%) |
126 (0.031%) |
50 (0.029%) |
0.702 | 176 (0.031%) |
<0.001 | |
Mouth Swelling (10075203) |
499 (0.052%) |
167 (0.042%) |
0.029 | 666 (0.049%) |
90 (0.022%) |
39 (0.023%) |
0.900 | 129 (0.023%) |
<0.001 | |
Tongue Discomfort (10077855) |
446 (0.046%) |
129 (0.033%) |
<0.001 | 575 (0.042%) |
93 (0.023%) |
30 (0.018%) |
0.195 | 123 (0.022%) |
<0.001 | |
Tongue Oedema (10043967) |
455 (0.047%) |
107 (0.027%) |
<0.001 | 562 (0.041%) |
66 (0.016%) |
22 (0.013%) |
0.332 | 88 (0.015%) |
<0.001 | |
Salivary Hypersecretion (10039424) |
234 (0.024%) |
102 (0.026%) |
0.558 | 336 (0.025%) |
110 (0.027%) |
34 (0.020%) |
0.108 | 144 (0.025%) |
0.839 | |
- Note: Chi-squared test (χ2) and Fisher's exact test had been used with a significance level (Sig.) <0.05.
- Abbreviation: mRNA, messenger RNA.
Among females, dysgeusia, ageusia, dry mouth, taste disorder, toothache, and mouth ulceration were significantly more associated with viral vector-based than mRNA-based vaccines. On the other hand, oral paraesthesia, oral hypoesthesia, swollen tongue, lip oedema, and tongue oedema were significantly more associated with mRNA-based vaccines (Figure 2).
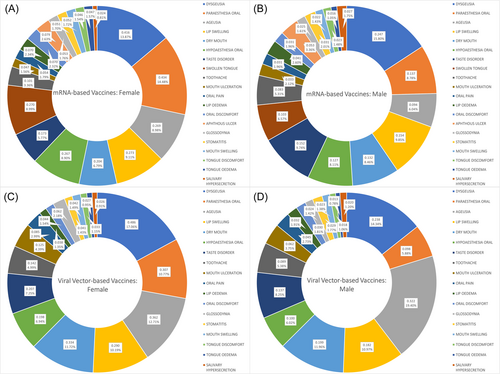
Among males, ageusia, lip swelling, dry mouth, and mouth ulceration were significantly more associated with viral vector-based than mRNA-based vaccines. On the other hand, oral paraesthesia, oral hypoesthesia, and swollen tongue were significantly more associated with mRNA-based vaccines (Figure 2).
3.5 Age-specific prevalence of oral SEs
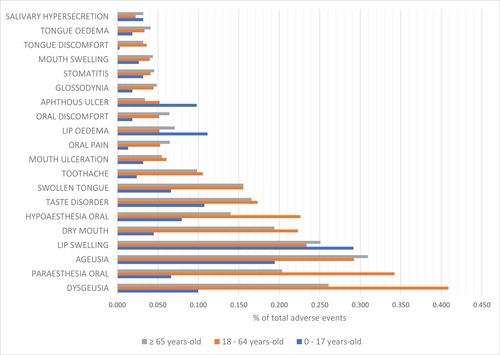
Lip swelling (0.292%) was the most common oral SE among the minors group (0–17 years old), followed by ageusia (0.194%), lip oedema (0.111%), taste disorder (0.107%), and dysgeusia (0.100%). Dysgeusia (0.409%) was the most common oral SE among the adults' group (18–64 years old), followed by oral paraesthesia (0.342%), ageusia (0.292%), lip swelling (0.234%), oral hypoesthesia (0.226%), dry mouth (0.223%), taste disorder (0.173%), and swollen tongue (0.156%). Ageusia (0.309%) was the most common oral SE among the seniors' group (≥65 years old), followed by dysgeusia (0.261%), lip swelling (0.251%), oral paraesthesia (0.203%), dry mouth (0.194%), taste disorder (0.166%), and swollen tongue (0.156%) (Figure 3).
Viral vector-based vaccines were associated with a significantly higher frequency of dysgeusia (Sig. = 0.002), oral paraesthesia (Sig. = 0.032), dry mouth (Sig. < 0.001), and toothache (Sig. < 0.001) among the minors age group (0–17 years old) compared with mRNA-based vaccines. Similarly, dysgeusia (Sig. = 0.004), dry mouth (Sig. < 0.001), toothache (Sig. < 0.001), and mouth ulceration (Sig. < 0.001) were more significantly associated with viral vector-based vaccines than mRNA-based vaccines in the seniors' group (≥65 years old) (Figure 4).
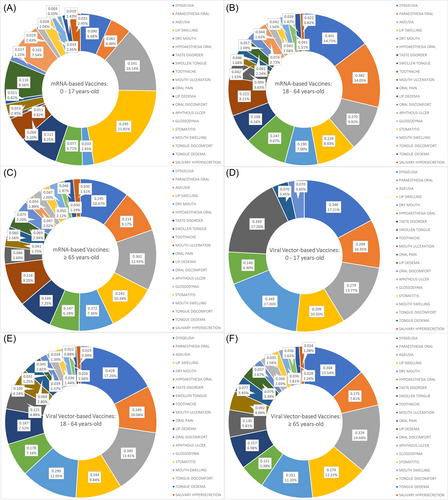
In the adults age group (18–64 years old), dysgeusia (Sig. = 0.013), ageusia (Sig. < 0.001), dry mouth (Sig. < 0.001), taste disorder (Sig. = 0.009), mouth ulceration (Sig. < 0.001), and oral pain (Sig. < 0.001) were more significantly common in the viral vector-based vaccines group (Table 4).
| Preferred term | mRNA-based vaccines | Viral vector-based vaccines | Sig. (mRNA vs. viral vector) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
0–17 years old |
18–64 years old |
≥65 years old |
Sig. | 0–17 years old |
18–64 years old |
≥65 years old |
Sig. | 0–17 years old |
18–64 years old |
≥65 years old |
|
Dysgeusia (10013911) |
34 (0.090%) |
4498 (0.401%) |
542 (0.245%) |
<0.001 | 5 (0.348%) |
2011 (0.428%) |
254 (0.304%) |
<0.001 | 0.002 | 0.013 | 0.004 |
Paraesthesia Oral (10057372) |
22 (0.061%) |
4104 (0.382%) |
462 (0.214%) |
<0.001 | 3 (0.209%) |
1144 (0.249%) |
145 (0.175%) |
<0.001 | 0.032 | <0.001 | 0.034 |
Ageusia (10001480) |
72 (0.191%) |
3031 (0.270%) |
669 (0.302%) |
<0.001 | 4 (0.279%) |
1621 (0.345%) |
275 (0.329%) |
0.697 | 0.460 | <0.001 | 0.233 |
Lip Swelling (10024570) |
107 (0.295%) |
2463 (0.229%) |
521 (0.242%) |
0.026 | 3 (0.209%) |
1121 (0.244%) |
227 (0.274%) |
0.265 | 0.557 | 0.084 | 0.112 |
Dry Mouth (10013781) |
12 (0.033%) |
2046 (0.190%) |
371 (0.172%) |
<0.001 | 5 (0.349%) |
1373 (0.299%) |
208 (0.251%) |
0.059 | <0.001 | <0.001 | <0.001 |
Hypoaesthesia Oral (10057371) |
28 (0.077%) |
2649 (0.247%) |
317 (0.147%) |
<0.001 | 2 (0.140%) |
816 (0.178%) |
100 (0.121%) |
0.001 | 0.410 | <0.001 | 0.086 |
Taste Disorder (10082490) |
42 (0.111%) |
1882 (0.168%) |
375 (0.169%) |
0.029 | 0 (0%) | 876 (0.187%) |
131 (0.157%) |
0.047 | 0.206 | 0.009 | 0.445 |
Swollen Tongue (10042727) |
25 (0.069%) |
2398 (0.223%) |
466 (0.216%) |
<0.001 | 0 (0%) | 0 (0%) | 0 (0%) | N/A | 0.320 | <0.001 | <0.001 |
Toothache (10044055) |
4 (0.011%) |
1060 (0.099%) |
186 (0.086%) |
<0.001 | 5 (0.349%) |
556 (0.121%) |
108 (0.130%) |
0.039 | <0.001 | <0.001 | 0.001 |
Mouth Ulceration (10028034) |
12 (0.033%) |
448 (0.042%) |
88 (0.041%) |
0.721 | 0 (0%) | 483 (0.105%) |
76 (0.092%) |
0.260 | 0.491 | <0.001 | <0.001 |
Oral Pain (10031009) |
4 (0.011%) |
491 (0.046%) |
129 (0.060%) |
<0.001 | 1 (0.070%) |
319 (0.069%) |
64 (0.077%) |
0.736 | 0.058 | <0.001 | 0.093 |
Lip Oedema (10024558) |
42 (0.116%) |
654 (0.061%) |
148 (0.069%) |
<0.001 | 0 (0%) | 142 (0.031%) |
63 (0.076%) |
<0.001 | 0.198 | <0.001 | 0.493 |
Oral Discomfort (10030973) |
6 (0.017%) |
612 (0.057%) |
161 (0.075%) |
<0.001 | 1 (0.070%) |
179 (0.039%) |
31 (0.037%) |
0.819 | 0.146 | <0.001 | <0.001 |
Aphthous Ulcer (10002959) |
37 (0.102%) |
799 (0.074%) |
102 (0.047%) |
<0.001 | 0 (0%) | 0 (0%) | 0 (0%) | N/A | 0.227 | <0.001 | <0.001 |
Glossodynia (10018388) |
7 (0.019%) |
476 (0.044%) |
95 (0.044%) |
0.080 | 0 (0%) | 208 (0.045%) |
50 (0.060%) |
0.132 | 0.599 | 0.796 | 0.070 |
Stomatitis (10042128) |
12 (0.033%) |
463 (0.043%) |
107 (0.050%) |
0.255 | 0 (0%) | 164 (0.036%) |
29 (0.035%) |
0.771 | 0.491 | 0.038 | 0.094 |
Mouth Swelling (10075203) |
10 (0.028%) |
456 (0.042%) |
101 (0.047%) |
0.243 | 0 (0%) | 158 (0.034%) |
30 (0.036%) |
0.754 | 0.530 | 0.022 | 0.215 |
Tongue Discomfort (10077855) |
1 (0.003%) |
441 (0.041%) |
65 (0.030%) |
<0.001 | 0 (0%) | 118 (0.026%) |
30 (0.036%) |
0.196 | 0.843 | <0.001 | 0.404 |
Tongue Oedema (10043967) |
7 (0.019%) |
414 (0.039%) |
99 (0.046%) |
0.044 | 0 (0%) | 100 (0.022%) |
23 (0.028%) |
0.486 | 0.599 | <0.001 | 0.028 |
Salivary Hypersecretion (10039424) |
12 (0.033%) |
241 (0.022%) |
76 (0.035%) |
0.002 | 0 (0%) | 107 (0.023%) |
20 (0.024%) |
0.836 | 0.491 | 0.748 | 0.130 |
- Note: Chi-squared test (χ2) and Fisher's exact test had been used with a significance level (Sig.) <0.05.
- Abbreviation: mRNA, messenger RNA.
4 DISCUSSION
This cumulative, big-data-based, retrospective analysis aimed to assess the prevalence of oral SEs potentially associated with COVID-19 vaccination in the EEA. The present analysis revealed a low prevalence of oral SEs, with taste, other sensory and anaphylactic SEs being the most common SEs, similar to what was found earlier among the US population. Females and mRNA-based vaccines were associated with higher prevalence of oral SEs. The high frequency of taste disorders could be confounded by several factors such as increased public awareness, breakthrough infections, and long COVID-19.
A recent retrospective analysis for the VAERS reports of oral SEs following COVID-19 vaccination revealed that oral paraesthesia (0.872 case per each 100 received reports) was the most commonly reported SE in the United States, followed by lip swelling (0.844%), ageusia (0.722%), oral hypoesthesia (0.648%), swollen tongue (0.628%), and dysgeusia (0.617%).14 Our study results are consistent with what was found in the United States, as taste, other sensory and anaphylactic SEs, e.g., lip and tongue swelling, were the most common oral SEs. In the present analysis, dysgeusia was the most common (0.381%), followed by oral paraesthesia (0.315%), ageusia (0.296%), lip swelling (0.243%), dry mouth (0.215%), oral hypoaesthesia (0.210%), swollen tongue (0.207%), and taste disorder (0.173%).
Initially published data on COVID-19 vaccine safety by manufacturers and drug regulators in Europe, the United States, Canada, and the United Kingdom provided scanty information about the possibility of oral SEs. These rare or very rare oral SEs included peripheral facial paralysis (Bell's palsy), lymph node enlargement, facial swelling, and orofacial reactions linked to allergy/anaphylaxis.13-17, 25-27 Arguably, Cirillo observed a heterogenicity in the acknowledgment of orofacial SEs in the US compared with Europe.25 The present study did not only cover these oral SEs suggested by the regulators but also found numerous overlooked oral SEs. The need for the wider use of hybrid surveillance systems has been called for since the beginning of COVID-19 mass vaccination to monitor the rare or very rare nonlife-threatening SEs, including oral SEs.28, 29
Although a causative relationship between COVID-19 vaccines and oral SEs has not been verified yet, the probable pathophysiological pathways may include immune cross-reactivity, autoimmune dysregulation, hypersensitivity reactions, molecular mimicry, and allergy to vaccine ingredients.30 Moreover, immune dysregulation can be linked with the aggravation of underlying, often undiagnosed, conditions in susceptible persons. Vaccine-induced reactivation of latent viral infections such as herpes simplex virus type 1 and the varicella-zoster virus may be responsible for some forms of oral symptoms, such as paraesthesia, Bells' palsy, mouth discomfort and ulcerations.31-34
The present study found that females had significantly higher levels of the most common (top 20) oral SEs following COVID-19 vaccination, which is similar to what was found earlier in the United States.14 The same pattern was noticed by Di Spirito et al.13 in their systematic review, where 68.8% of the reported cases were females. However, it is unclear why females had higher reported oral SEs; the prevailing evidence from passive and active surveillance studies confirmed the hypothesis that females' immune response could be stronger, thus triggering more frequent and severe postvaccination side effects generally.35-37 Whether sex-related differences of oral SEs are tailored by mere biologic factors or influenced by sociocultural patterns of femineity versus masculinity, it is strongly required to investigate the real aetiology of these disparities.37
Oral paraesthesia (Sig. < 0.001), swollen tongue (Sig. < 0.001), oral discomfort (Sig. < 0.001), and mouth swelling (Sig. = 0.036) were significantly more common in the mRNA-based vaccines group. Similarly, the VAERS reports indicated that COVID-19 mRNA-based vaccines were associated with a higher frequency of oral paraesthesia (Sig. < 0.001), swollen tongue (Sig. < 0.001), oral discomfort (Sig. = 0.001), and mouth swelling (Sig. = 0.021).14 A recent comprehensive analysis of VAERS data by Sa et al.38 revealed that inflammatory SEs were less common after viral vector-based vaccines, while mRNA-based vaccines were associated with fewer coagulation disorders.
Our study revealed that taste-related SEs were more frequently reported in the viral vector-based vaccines group; however, the VAERS-based study did not detect significant differences between mRNA- and viral vector-based vaccine groups.14 Unlike other vaccines, COVID-19 vaccines were associated with a significantly higher frequency of taste-related SEs in the United States. Several hypotheses can be proposed to explain this finding: (i) the increased public awareness of taste disorders had probably increased during the COVID-19 pandemic due to utilizing taste disorders in differential diagnosis and case triage, (ii) the increased possibility of breakthrough infections that could be associated with taste disorders as clinical complications, and (iii) suffering from taste disorders as a result of long COVID-19.39-41
Hertel et al.42 conducted a historical cohort study (n = 217 863) using data retrieved from the TriNetX database (USA), and their results suggested that the incidence of oral lichen planus (OLP) and oral lichenoid reactions was a rare SE of COVID-19 vaccines, with a significantly higher risk of developing these lesions in vaccinated (0.067%) than nonvaccinated (0.027%) individuals. On the other hand, our study and the VAERS-based study found that the prevalence of OLP was (0.004% and 0.006%, respectively) very uncommon.14
4.1 Strengths
The anatomo-physiological classification used in this study to distinguish oral SEs seemed an adequate solution to deal with a large amount of unorganized data. In addition, this methodologic approach excluded nonvaccination-related symptoms that could mimic postvaccine effects. Using a pan-European dataset like EudraVigilance, which is systematically classified according to vaccine type, sex, and age group, facilitated subgroup analysis to determine high-risk groups, if any. From a clinical practice viewpoint, the subgroup analysis can be useful for oral medicine specialists.
4.2 Limitations
First, all limitations of passive surveillance systems are inherited in this analysis; therefore, the prevalence of oral SEs calculated here should be used as indicative rather than true values. Second, selecting a single, yet multinational, database as an information source (EudraVigilance) may limit the generalizability of the study findings because of the ethnic backgrounds of the included individuals. Third, this analysis did not evaluate patients' medical histories or oral SEs onset or duration, which could have better explained their potential etiologies that might be causally linked with COVID-19 vaccines. Fourthly, the subtle differences between clinical signs and symptoms affecting the oral cavity following inoculations are also prone to self-reported bias.
4.3 Implications
This study results are expected to contribute to the current limited knowledge of oral SEs associated with COVID-19 vaccination. The cumulated and verified large-scale data can be compared to available sources to prepare reports/recommendations to reassure populations not only in the EEA. Our findings may support general medical/dental practitioners, oral medicine, and oral surgery specialists during differential diagnosis processes of noncharacteristic oral pathologies, with various manifestations. Moreover, these findings, as part of global pharmacovigilance protocol, provide an evidence-based, rational basis to manage the “unexplained” symptoms that may occur after COVID-19 vaccination.
5 CONCLUSION
The present study revealed a low prevalence of oral SEs, with taste-related (e.g., dysgeusia and ageusia), other sensory (e.g., oral paraesthesia and oral hypoaesthesia) and anaphylactic (e.g., lip swelling, swollen tongue, lip oedema, and mouth swelling) SEs being the most common SEs in Europe, similar to what was found earlier among the US population. Females and mRNA-based vaccines were associated with higher prevalence of oral SEs. The high frequency of taste-related disorders could be confounded by several factors such as increased public awareness, breakthrough infections, and long COVID-19. Future studies are required to explore the potential risk factors of oral sensory and anaphylactic SEs to verify whether they are causally linked to COVID-19 vaccines.
AUTHOR CONTRIBUTIONS
Conceptualization: Abanoub Riad; methodology: Abanoub Riad, and Sameh Attia; validation: Abanoub Riad, Arkadiusz Dziedzic, and Sameh Attia; formal analysis: Abanoub Riad; investigation: Nelly schulz-Weidner; writing—original draft preparation: Abanoub Riad, Arkadiusz Dziedzic and Sameh Attia; writing—review and editing: Nelly schulz-Weidner, and Hans-Peter Howaldt; supervision: Abanoub Riad; project administration: Abanoub Riad; funding acquisition: Sameh Attia and Hans-Peter Howaldt All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
Open Access funding enabled and organized by Projekt DEAL. The work of A.R. is supported by the NPO “Systemic Risk Institute” number LX22NPO5101, funded by the European Union - Next Generation EU (Ministry of Education, Youth and Sports, NPO: EXCELES).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available at: https://www.adrreports.eu/en/search.html




